Published online Sep 26, 2017. doi: 10.4330/wjc.v9.i9.715
Peer-review started: January 14, 2016
First decision: March 7, 2016
Revised: October 9, 2016
Accepted: October 22, 2016
Article in press: October 24, 2016
Published online: September 26, 2017
Processing time: 622 Days and 14.8 Hours
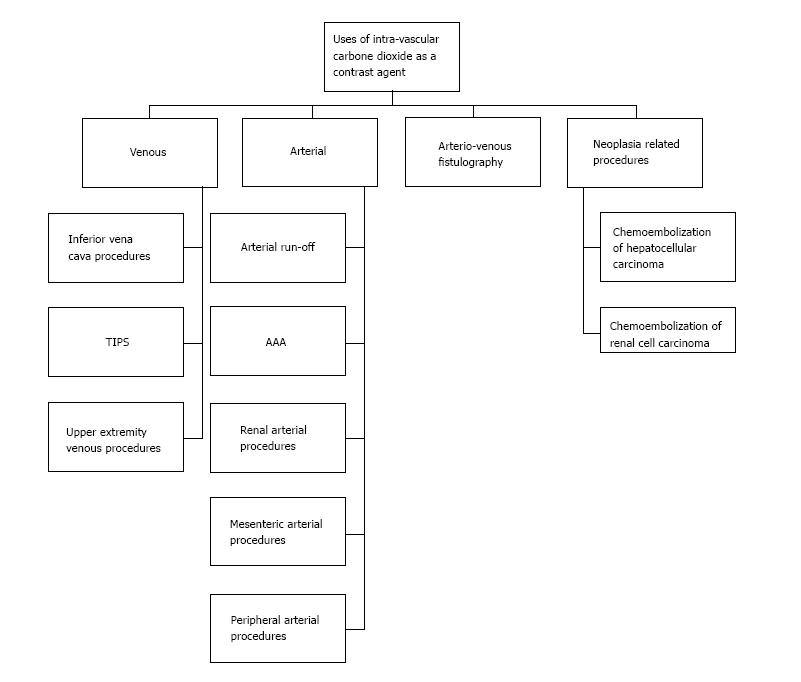
Use of X-ray contrast allows us to differentiate between two or more adjacent structures on radiographic studies. The X-ray contrast agent can be the one with increase X-ray absorption, like iodine and a barium X-ray contrast agent or the one with decrease X-ray absorption like air and carbon dioxide contrast agent. Each contrast agent possesses different risks and benefits in various ways. Carbon dioxide as an intravascular contrast agent can be used as an alternative intravascular contrast agent and has superior results in some cases. In patients with renal dysfunction or iodinated contrast allergy, the use of Iodinated Contrast Agent poses the risk of considerable morbidity. Similarly, use of Gadolinium is discouraged in subject with severe renal dysfunction. Use of carbon dioxide (CO2) as an intravascular contrast, offers an alternative in such patients for certain procedures, as it is not nephrotoxic and it does not incite allergic reactions. It is inexpensive, readily available and due to its unique physical properties, it can be used to image a wide variety of vascular beds and chambers. The aim of this paper is to systemically review the current literature to describe the indications, contraindications, adverse effects, instruments, precautions, latest methodologies and data supporting for the use of CO2 as a contrast agent.
Core tip: In patients with renal dysfunction or iodinated contrast allergy, use of iodinated contrast agent poses the risk of considerable morbidity. Similarly, use of gadolinium is discouraged in subject with severe renal dysfunction. Use of carbon dioxide (CO2) as an intravascular contrast offers an alternative in such patients for certain procedures, as it is not nephrotoxic and it does not incite allergic reactions. It is inexpensive, readily available and due to its unique physical properties it can be used to image a wide variety of vascular beds and chambers. This article describes the indications, contraindications, adverse effects, instruments, precautions, latest methodologies and data supporting for the use of CO2 as a contrast agent.
