Published online Aug 26, 2017. doi: 10.4330/wjc.v9.i8.685
Peer-review started: December 19, 2016
First decision: January 28, 2017
Revised: May 25, 2017
Accepted: June 6, 2017
Article in press: June 8, 2017
Published online: August 26, 2017
Processing time: 249 Days and 4.6 Hours
To test the safety and effectiveness of hypertonic saline solution (HSS + F) as a strategy for weight loss and prevention of further deterioration of renal function.
Patients admitted with acute decompensated heart failure (ADHF) who received HSS + F were included in the study. After a period of a standard ADHF treatment, our patients received an intravenous infusion of furosemide (250 mg) combined with HSS (150 mL of 3% NaCl) twice a day for a mean duration of 2.3 d. Our primary outcomes were weight loss and a change in serum creatinine per day of treatment. The parameters of the period prior to treatment with HSS + F were compared with those of the period with HSS + F.
A total of 47 patients were included. The mean creatinine on admission was 155 μmol/L ± 65 μmol/L, the ejection fraction was 40% ± 17%. The experimental treatment (HSS + F) resulted in greater weight loss per day of treatment than the standard treatment (-1.4 kg/d ± 1.4 kg/d vs -0.4 kg/d ± 1.0 kg/d, P = 0.0168). Importantly, the change in creatinine was not significantly different.
This study supports the effectiveness of HSS + F on weight loss in patients with ADHF. The safety profile, particularly with regard to renal function, leads us to believe that HSS + F may be a valuable option for those patients presenting with ADHF who do not respond to conventional treatment with intravenous furosemide alone.
Core tip: Hypertonic saline solution (HSS) has been proposed in recent years as a potential therapy to facilitate diuresis in patients with decompensated heart failure and to overcome diuretic resistance. This study supports the effectiveness of HSS + F on weight loss in patients with acute decompensated heart failure and a high burden of comorbidities, despite a proportion of patients having preserved ejection fraction, right heart failure and advanced renal failure. The administration of small intravenous boluses of HSS in conjunction with intravenous furosemide can be a feasible and inexpensive therapeutic option which can prevent the use of costlier and more invasive treatments such as ultrafiltration, hemodialysis and inotropic infusion.
- Citation: Lafrenière G, Béliveau P, Bégin JY, Simonyan D, Côté S, Gaudreault V, Israeli Z, Lavi S, Bagur R. Effects of hypertonic saline solution on body weight and serum creatinine in patients with acute decompensated heart failure. World J Cardiol 2017; 9(8): 685-692
- URL: https://www.wjgnet.com/1949-8462/full/v9/i8/685.htm
- DOI: https://dx.doi.org/10.4330/wjc.v9.i8.685
Heart failure (HF) is a well-recognized major public health problem affecting about 26 million people worldwide[1]. Its impact in terms of mortality, morbidity, quality of life and cost is considerable. Acute decompensated heart failure (ADHF) is a leading cause of hospitalization and a common issue in emergency departments. Loop diuretics have long been recognized as the key for the treatment of ADHF[2], however, high doses can cause adverse effects, including electrolyte abnormalities and deterioration of renal function. In addition, patients can develop resistance to diuretics and congestive symptoms can persist despite treatment with high doses[3]. Currently available treatment options include higher doses or a continuous infusion of intravenous diuretics[4,5], a combination of different classes of diuretics for their synergistic effects[6,7], and in severe/advanced cases, parenteral inotropes[8-10] and ultrafiltration[11,12]. The last two options are not associated with a better prognosis, and in fact, can cause deleterious effects and their use is limited by the cost and availability[11-14].
The hypertonic saline solution (HSS) has been proposed in recent years as an adjunctive therapy for intravenous loop diuretics to improve or restore their initial pharmacological efficacy[3]. Among the proposed mechanisms to explain the benefits of HSS, it has been reported that it would prevent intravascular depletion due to diuretics[15,16] and thus would maintain renal flow and the glomerular filtration rate (GFR) during intensive treatment of intravenous furosemide[17].
