Published online Sep 26, 2017. doi: 10.4330/wjc.v9.i9.742
Peer-review started: January 16, 2017
First decision: February 20, 2017
Revised: June 30, 2017
Accepted: July 14, 2017
Article in press: July 17, 2017
Published online: September 26, 2017
Processing time: 255 Days and 22.7 Hours
To identify predictors of need for repeat procedures after initial atrial fibrillation (AF) ablation.
We identified a cohort undergoing first time AF ablation at our institution from January 2004 to February 2014 who had cardiac magnetic resonance (CMR) imaging performed prior to ablation. Clinical variables and anatomic characteristics (determined from CMR) were assessed as predictors of need for repeat ablation. The decision regarding need for and timing of repeat ablation was at the discretion of the treating physician.
From a cohort of 331 patients, 142 patients (43%) underwent repeat ablation at a mean of 13.6 ± 18.4 mo after the index procedure. Both male gender (81% vs 71%, P = 0.05) and lower ejection fraction (57.4% ± 10.3% vs 59.8% ± 9.4%, P = 0.04) were associated with need for repeat ablation. On pre-ablation CMR, mean pulmonary vein (PV) diameters were significantly larger in all four PVs among patients requiring repeat procedures. In multivariate analysis, increased right superior PV diameter significantly predicted need for repeat ablation (odds ratio 1.08 per millimeter increase in diameter, 95%CI: 1.00-1.16, P = 0.05). There were also trends toward significance for increased left and right inferior PV sizes among those requiring repeat procedures.
Increased PV size predicts the need for repeat AF ablation, with each millimeter increase in PV diameter associated with an approximately 5%-10% increased risk of requiring repeat procedures.
Core tip: Among patients undergoing initial atrial fibrillation ablation, those with larger pulmonary vein (PV) size determined by pre-procedure cardiac magnetic resonance imaging had an increased likelihood of needing repeat ablation procedures. Each millimeter increase in PV diameter was associated with an approximately 5%-10% increased risk of requiring repeat procedures.
- Citation: Desai Y, Levy MR, Iravanian S, Clermont EC, Kelli HM, Eisner RL, El-Chami MF, Leon AR, Delurgio DB, Merchant FM. Clinical and anatomic predictors of need for repeat atrial fibrillation ablation. World J Cardiol 2017; 9(9): 742-748
- URL: https://www.wjgnet.com/1949-8462/full/v9/i9/742.htm
- DOI: https://dx.doi.org/10.4330/wjc.v9.i9.742
Although catheter ablation can be an effective treatment strategy for atrial fibrillation (AF), approximately 1 in 6 patients will undergo repeat ablation within 1 year of their initial ablation procedure[1]. This has motivated the search for clinical and demographic parameters that might predict an increased likelihood of AF recurrence and need for repeat ablation.
Although many studies have assessed predictors of AF recurrence after ablation, it is unclear whether there are additional relevant predictors of need for repeat ablation. Among clinical variables, the pattern of AF (paroxysmal vs persistent), congestive heart failure, hypertension, tobacco use and gender have all been associated with risk of AF recurrence[2-4], as have serum biomarkers such as C-reactive protein (CRP)[5]. Anatomic characteristics identified on cardiac imaging have also been evaluated as predictors of AF recurrence. Prior studies have suggested that larger left atrial (LA) size and lower left ventricle ejection fraction (LVEF) are associated with increased AF recurrence after ablation, although a meta-analysis demonstrated significant heterogeneity across studies in the predictive capacity of these parameters[6]. Although the pulmonary veins (PVs) are known to play an important role in the pathophysiology of AF and prior studies have assessed differences in PV anatomy and geometry between patients with and without AF, the role of PV anatomic features as predictors of AF recurrence and need for repeat ablation have not been well characterized.
In this analysis, we sought to identify predictors of the need for repeat ablation in a cohort of patients undergoing initial AF ablation.
The Emory University institutional review board approved the study protocol. Patients at Emory University Hospital Midtown undergoing initial catheter ablation for AF between January 2004 and February 2014 who had pre-procedure cardiac magnetic resonance (CMR) imaging performed were included in this analysis. Baseline demographic data, clinical covariates, and procedural details were ascertained by review of electronic medical records. The decision to perform AF ablation along with specific details of the ablation strategy and peri-procedural management was performed at the discretion of the treating physician. PV isolation was the primary goal of all procedures, with additional substrate modification performed at operator discretion. The decision regarding need for and timing of repeat ablation was also left to the discretion of each operator.