- Citation: Ali F, Mangi MA, Rehman H, Kaluski E. Use of carbon dioxide as an intravascular contrast agent: A review of current literature. World J Cardiol 2017; 9(9): 715-722
- URL: https://www.wjgnet.com/1949-8462/full/v9/i9/715.htm
- DOI: https://dx.doi.org/10.4330/wjc.v9.i9.715
In medical parlance, contrast is a mean which allows us to differentiate between two or more adjacent elements on a radiographic study. There are essentially two prototypes of X-ray contrast agents: (1) Positive agents (which increase X-ray absorption: Iodine or barium based); and (2) negative agents [decrease X-ray absorption: Air, carbon dioxide (CO2)][1]. In animal experiments (1940s) and later in human studies (1950s), CO2 enabled investigators to delineate both right and left heart structures. With the introduction of digital subtraction angiography (DSA) in 1980s, the image quality improved significantly[1]. Conditions where use of iodinated contrast agent (ICA) are precluded such as impaired kidney functions, dye allergy, CO2 may be used as an alternate contrast agent with comparable results and in some cases superior results[2-4].
Understanding of physical properties of CO2 is central for its use. Administration of CO2 needs extreme care. It is a colorless, odorless and significantly compressible gas. CO2 has low molecular weight as compared to ICA, is less viscous then blood and ICA and due to this property it can be used to image small collateral vessels. It displaces the blood in the vessels and acts as a negative contrast agent. This property creates a significant gradient between the radiographic density of the vessel wall and the lumen. DSA technique uses this difference in the densities to provide a contrast image. CO2 is more soluble than oxygen (O2) and dissolves in the blood within 2-3 min after injection. When mixed with water it creates carbonic acid (H2CO3) which dissociates into bicarbonate (HCO3-) and hydrogen (H+) ions carried by blood flow to the lungs. Reverse reaction happens in the lungs where the breakdown product of H2CO3, CO2 is then exhaled. These chemical reactions are facilitated by enzymes called carbonic anhydrases.
During its use, monitoring of vital signs is required. Capnography if available would be useful in monitoring the ventilation.
There are 3 commonly used methods of administering CO2. Preferred method is via automated injectors (automated CO2 mmanders). Hand held syringes have been used in the past but are not commonly used now due to increased risk of complications such as air contamination and explosive over dosage[5,6].
Automated CO2 mmanders: Have the utility of being handy, portable, safe and easy to use but their high cost (approximately 3000 USD)[7] make them an unpopular choice.
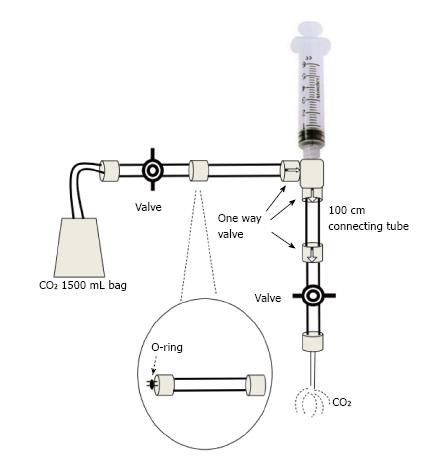
The modified plastic bag system with O-ring: Is a preferred method by some experts. Kit packs consisting of bag, tube and valves are available commercially (custom waste management kit by Merit Medical, South Jordan, UT; or Angio-Dynamics Queensbury, NY)[8,9]. The usual source of CO2 is an Aluminum or steel cylinder of medical grade CO2 which is about 99.99% pure, fitted in a series circuit with a valve, a gauge, a regulator, a diaphragm and an antibacterial filter. A 1500 mL plastic bag with a single port connected with a low pressure tube and a 2-way stopcock at the distal end of the tube is then connected to the CO2 cylinder. It is then filled and manually purged at least 3 times. The filled bag is then connected at its 2-way stopcock end with an O-ring connector which on the other end is connected with the delivery syringe (20-60 mL). There is a 1-way valve between the O-ring and the syringe. The syringe is then connected with another 1-way valve and then with a 100 cm connecting tube. The distal end of this tube has one more 1-way valve which is then connected with a 3-way valve. This 3-way valve can then be connected with the angiographic catheter. On the other port an additional syringe for back-bleeding or eliminating the air from the system can be attached (Figure 1). To fill the delivery syringe the plunger is simply retracted. The 1-way valves will allow the CO2 in the plastic bag to move into the syringe. The plunger can then be advanced at the desired rate and amount. The 1-way valves will allow the gas to move towards the 3-way valve which can then be adjusted depending on the ports required to be used. The angiographic catheter is at times filled with blood which can be cleared by using the additional syringe attached at the 3-way valve. Forceful boluses of 3-5 mL CO2 can be used to clear the catheter from any remaining fluid. The catheter can then be flushed with 1-3 mL of CO2 every 2-3 min. All the connections of the circuit need to be air tight to avoid any air aspiration or embolism. The plastic bag should be filled enough to remain flaccid as tightly filled bag may pose risk of overdose due to gas compression[9].