Compared to the administration of high doses of intravenous furosemide alone, concomitant use of HSS (HSS + F) has shown, in patients with ADHF, a more rapid and complete resolution of the signs and symptoms of congestion by increasing urine volume and by potentiating weight loss[16,18,19], the potential to protect against deterioration of renal function during intensive diuretic therapy[15,20], an improvement of cardiac biomarkers and echocardiographic parameters[19,21,22], a reduced length of hospital stay and frequency of re-hospitalizations[23] and a good safety profile[24].
Therefore, the aim of the present report was to test the safety and effectiveness of HSS + F as a strategy for weight loss and prevention of further deterioration of renal function compared to the usual intensive treatment with intravenous furosemide alone.
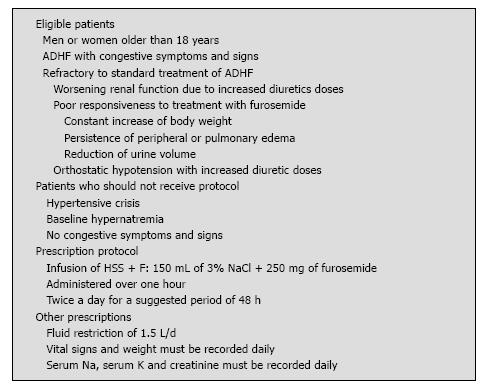
Patients admitted with ADHF and who received HSS + F between January 2012 and December 2013 at the Quebec University Hospital Centre were included for the analysis. The decision to prescribe HSS + F following the standard treatment for a given patient was left to the discretion of the treating cardiologist, who had received at the beginning of the study a list of the suggested inclusion and exclusion criteria (Figure 1). All clinical, echocardiographic and laboratory data were prospectively collected in a dedicated database. Institutional review board approval and patient consent were not required because of the nature of this study.
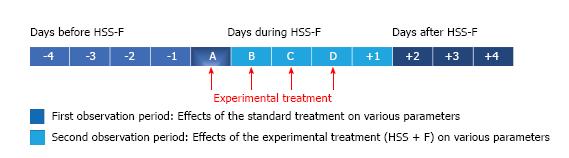
On admission for ADHF, most patients were on fluid restriction of 1.5 L/d, received an intravenous furosemide dose that was adjusted according to the clinical response and the conventional HF treatment that was considered appropriate by the treating physician based on current recommendations[25,26]. When patients were considered to be refractory to this treatment, based on a poor response to standard therapy (weight, creatinine, clinical judgment), intravenous furosemide was replaced by an intravenous infusion of HSS + F (150 mL of 3% NaCl + 250 mg of furosemide) administered over one hour twice a day for a suggested period of 48 h that could be extended or shortened depending on the clinical response. Patients underwent the usual daily medical examination for the evaluation of the signs and symptoms of HF. Vital signs and weight were recorded daily; serum creatinine, sodium (Na) and potassium (K) levels were closely monitored during treatment. Moreover, the clinician’s impression concerning the treatment effectiveness was recorded in the medical notes. Patients were all compared to themselves with a before and after study design. The effects of treatment with intravenous furosemide alone (standard treatment) were compared to the treatment with intravenous furosemide plus HSS (HSS + F) (experimental treatment) administered following the standard treatment. The results available from days one to four prior to the initiation of saline treatment were analyzed and compared to the experimental treatment d (Figure 2).
Our primary outcomes were the decrease in weight and the change of creatinine per day of treatment. Secondary outcomes were the effect of the experimental treatment on the serum Na and K levels and its safety profile regarding neurological events.
The effects of HSS + F on weight and creatinine were studied in the “treatment impasse” subgroup defined as all patients with increased weight and creatinine per day of treatment despite standard therapy.