All patients included in this analysis underwent pre-procedure gadolinium-enhanced CMR to delineate LA and PV anatomy. CMR was performed on a 1.5 Tesla Philips Intera® magnetic resonance imaging (MRI) scanner (Amsterdam, The Netherlands) using a five-element phased-array cardiac coil. PV anatomy was defined using turbospin echo and gradient echo imaging in axial and double oblique planes following administration of gadopentetate dimeglumine (Magnevist®) or gadobenate dimeglumine (MultiHance®) at a dose of 0.075-0.10 mmol/kg. Orthogonal projections of angiographic images were used to measure PV and LA dimensions[7].
Continuous variables are presented as mean ± SD, and categorical data are summarized as frequencies and percentages. Comparisons across groups were performed using the Student’s t test or χ2 test, as appropriate. A binomial logistic regression of variables with univariate P-value ≤ 0.1 was used for the multivariate analysis. For all comparisons, a two-tailed P < 0.05 was considered to be statistically significant. Analysis was performed using MATLAB software (Mathworks, Inc., Natick, MA, United States).
A cohort of 331 patients underwent first time AF ablation with pre-ablation CMR scans. Of the entire cohort, 142 (43%) underwent repeat ablation at a mean of 13.6 ± 18.4 mo after the initial procedure. Among repeat procedures, 61% were performed primarily for recurrent AF and the remaining were performed primarily for organized atrial tachycardias. Touch-up lesions were performed on at least one PV for 69% of patients upon repeat ablation.
Across the entire cohort at the initial procedure, mean age was 58.4 ± 10.3 years and 24% had persistent AF, without significant differences between those undergoing a single vs repeat procedures. During the index ablation, 91% of patients had radiofrequency (RF) ablation and the remaining had Cryoballoon ablation, again without significant differences in the single vs repeat procedure groups. In addition to PV isolation, 101 (31%) patients underwent additional substrate modification during the initial procedure, including 79 patients who underwent linear lesions (either mitral annulus or LA roof) and 55 patients who had LA complex fractionated atrial electrograms (CFAEs) ablated. Duration of the first ablation procedure, defined as the elapsed time between initial and final ablation lesions, was longer in patients who required repeat procedures, although the difference was not significant (148.4 ± 53.5 min vs 138.3 ± 55.2 min, P = 0.11).
Baseline clinical characteristics, stratified by patients with and without repeat ablation are shown in Table 1. Males were more likely to undergo repeat ablation (81% vs 71%, P = 0.05). Left ventricular ejection fraction was lower in patients undergoing repeat ablation, although the absolute difference between groups was small (57.4% ± 10.3% vs 59.8% ± 9.4%, P = 0.04). Other clinical parameters, including the prevalence of hypertension, coronary artery disease, diabetes mellitus and obstructive sleep apnea were similar between groups. Medications at the time of initial ablation were also similar.