Underwater seal: This is a relatively simple, inexpensive and easier method but there may be a slight risk of air contamination and or inadvertent explosive administration of CO2 into the patient[10]. In this system, the CO2 source is connected to a regulator, a particle filter and a 3-way tap by connecting tubes. One end of 3-way valve has a sliding 2-way valve connected to a 60 cc syringe. The other end of 3-way valve has a tube serving as a simple under water seal by having the other end of the tube dipped in a bowel of saline. When the CO2 source is turned on and the 3-way valve is on to the syringe, the syringe will get filled without pulling the plunger, by the positive pressure of the CO2 coming from the source. Once the syringe is filled the 2-way valve is turned off and 3-way valve is turned to the under-water seal. Bubbles of CO2 would be seen in the bowel of saline coming out of the tube’s end. The CO2 source is then turned off and the 3-way valve is then turned on to the syringe and the water seal. The CO2 can then be purged through the water seal. This process of filling and purging can be repeated at least 3 times to make sure that there is only CO2 and no air in the tubing and syringe system. Then the filled syringe along with a 2-way valve turned off, can then be disconnected and attached to the angiography catheter. Right before it is connected to the angiography catheter, the 2-way valve is turned on to release the positive pressure in the syringe to come down to atmospheric pressure. This will avoid explosive administration and or over dosing of pressurized CO2 in the syringe but at the same time this may create a very small risk of air contamination. Only fully filled syringes should be used while using this method as half-filled syringes when opened to atmospheric pressure will certainly lead to higher risk of air contamination. The innovators of this system also described their experience of 5 years in over 250 patients and no directly related complications were noticed[10].
Typically 30-40 mL of CO2 is injected for abdominal aortography or IVC visualization. Twenty to thirty milliliter is used for lower extremity vessels and other aortic branches like celiac, superior mesenteric or renal arteries. The left renal artery which is more posteriorly located can be filled even with 10 mL if injected with patient lying on the right side. Injections can be repeated at approximately 3 min intervals. Thirty to fifty milliliter may be used for runoff studies by injecting at low rate of 10 mL/s.
The diagnostic accuracy is acceptable in comparison to contemporary ICA and in some conditions such as TIPS, CO2 is even rendered superior to the ICA.
Aortic aneurysm repairs: CO2 has been used in endovascular repairs of aortic aneurysms[11-14]. A recent prospective study of 72 patients with abdominal aortic aneurysm (AAA) endovascular repair demonstrated that CO2 has overall sensitivity of 84% and specificity of 72% as compared to ICA as the standard criterion for detection of endoleaks and in patients who are at risk of nephrotoxicity from ICA, CO2 can be used as an acceptable alternative to ICA[15]. Another study describes the outcomes of CO2-guided procedures are similar to those which are ICA-guided[14]. Additional benefit of CO2 use in endovascular repair of AAA is that an accessory catheter which is otherwise required for ICA may not be required for CO2 injection as it can be administered through the endograft sheath or femoral access sheath[13].
Aortography: CO2 may be used for aortography and for runoff studies in most patients[16]. If needed supplemental ICA imaging may be used in order to obtain additional information. To get the retrograde aortogram, CO2 may be injected retrograde through the femoral artery by percutaneous catheterization with a 4-Fr end-hold catheter (Cobra-shaped or shepherd hook catheter) or catheters with side-holes (Omni-flush, pigtail, Racquet, multipurpose). Contra-lateral superficial femoral arterial views can also be taken through the same port by moving the catheter into the contralateral superficial femoral artery. For antegrade views micro-catheters of 3-Fr may be used for popliteal, tibial and peroneal arteries. Use of intra-arterial nitroglycerine and or leg elevation may be done for better visualization of smaller vessels such as tibial and plantar branches.