| Parameter | Single ablation (n = 189) | Repeat ablation (n = 142) | P value |
| Age (yr) | 59.2 ± 10.8 | 57.4 ± 9.5 | 0.12 |
| Male gender | 135 (71) | 115 (81) | 0.05 |
| Left ventricular ejection fraction | 59.8 ± 9.4 | 57.4 ± 10.3 | 0.04 |
| Hypertension | 114 (61) | 79 (57) | 0.46 |
| Coronary artery disease | 29 (16) | 15 (11) | 0.22 |
| Diabetes mellitus, type II | 13 (7) | 13 (9) | 0.43 |
| CVA or TIA | 3 (2) | 1 (1) | 0.47 |
| Obstructive sleep apnea | 39 (21) | 24 (17) | 0.42 |
| Congestive heart failure | 13 (7) | 7 (5) | 0.48 |
| Persistent atrial fibrillation | 41 (22) | 37 (27) | 0.31 |
| Medications at initial ablation | |||
| Beta blocker | 90 (49) | 72 (53) | 0.45 |
| Calcium channel blocker | 28 (15) | 24 (18) | 0.53 |
| ACE-I or ARB | 45 (24) | 32 (24) | 0.89 |
| Statin | 66 (35) | 43 (32) | 0.47 |
| Warfarin | 100 (54) | 88 (64) | 0.06 |
| Direct OAC | 62 (33) | 33 (24) | 0.07 |
| Anti-arrhythmic drug | |||
| Class III | |||
| Amiodarone | 19 (10) | 12 (9) | 0.68 |
| Dronedarone | 27 (15) | 19 (14) | 0.89 |
| Sotalol | 33 (18) | 23 (17) | 0.85 |
| Dofetilide | 9 (5) | 12 (9) | 0.15 |
| Class Ic (Flecainide or Propafenone) | 54 (29) | 41 (30) | 0.83 |
| Procedural data | |||
| Ablation time (min) | 138.3 ± 55.2 | 148.4 ± 53.5 | 0.11 |
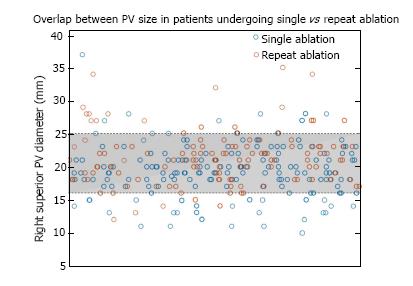
Anatomic predictors of need for repeat ablation identified by CMR are presented in Table 2. Mean left and right PV ostial diameters were significantly larger in patients undergoing repeat ablation: Right superior PV, 21.5 mm ± 4.3 mm vs 19.4 mm ± 4.0 mm (P < 0.01); right inferior PV, 19.6 mm ± 5.8 mm vs 18.0 mm ± 3.5 mm (< 0.01); left superior PV, 18.7 mm ± 3.0 mm vs 17.7 mm ± 3.4 mm (P < 0.01); left inferior PV, 18.6 mm ± 5.0 mm vs 17.0 mm ± 2.7 mm (P < 0.01). Although on average patients requiring repeat procedures had larger PVs, there was significant overlap in the distributions, making it difficult to identify clinically meaningful thresholds to predict an increased risk of need for repeat ablation. For example, in the distribution of right superior PV diameter, only 5% of patients with a single ablation had diameters > 25 mm, and among patients requiring repeat procedures, only 4% had right superior PV diameters < 16 mm (Figure 1). However, 80% of the measurements fell between 16 and 25 mm with significant overlap between those undergoing a single vs repeat procedures (Figure 1). Cumulative PV diameter was also significantly larger in patients who required repeat ablation: 78.5 ± 11.2 mm vs 71.6 ± 9.5 mm (P < 0.01), although there was still significant overlap in size compared with those who did not undergo repeat procedures. Of the 142 patients in the repeat ablation group, 96 (68%) required PV touch-up lesions during the second ablation. Patients who required touch-up lesions were more likely to have larger left inferior PV diameter on MRI before initial ablation: 19.1 ± 5.7 mm vs 17.5 ± 3.0 mm (P = 0.045). Sizes of the other PVs were not significantly different between those who did and did not require PV touch-up at the second procedure.
| Pre-ablation size parameters | Single ablation (n = 189) | Repeat ablation (n = 142) | P value |
| Right atrial area (cm2)1 | 23.0 ± 5.8 | 24.4 ± 5.4 | 0.08 |
| Left atrial area (cm2)1 | 28.0 ± 5.3 | 29.3 ± 6.2 | 0.13 |
| Pulmonary vein ostial diameter (mm) | |||
| Right superior vein | 19.4 ± 4.0 | 21.5 ± 4.3 | < 0.01 |
| Right inferior vein | 18.0 ± 3.5 | 19.6 ± 5.8 | < 0.01 |
| Left superior vein | 17.7 ± 3.4 | 18.7 ± 3.0 | < 0.01 |
| Left inferior vein | 17.0 ± 2.7 | 18.6 ± 5.0 | < 0.01 |
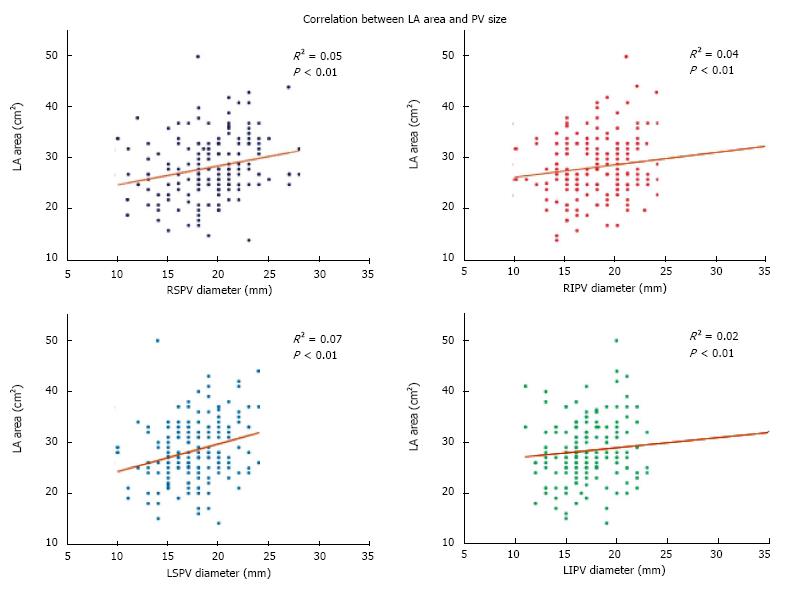
Mean right (24.4 ± 5.4 cm2vs 23.0 ± 5.8 cm2, P = 0.08) and left (29.3 ± 6.2 cm2vs 28.0 ± 5.3 cm2, P = 0.13) atrial areas assessed by CMR were numerically larger in patients with repeat ablation, although the differences were not significant. Of note, due to evolution in the protocol for measuring atrial volumes by CMR at our institution, right and LA area data were only available for 204 out of 331 patients. There was a statistically significant but modest direct correlation between PV size and LA area for all but the left inferior PV (Figure 2). A multivariate linear regression of all 4 PVs with LA area was also significant (R2 = 0.11, P < 0.01), demonstrating a direct relationship between PV and LA size. Male gender was also associated with larger PV size, although the results were only significant for the right superior PV [odds ratio (OR) = 1.10, 95%CI: 1.03-1.18, P < 0.01] and left superior PV (OR = 1.19, 95%CI: 1.09-1.31, P < 0.01).
Results of an analysis to identify multivariate clinical and anatomic predictors of need for repeat ablation are presented in Table 3. The only multivariate predictor of need for repeat ablation was larger right superior PV diameter (OR = 1.08 per millimeter increase in diameter, 95%CI: 1.00-1.16, P = 0.05). There were also trends toward significance in multivariate analysis for increased left and right inferior PV dimensions as predictors of need for repeat ablation. Clinical variables including male gender and LVEF were no longer significant predictors of need for repeat ablation after multivariate adjustment. It should be noted that despite a univariate P < 0.1 (P = 0.08), we excluded RA area from the multivariate analysis because only a small percentage of patients had data available.
| Variable | Odds ratio (95%CI) | P value |
| Clinical parameters | ||
| Male gender | 1.53 (0.77-3.05) | 0.23 |
| LVEF | 0.98 (0.95-1.01) | 0.25 |
| Warfarin | 1.04 (0.43-2.51) | 0.92 |
| Direct OAC | 0.59 (0.24-1.46) | 0.25 |
| Anatomic parameters | ||
| Right superior PV diameter | 1.08 (1.00-1.16) | 0.05 |
| Right inferior PV diameter | 1.07 (0.99-1.15) | 0.09 |
| Left superior PV diameter | 1.05 (0.95-1.16) | 0.36 |
| Left inferior PV diameter | 1.10 (0.99-1.22) | 0.07 |
In this cohort of 331 patients undergoing first time AF ablation, both clinical parameters including male gender and LVEF and anatomic characteristics assessed by CMR, most notably increased PV size, were associated with need for repeat ablation. However, in multivariate analysis, only increased PV size remained a significant predictor, suggesting that clinical factors may have limited utility in predicting the likelihood of repeat ablation. These findings also highlight the possibility that pre-procedure imaging may be useful in counseling patients undergoing initial AF ablation on the likelihood of needing repeat procedures and may facilitate more informed decision-making.