Renal artery angiography (Figure 3): CO2 can be used in the assessment of renal artery stenosis, aneurysms, AV (arterio-venous) malformations, AV fistulas, renal artery stenting, invading tumors in renal veins or arteries, renal cell carcinomas, evaluation of transplanted kidney vascular stenosis and for its angioplasty and/or stenting, anastomotic stenosis, diffuse arterial disease related to chronic rejection and AV fistulas after renal transplant biopsy (in which case it may be superior to ICA)[17,18]. In such cases CO2 may be used as initial imaging modality to get an overview and then small dose ICA may be used for confirmation of the findings[11]. CO2 does not adequately fill the distal portion of renal artery very well in a supine patient, as it is located posterior to the aorta. In this situation, the patient may be turned on the side to bring the renal arteries superior with respect to the aorta. Recent studies have also demonstrated the use of CO2 in combination with intravascular ultrasound for successful vascular stenting. In a study of 18 patients, 27 successful renal artery stenting procedures were done using CO2 and intravascular ultrasound with good outcomes[19,20].
Inferior venae cava imaging: CO2 can be used for the placement of inferior venae cava (IVC) filters, IVC venous anomalies and thrombus visualization, recanalization of occlusion and estimation of IVC diameters (accuracy of about 97%). In a study of 50 patients, CO2 was used for IVC filter placement at the bedside in ICU setting. Only 2 of these patients required additional ICA for better visualization. The study concluded with positive results and favored the use of CO2 as first line contrast agent in ICU patients requiring IVC filter[21].
Portal vein imaging (portography): A very important utility of CO2 is in the delineation of the portal vein anatomy (wedged hepatic venography) during TIPS procedure (Figure 4). CO2 is found to be superior to ICA for this use and can be used as first line contrast agent for portography[22]. The reason is buoyancy and low viscosity of CO2 making it travelling through the sinusoids easily and deeply. In liver transplants, anastomosis can also be visualized using CO2. In a study of 16 patients, the utility of CO2 was compared with ICA for balloon-occluded retrograde trans-venous venography (BRTV) and obliteration (BRTO) for gastric varices and it was found that varices were visualized better with CO2 than with ICA and even in cases where ICA could not reach the varices, CO2 successfully delineated these varices (in 7 out of 16 patients), leading to successful obliteration of the varices[23]. According to some estimates, success rate of portal vein visualization with CO2 wedged hepatic venography is approximately 90%. A diagnostic catheter of 5-Fr can be used for wedged hepatic venography. Using the femoral or jugular vein approach, catheter can be advanced into a peripheral hepatic vein for wedging. It is also being used for multi-detector CT cholangiopancreatography. In a study of 73 patients, the feasibility of CO2 enhanced CT cholangiopancreatography was assessed and found to be very useful for interventional procedures[24].
Splenoportography: Can also be done by using CO2 in selected patients[25] such as a patient in which portal vein imaging study for patency is inconclusive[26]. Twenty-two to twenty-five gauge needle can be used to inject CO2 into the parenchyma of spleen. This is useful in pediatric patients as it obviates the catheterization of femoral artery for arterial portography. Endoscopic ultrasound guided direct portal venography with CO2 by using a small FNA needle has also been used in animal studies with favorable results[27].
Tumor embolization procedures: CO2 can be used for the following oncological embolization procedures: Embolization of renal cell carcinoma and its metastatic lesions in the bone, hepatocellular carcinoma[28,29], radiofrequency ablation and transcatheter arterial chemo-embolization of hepatocellular carcinoma (by using intra-arterial CO2 for enhancement for ultrasonography guidance)[30], uterine artery embolization in uterine leiomyoma[31]. These procedures can be optimized by using super-selective angiographic techniques with help of micro-catheters of 3 Fr.
Upper extremity venography: Can be performed using the CO2[32]. It can be useful for AV-fistula formation[33], insertions of trans-venous pacer wires[34], central venous catheters and for the delineation of any atypical vascular anatomy. The preferable site of injection is antecubital vein and a 21 gauge catheter may be used. In a series of 146 AV fistulography procedures using CO2 as the first line contrast agent, 141 cases required AV fistula intervention and in 115 of these cases intervention was performed successfully using CO2 alone. Rest of the cases required ICA for various reasons in addition to CO2 for intervention[35]. For AV fistula assessment, one needs to be careful of not letting CO2 reflux into arterial system due to potential risk of neurologic sequelae including infarction. Also there is a potential of overestimation of fistula stenosis.
Gastrointestinal bleeding: Due to increased compressibility and low viscosity it may be useful in detecting the site of occult bleeding or ongoing blood loss such as the gastrointestinal tract, with higher sensitivity than ICA. CO2 can also be used in selected angiographies for chronic mesenteric ischemia[36].