In our cohort, male gender was more prevalent among those requiring repeat ablation. Our findings regarding male gender are consistent with the results from the STOP-AF trial, in which the only clinical parameter predictive of early recurrence was male sex[4]. Interestingly, in our analysis, male gender was correlated with PV diameter, so it is conceivable that male gender is a marker for larger PV size and was thus no longer significant in multivariate analysis once PV size was taken into account.
Left ventricular ejection fraction was lower in patients undergoing repeat ablation, which is also consistent with previous findings looking at predictors of AF recurrence[8]. It should be noted, however, that in our analysis mean ejection fractions were in the normal range in both groups (single and repeat ablations) and the absolute difference in LVEF, although significant, was small. Such small differences in LVEF within the normal range are unlikely to have any clinically meaningful impact in helping to risk stratify patients likely to need multiple procedures.
None of the other clinical parameters in our study were significantly different between the cohorts who had a single ablation vs those who required repeat procedures. This corroborates the recent findings of Al-Hijji et al[9], who studied predictors of repeat catheter ablation in a large study of over 8600 patients, and found no association between congestive heart failure, hypertension and diabetes and need for repeat ablation. Other studies have implicated obstructive sleep apnea in the pathophysiology of AF[10], and, indeed, the total prevalence within our study population was 19%-greater than typical estimates of between 3%-7% in the general population[11]. However, the proportion of patients with OSA was not significantly different among patients requiring repeat ablation in our cohort. Broadly speaking, our data along with others suggest that clinical variables likely have limited utility in identifying those patients most likely to require repeat ablation procedures.
In contrast to clinical variables, several anatomic predictors assessed by pre-ablation CMR were significantly different between those undergoing single vs. repeat ablations in our cohort. Previous studies have assessed anatomic predictors of AF recurrence after ablation. Two studies which used pre-ablation CT to characterize PV and LA anatomy found that anomalous PV anatomy (e.g., presence of left common PV trunk or presence of middle accessory PVs) was not correlated with procedure outcome[12,13]. To our knowledge, only one other study investigated the effect of PV size. Our findings corroborate the results of Hauser et al[14] who reported that patients with at least one PV ostial area larger than 461 mm2 were more likely to have early recurrence of AF and those with at least one PV area larger than 371 mm2 were more likely to have late recurrence. The results of our multivariate analysis suggest that an increase in PV diameter of one millimeter is associated with a roughly 5%-10% increased likelihood of requiring a repeat ablation.
Although the pathophysiology of AF is not fully understood, it is known that the myocardial sleeves extending around the PVs are sites of enhanced automaticity and anisotropic conduction which may facilitate re-entry and provide some of the triggers and substrate necessary for AF[15]. Several hypotheses may explain why larger PVs are associated with an increased likelihood of need for repeat ablation. Since the majority of patients in our study had point-by-point RF ablation, it is conceivable that with larger veins, permanent and transmural isolation is more difficult to achieve due to the need for larger/wider circumferential lesions resulting in a higher likelihood of gaps or recovery of conduction. Patients who required repeat ablation had numerically (although not statistically significant) longer initial ablation times, which may reflect a wider area requiring ablation around larger PVs. In contrast, rather than a purely anatomic explanation, it is also conceivable that larger PV size may be associated with larger LA size and reflect a more advanced atrial substrate or a higher prevalence of risk factors which may contribute to recurrence after ablation and need for repeat procedures.
Previous studies have shown that LA size is larger in patients with AF, and that larger LA size is an independent predictor of AF recurrence after ablation[16]. However, the association between LA size and PV size is inconsistent and not all studies have demonstrated a direct relationship[17]. In our cohort, LA size was weakly correlated with PV diameter. LA size was numerically larger in patients requiring repeat ablation, but the difference was not statistically significant. However, due to an evolution in the technique for measurement and reporting of atrial volumes at our institution during the course of this study, we were only able to report LA area in 204 of the 331 patients (61%), which raises the possibility that we were underpowered to detect a significant difference in LA size.
We used repeat ablation, as opposed to AF recurrence, as the primary endpoint for this study. Whereas AF recurrence is an objective measure and much has been reported about predictors of AF recurrence after ablation, need for repeat ablation is a more subjective endpoint and has been less well validated. Although thresholds for performing repeat ablation may vary between providers and across different patient circumstances, the need for repeat procedures has an important impact on resource utilization and is an important metric when counseling patients on expected outcomes after an initial procedure. We chose not to report data on AF recurrence in this cohort. During the time course covered by this analysis, many institutions, including ours, have evolved to more rigorous monitoring for recurrent arrhythmias after ablation, as reflected in the most recent HRS/EHRA/ECAS consensus statement on AF ablation[18]. Given this evolution, along with increasing numbers of patients with implantable devices capable of detecting AF, it is likely that our ability to detect clinically silent recurrent AF has improved significantly which would confound the results of any analysis looking at AF recurrence as an endpoint.
As an additional limitation, we cannot rule out the possibility that some patients underwent repeat procedures at another facility after having an initial ablation performed at our institution and therefore, would not have been captured as needing repeat procedures for the purpose of this analysis.
Due to evolution in the technique for measuring and reporting atrial volumes on CMR at our institution, we were only able to report right and LA volumes on a subset of patients in the cohort and therefore, may have been underpowered for analyses involving atrial volumes. Lastly, we did not have data available to assess other anatomic parameters that may affect ablation outcomes, such as mitral valve pathology and PV anatomic variants.
Our data demonstrate that increased PV size is an important predictor of outcomes after AF ablation, with each millimeter increase in PV diameter associated with a roughly 5%-10% increased risk of needing a repeat procedure. These findings suggest that results of pre-procedure cross-sectional imaging may be useful in counseling patients undergoing initial AF ablation on the likelihood of needing repeat procedures and may facilitate more informed decision-making. Additional study will be needed to determine whether ablation strategies can be altered at the time of initial ablation in patients with large PVs to mitigate the increased risk of needing repeat procedures.
Although many studies have assessed predictors of atrial fibrillation (AF) recurrence after ablation, it is unclear whether there are additional relevant predictors of need for repeat ablation. In this study, the authors analyzed clinical and anatomic predictors of need for repeat AF ablation.
A significant percentage of patients require repeat procedures after initial AF ablation and tools to identify those at highest risk of needing repeat procedures would be useful.
Larger pulmonary vein (PV) size determined by pre-procedure cardiac magnetic resonance imaging had an increased likelihood of needing repeat ablation procedures. Each millimeter increase in PV diameter was associated with an approximately 5%-10% increased risk of requiring repeat procedures.
The data suggest that pre-procedure magnetic resonance imaging may be useful in identifying individuals at highest risk for needing repeat AF ablation procedures.
The manuscript is well written and highlights a popular topic with AF recurrence after pulmonary vein isolation.
Manuscript source: Unsolicited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Boos CJ, Celikyurt YU, Letsas K S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Piccini JP, Lopes RD, Kong MH, Hasselblad V, Jackson K, Al-Khatib SM. Pulmonary vein isolation for the maintenance of sinus rhythm in patients with atrial fibrillation: a meta-analysis of randomized, controlled trials. Circ Arrhythm Electrophysiol. 2009;2:626-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 214] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 2. | Al Chekakie MO, Akar JG, Wang F, Al Muradi H, Wu J, Santucci P, Varma N, Wilber DJ. The effects of statins and renin-angiotensin system blockers on atrial fibrillation recurrence following antral pulmonary vein isolation. J Cardiovasc Electrophysiol. 2007;18:942-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Themistoclakis S, Schweikert RA, Saliba WI, Bonso A, Rossillo A, Bader G, Wazni O, Burkhardt DJ, Raviele A, Natale A. Clinical predictors and relationship between early and late atrial tachyarrhythmias after pulmonary vein antrum isolation. Heart Rhythm. 2008;5:679-685. [PubMed] [DOI] [Full Text] |