Contrast ultrasonography: CO2 can be used to enhance sonography by employing CO2 microbubbles. In a study where conventional sonography was compared with CO2 micro-bubble enhanced sonography; the former detected only 6 tumors however with CO2-microbubble enhanced sonography 14 tumors were detected and then treated successfully with radiofrequency ablation using CO2-microbubbles enhanced sonography[37].
Overall CO2 is a relatively safe agent[38]. In a study of 800 subjects, only one complication of transient colonic ischemia was reported. In another study of 1200 subjects only 7 subjects developed some kind complication. Livedo reticularis, bowel ischemia and renal dysfunction have been described after in 1 patient with CREST syndrome[38] (Table 1).
| Characteristics | Carbon dioxide | Iodinated contrast agents |
| Overall sensitivity | Less | Higher |
| Overall specificity | Less | Higher |
| Nephrotoxicity | No | Yes |
| Allergenic | No | Yes |
| Cost | Low | High |
| Ease of administration | Cumbersome | Easier |
| Limitations | Visibility and air contamination | Dose related toxicity and allergy |
| Delivery via small caliber catheters | Possible | Difficult |
| Radiation exposure | Increased if digital subtraction angiography used | Standard |
| Dose | Rate related toxicity | Volume related nephrotoxicity |
| Contraindications | Pulmonary-systemic communications; not for use in heart, brain or spinal vasculature | Allergy, nephrotoxicity |
| Hepatotoxicity | Rare | Rare |
| Quality of image | Good | Better |
| Procedure duration | Increased | Standard |
The adverse effects are primarily either dose related or buoyancy related. Majority of the adverse effects are due to “vapor-lock phenomenon” which result when large amounts of CO2 are injected or a small amount is injected too frequently with very short intervals causing trapping of CO2 gas column in the vessel and consequently obstructing the vessel. This may lead to ischemia of the tissues. Cases with transient mesenteric ischemia and ischemic colitis secondary to “vapor lock phenomenon” have been described in the literature. Similar mechanism may potentially precipitate right sided heart failure. Sometimes CO2 bubbles may accumulate in an aortic aneurysm and may cause blood flow obstruction, leading to tissue ischemia. Even a transient occlusion of inferior mesenteric artery may result in mesenteric ischemia. Typically, this happens with the use of excessive dose of CO2. Similarly a vapor lock may happen in the pulmonary artery and this may lead to significant hypotension. Air contamination may also cause vapor-lock phenomenon that is typically worse and more persistent. Usually CO2 bubbles in the pulmonary artery dissolve within 30 s. If they persist beyond 30 s then either air contamination or CO2 over-dosage should be suspected and the tubing system should be checked for any air leak. For hypotension secondary to vapor lock phenomenon, patient should be placed in Trendelenburg position or lateral decubitus positions. Aspirating the air using a catheter from the pulmonary artery should also be considered.
Due to its buoyancy the visualization of a dependent (inferior or caudal positioned) vessels may be suboptimal (such as visualization of renal arteries in supine position). This problem can be circumvented by putting the patient in lateral decubitus position. CO2 may get trapped in organs which are non-dependant and cause decreased blood flow or ischemia such as in transplanted kidneys or mesenteric vessels. If we place the patient in lateral decubitus position, the CO2 may remain trapped in right atrium instead of moving into pulmonary arteries. Changing the body position may help clearance in these situations. Similarly using low volumes of CO2 with adequate time intervals may help avoiding these adverse effects. Although 100 mL is the recommended maximum volume for arterial use and 50 mL for venous use, by using above mentioned precautions, larger total volume may be used. Vessels which are more anterior such as superior mesenteric artery (SMA), CO2 is useful in their evaluation, particularly for proximal mesenteric stenosis. For more distal assessment ICA probably provides superior imaging.
Due to its dissolution in blood soon after injection vessels with slower flow may not have adequate visualization and this may lead to overestimation of stenosis. Similarly due to the expansive nature of CO2 and elasticity of vessels, CO2 may lead to overestimation of the vessel diameter. This may cause errors in estimation of balloon or stent size during intervention procedures.
The use of CO2 for cerebral, spinal and or cardiac procedures should be avoided as there is a potential risk of ischemia to vital organs[39]. In animal models neurotoxicity has been reported after cerebral use. For the same reasons, before the use of CO2 presence of atrial or ventricular septal defects or pulmonary arterio-venous malformation should be ruled out to avoid the risk of paradoxical embolism to CNS and or coronary embolization. CO2 may also aggravate or worsen pulmonary arterial pressure therefore the use of this agent should be avoided in pulmonary hypertension.