| 4. | Andrade JG, Khairy P, Macle L, Packer DL, Lehmann JW, Holcomb RG, Ruskin JN, Dubuc M. Incidence and significance of early recurrences of atrial fibrillation after cryoballoon ablation: insights from the multicenter Sustained Treatment of Paroxysmal Atrial Fibrillation (STOP AF) Trial. Circ Arrhythm Electrophysiol. 2014;7:69-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 116] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 5. | Dernellis J, Panaretou M. C-reactive protein and paroxysmal atrial fibrillation: evidence of the implication of an inflammatory process in paroxysmal atrial fibrillation. Acta Cardiol. 2001;56:375-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 154] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 6. | Balk EM, Garlitski AC, Alsheikh-Ali AA, Terasawa T, Chung M, Ip S. Predictors of atrial fibrillation recurrence after radiofrequency catheter ablation: a systematic review. J Cardiovasc Electrophysiol. 2010;21:1208-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Merchant FM, Levy MR, Iravanian S, Clermont EC, Kelli HM, Eisner RL, El-Chami MF, Leon AR, Delurgio DB. Pulmonary vein anatomy assessed by cardiac magnetic resonance imaging in patients undergoing initial atrial fibrillation ablation: implications for novel ablation technologies. J Interv Card Electrophysiol. 2016;46:89-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Cha YM, Friedman PA, Asirvatham SJ, Shen WK, Munger TM, Rea RF, Brady PA, Jahangir A, Monahan KH, Hodge DO. Catheter ablation for atrial fibrillation in patients with obesity. Circulation. 2008;117:2583-2590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 9. | Al-Hijji MA, Deshmukh AJ, Yao X, Mwangi R, Sangaralingham LR, Friedman PA, Asirvatham SJ, Packer DL, Shah ND, Noseworthy PA. Trends and predictors of repeat catheter ablation for atrial fibrillation. Am Heart J. 2016;171:48-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Zhang L, Hou Y, Po SS. Obstructive Sleep Apnoea and Atrial Fibrillation. Arrhythm Electrophysiol Rev. 2015;4:14-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1329] [Cited by in RCA: 1501] [Article Influence: 88.3] [Reference Citation Analysis (1)] |
| 12. | Hof I, Chilukuri K, Arbab-Zadeh A, Scherr D, Dalal D, Nazarian S, Henrikson C, Spragg D, Berger R, Marine J. Does left atrial volume and pulmonary venous anatomy predict the outcome of catheter ablation of atrial fibrillation? J Cardiovasc Electrophysiol. 2009;20:1005-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 135] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 13. | Stabile G, Anselmino M, Soldati E, De Ruvo E, Solimene F, Iuliano A, Sciarra L, Bongiorni MG, Calò L, Gaita F. Effect of left atrial volume and pulmonary vein anatomy on outcome of nMARQ™ catheter ablation of paroxysmal atrial fibrillation. J Interv Card Electrophysiol. 2017;48:201-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Hauser TH, Essebag V, Baldessin F, McClennen S, Yeon SB, Manning WJ, Josephson ME. Prognostic value of pulmonary vein size in prediction of atrial fibrillation recurrence after pulmonary vein isolation: a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson. 2015;17:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Chen SA, Chen YJ, Yeh HI, Tai CT, Chen YC, Lin CI. Pathophysiology of the pulmonary vein as an atrial fibrillation initiator. Pacing Clin Electrophysiol. 2003;26:1576-1582. [PubMed] |
| 16. | Sohns C, Sohns JM, Vollmann D, Lüthje L, Bergau L, Dorenkamp M, Zwaka PA, Hasenfuß G, Lotz J, Zabel M. Left atrial volumetry from routine diagnostic work up prior to pulmonary vein ablation is a good predictor of freedom from atrial fibrillation. Eur Heart J Cardiovasc Imaging. 2013;14:684-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | den Uijl DW, Tops LF, Delgado V, Schuijf JD, Kroft LJ, de Roos A, Boersma E, Trines SA, Zeppenfeld K, Schalij MJ. Effect of pulmonary vein anatomy and left atrial dimensions on outcome of circumferential radiofrequency catheter ablation for atrial fibrillation. Am J Cardiol. 2011;107:243-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 18. | Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, Crijns HJ, Damiano RJ Jr, Davies DW, DiMarco J, Edgerton J, Ellenbogen K, Ezekowitz MD, Haines DE, Haissaguerre M, Hindricks G, Iesaka Y, Jackman W, Jalife J, Jais P, Kalman J, Keane D, Kim YH, Kirchhof P, Klein G, Kottkamp H, Kumagai K, Lindsay BD, Mansour M, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Nakagawa H, Natale A, Nattel S, Packer DL, Pappone C, Prystowsky E, Raviele A, Reddy V, Ruskin JN, Shemin RJ, Tsao HM, Wilber D; Heart Rhythm Society Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm. 2012;9:632-696.e21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1237] [Cited by in RCA: 1317] [Article Influence: 101.3] [Reference Citation Analysis (0)] |