There are some relative contraindications for the use of CO2 for upper extremity which are similar for other uses as well and these are the presence of cardiac septal defects, pulmonary AV malformations, pulmonary hypertension and severe emphysema. In a series of 146 arteriovenous fistulography procedures, in 3 cases when manual injection of CO2 into the brachial artery was performed, a reflux of the gas into the thoracic aorta occurred precipitating transient loss of consciousness[35].
Typically, CO2 angiography does not cause any significant changes in the serum osmolality or blood gas values[40] unless excessive quantities of CO2 are used or significant derangements of pulmonary function happen. Caution is required in cases where pulmonary functions are compromised such as in chronic obstructive pulmonary disease, as clearance of CO2 may be decreased. Doses of CO2 for diagnostic purposes are typically between 20-40 cc and it has no effect on vital signs. Any change in vital signs should prompt the considerations for air contamination or air trapping.
Peristaltic and breathing movements sometime may decrease the image quality of mesenteric CO2 angiography. This problem may be avoided by selective or superselective CO2 injection into the mesenteric arterial branch, getting additional mask images or using intravenous glucagon to suppress the peristalsis.
While using CO2, sedation should be avoided or minimized as any of the side effects of CO2 overdosing or air contamination may be missed in the presence of heavy sedation. During the procedure patients vital signs (pulse-oxymetry, blood pressure, heart rate, respiratory rate, ECG, and if possible capnography) need to be monitored closely. Any change in these parameters should raise the suspicion of CO2 over dosage or air contamination.
The utility of CO2 as contrast agent for CT angiography for abdominal aorta and peripheral vessels is also currently being evaluated[41,42]. In an animal study the use of CO2 micro-bubbles mixed in saline was compared with conventional CO2 gas and ICA and demonstrated that vessels can be depicted using X-ray angiography and CO2 micro-bubbles as enhancement[43]. CO2 bubbles sometimes may provide better visualization then plain CO2 gas with additional benefit of low dose requirement[44].
CO2 is useful in cases where ICA cannot be used due to allergy or impaired kidney functions. CO2 may be superior to ICA in certain procedures such as in TIPS.
Manuscript source: Unsolicited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Landesberg G, Maruyama H S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ
| 1. | Hawkins IF, Caridi JG. Carbon dioxide (CO2) digital subtraction angiography: 26-year experience at the University of Florida. Eur Radiol. 1998;8:391-402. [PubMed] |
| 2. | Shipovskiĭ VN, Kurbanov RV, Saakian AM, Marov KB. Carboxyangiography: a new type of opacification in angiographic practice--first clinical experience. Angiol Sosud Khir. 2010;16:73-79. [PubMed] |
| 3. | Hawkins IF, Cho KJ, Caridi JG. Carbon dioxide in angiography to reduce the risk of contrast-induced nephropathy. Radiol Clin North Am. 2009;47:813-825, v-vi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Chanis G. [Carbon dioxide angiography: technique and indications]. Rev Med Panama. 2002;27:18-25. [PubMed] |
| 5. | Corazza I, Rossi PL, Feliciani G, Pisani L, Zannoli S, Zannoli R. Mechanical aspects of CO2 angiography. Phys Med. 2013;29:33-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Kawasaki D, Fujii K, Fukunaga M, Masutani M, Nakata A, Masuyama T. Safety and efficacy of endovascular therapy with a simple homemade carbon dioxide delivery system in patients with ileofemoral artery diseases. Circ J. 2012;76:1722-1728. [PubMed] |
| 7. | AngioAdvancements. Products. [accessed 2015 Mar 21]. Available from: http://www.angioadvancements.com/Products.html. |
| 8. | Funaki B. Carbon dioxide angiography. Semin Intervent Radiol. 2008;25:65-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Hawkins IF, Caridi JG, Klioze SD, Mladinich CR. Modified plastic bag system with O-ring fitting connection for carbon dioxide angiography. AJR Am J Roentgenol. 2001;176:229-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Cronin P, Patel JV, Kessel DO, Robertson I, McPherson SJ. Carbon dioxide angiography: a simple and safe system of delivery. Clin Radiol. 2005;60:123-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Knipp BS, Escobar GA, English S, Upchurch GR, Criado E. Endovascular repair of ruptured aortic aneurysms using carbon dioxide contrast angiography. Ann Vasc Surg. 2010;24:845-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Lee AD, Hall RG. An evaluation of the use of carbon dioxide angiography in endovascular aortic aneurysm repair. Vasc Endovascular Surg. 2010;44:341-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Criado E, Kabbani L, Cho K. Catheter-less angiography for endovascular aortic aneurysm repair: a new application of carbon dioxide as a contrast agent. J Vasc Surg. 2008;48:527-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Chao A, Major K, Kumar SR, Patel K, Trujillo I, Hood DB, Rowe VL, Weaver FA. Carbon dioxide digital subtraction angiography-assisted endovascular aortic aneurysm repair in the azotemic patient. J Vasc Surg. 2007;45:451-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Huang SG, Woo K, Moos JM, Han S, Lew WK, Chao A, Hamilton A, Ochoa C, Hood DB, Rowe VL. A prospective study of carbon dioxide digital subtraction versus standard contrast arteriography in the detection of endoleaks in endovascular abdominal aortic aneurysm repairs. Ann Vasc Surg. 2013;27:38-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Ho CF, Chern MS, Wu MH, Wu HM, Lin WC, Chang CY, Chen MC, Chou TY. Carbon dioxide angiography in lower limbs: a prospective comparative study with selective iodinated contrast angiography. Kaohsiung J Med Sci. 2003;19:599-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Cheng PM, Van Allan RJ. Superior sensitivity of angiographic detection of arteriovenous fistula after biopsy in a renal allograft with CO2 compared with iodinated contrast medium. J Vasc Interv Radiol. 2006;17:1963-1966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Pérez L, Lecannelier E, Fernández A, Parra J, Aburto G, Robles I. [Renal angioplasty with stent using CO2 as contrast medium: report of one case]. Rev Med Chil. 2007;135:365-369. [PubMed] |
| 19. | Kawasaki D, Fujii K, Fukunaga M, Fukuda N, Masuyama T, Ohkubo N, Kato M. Safety and efficacy of carbon dioxide and intravascular ultrasound-guided stenting for renal artery stenosis in patients with chronic renal insufficiency. Angiology. 2015;66:231-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Kusuyama T, Iida H, Mitsui H. Intravascular ultrasound complements the diagnostic capability of carbon dioxide digital subtraction angiography for patients with allergies to iodinated contrast medium. Catheter Cardiovasc Interv. 2012;80:E82-E86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Schmelzer TM, Christmas AB, Jacobs DG, Heniford BT, Sing RF. Imaging of the vena cava in the intensive care unit prior to vena cava filter insertion: carbon dioxide as an alternative to iodinated contrast. Am Surg. 2008;74:141-145. [PubMed] |
| 22. | Maruyama H, Okugawa H, Ishibashi H, Takahashi M, Kobayashi S, Yoshizumi H, Yokosuka O. Carbon dioxide-based portography: an alternative to conventional imaging with the use of iodinated contrast medium. J Gastroenterol Hepatol. 2010;25:1111-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (1)] |
| 23. | Koizumi J, Hashimoto T, Myojin K, Itou C, Hara T, Sekiguchi T, Ichikawa T, Imai Y, Kagawa T, Nagata N. Carbon dioxide (CO2) vs iodinated contrast digital subtraction angiography during balloon-occluded retrograde transvenous obliteration (BRTO) using foam sclerosant for gastric varices. J Vasc Interv Radiol. 2012;23:1453-1459.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Sugimoto M, Yasuda H, Koda K, Suzuki M, Yamazaki M, Tezuka T, Kosugi C, Higuchi R, Watayo Y, Yagawa Y. Carbon dioxide-enhanced virtual MDCT cholangiopancreatography. J Hepatobiliary Pancreat Sci. 2010;17:601-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Yu SC, Cho KJ. Carbon dioxide wedged arterial splenoportography: a new technique--a case report and an experimental study. Acta Radiol. 2009;50:265-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Teng GJ. [Novel use of CO2 splenoportography: via spleen percutaneous fine needle puncture portography]. Zhonghua Yixue Zazhi. 2005;85:295-296. [PubMed] |
| 27. | Giday SA, Ko CW, Clarke JO, Shin EJ, Magno P, Jagannath SB, Buscaglia JM, Kantsevoy SV. EUS-guided portal vein carbon dioxide angiography: a pilot study in a porcine model. Gastrointest Endosc. 2007;66:814-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Wong AA, Charalel RA, Louie JD, Sze DY. Carbon dioxide contrast enhancement for C-arm CT utility for treatment planning during hepatic embolization procedures. J Vasc Interv Radiol. 2013;24:975-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Sonoda A, Nitta N, Ushio N, Nitta-Seko A, Tomozawa Y, Watanabe S, Ohta S, Takahashi M, Murata K. 320-detector-row computed tomography arteriography using CO2 gas to detect malignant liver tumors. Minim Invasive Ther Allied Technol. 2013;22:89-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 30. | Ohmoto K, Yoshioka N, Tomiyama Y, Shibata N, Kawase T, Yoshida K, Kuboki M, Yamamoto S. Use of intra-arterial carbon-dioxide-enhanced ultrasonography for guidance of radiofrequency ablation and transcatheter arterial chemoembolization in hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2006;29:1111-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 31. | Kim HS, Tsai J, Paxton BE. Safety and utility of uterine artery embolization with CO2 and a gadolinium-based contrast medium. J Vasc Interv Radiol. 2007;18:1021-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 32. | Heye S, Maleux G, Marchal GJ. Upper-extremity venography: CO2 versus iodinated contrast material. Radiology. 2006;241:291-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Heye S, Fourneau I, Maleux G, Claes K, Kuypers D, Oyen R. Preoperative mapping for haemodialysis access surgery with CO(2) venography of the upper limb. Eur J Vasc Endovasc Surg. 2010;39:340-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 34. | Winters SL, Curwin JH, Sussman JS, Coyne RF, Calhoun SK, Yablonsky TM, Schwartz JR, Quinlan K. Utility and safety of axillo-subclavian venous imaging with carbon dioxide (CO) prior to chronic lead system revisions. Pacing Clin Electrophysiol. 2010;33:790-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (1)] |
| 35. | Kariya S, Tanigawa N, Kojima H, Komemushi A, Shiraishi T, Kawanaka T, Sawada S. Efficacy of carbon dioxide for diagnosis and intervention in patients with failing hemodialysis access. Acta Radiol. 2010;51:994-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | Textor HJ, Wilhelm K, Strunk H, Schüller H, Schild HH. [The diagnosis of intra-abdominal hemorrhages with CO2 as the contrast medium]. Rofo. 1997;166:51-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 37. | Miyamoto N, Hiramatsu K, Tsuchiya K, Sato Y. Carbon dioxide microbubbles-enhanced sonographically guided radiofrequency ablation: treatment of patients with local progression of hepatocellular carcinoma. Radiat Med. 2008;26:92-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 38. | Johnson PL, Neperud J, Arnold J, Thomas J. Livedo reticularis and bowel ischemia after carbon dioxide arteriography in a patient with CREST syndrome. J Vasc Interv Radiol. 2011;22:395-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 39. | Culp WC, Porter TR, Culp WC, Vonk BN. Carbon dioxide in the aortic arch: coronary effects and implications in a swine study. Cardiovasc Intervent Radiol. 2003;26:128-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 40. | Barrera F, Durant TM, Lynch PR, Oppenheimer MJ, Stauffer HM, Stewart GH. In vivo visualization of intracardiac structures with gaseous carbon dioxide; cardiovascular-respiratory effects and associated changes in blood chemistry. Am J Physiol. 1956;186:325-334. [PubMed] |
| 41. | Penzkofer T, Slebocki K, Grommes J, Bruners P, Isfort PP, Heussen N, Schmitz-Rode T, Kuhl CK, Langer S, Mahnken AH. Carbon dioxide-contrasted computed tomography angiography: high pitch protocols and adapted injection parameters improve imaging quality. Rofo. 2013;185:128-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 42. | Mahnken AH, Penzkofer T, Grommes J, Isfort P, Bruners P, Langer S, Schmitz-Rode T, Mommertz G. Carbon dioxide-enhanced CT-guided placement of aortic stent-grafts: feasibility in an animal model. J Endovasc Ther. 2010;17:332-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 43. | Kariya S, Komemushi A, Nakatani M, Yoshida R, Sawada S, Tanigawa N. CO2 microbubble contrast enhancement in x-ray angiography. Clin Radiol. 2013;68:346-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 44. | Okumura Y, Kakuchi Y, Katano K, Takahashi S. [Visualization of stenosis in vascular access by bubble method under carbon dioxide (CO2) angiographic procedures]. Nihon Hoshasen Gijutsu Gakkai Zasshi. 2010;66:893-900. [PubMed] |