Continuous data are presented as mean ± SD or median [interquartile range (IQR)] depending on variable distribution, and categorical data are presented as frequencies (percentage). Differences between the weight reduction in the experimental treatment and the standard treatment periods were assessed by Wilcoxon Signed Rank test for paired samples. The same approach was used for all other analyses as the change of creatinine per day of treatment between these two periods and analyses in the “treatment impasse” subgroup. The level of statistical significance was set as P < 0.05. Data were analyzed using the SAS statistical software, version 9.3.
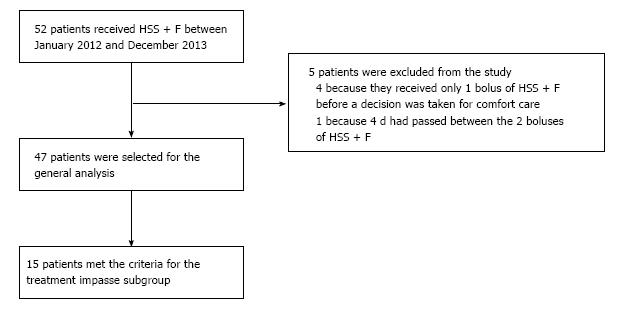
A total of 52 patients received HSS + F for ADHF. Five patients were excluded from the study; four because they received one bolus of HSS + F before the decision of giving end-of-life comfort care only, and one patient was not selected because four d had elapsed between the two boluses of HSS + F, making interpretation difficult.
Hence, a total of 47 patients (32 men, 68%), mean age of 77.6 ± 9.5 years, were included in the study (Figure 3). Thirty-two (68%) patients had chronic kidney disease based on an estimated GFR (eGFR) ≤ 60 mL/min per 1.73 m2. Moreover, the mean creatinine was 155 ± 65 μmol/L, leading to an eGFR of 42 ± 22 mL/min per 1.73 m2 on admission. Of note, 12 (25.5%) presented with a serum Na < 135 mmol/L. The left ventricle ejection fraction (LVEF) was 40% ± 17% and half of the patients had ≤ 40% (Table 1). In addition, 31 patients had pleural effusion, 11 had ascites, 11 presented with arterial hypotension and/or orthostatic hypotension and 13 had acute kidney injury defined as a 1.5-fold increase in serum creatinine or absolute increase in serum creatinine of ≥ 26.4 µmol/L from their baseline value[27,28].
| Variables | n = 47 |
| Age (yr) | 77.6 ± 9.5 |
| Males | 32 (68.1) |
| Body mass index (kg/m2) | 28.2 ± 7.2 |
| Hypertension | 46 (97.9) |
| Diabetes | 28 (59.6) |
| NYHA functional class (admission) | |
| III | 16 (38.1) |
| IV | 23 (54.8) |
| Coronary artery disease | 33 (70.2) |
| Ischemic heart failure | 29 (61.7) |
| Stroke or transient ischemic attack | 11 (23.4) |
| Vascular disease | 22 (46.8) |
| Atrial fibrillation | 27 (57.4) |
| Oxygen-dependent COPD | 4 (8.5) |
| Active cancer | 11 (23.4) |
| Baseline creatinine (µmol/L)1 | 140.1 ± 65.5 |
| Chronic kidney disease (eGFR ≤ 60 mL/min per 1.73 m2)1 | 32 (68.1) |
| Admission creatinine (µmol/L) | 154.8 ± 65.4 |
| Admission eGFR using MDRD (mL/min per 1.73 m2) | 42.2 ± 22.3 |
| Admission serum Na concentration < 135 mmol/L | 12 (25.5) |
| Echocardiographic data | |
| Left ventricle ejection fraction | 39.9 ± 17.4 |
| LVEF > 40% | 23 (48.9) |
| LVEF ≤ 40% | 24 (51.0) |
| Severe aortic stenosis | 1 (2.2) |
| Moderate and/or severe mitral regurgitation | 16 (34.8) |
| Severe tricuspid regurgitation | 6 (13.0) |
| Pulmonary hypertension ≥ 50 mmHg | 19 (41.3) |
| Severe diastolic dysfunction | 11 (23.9) |
| Right ventricular dysfunction/dilatation | 28 (60.9) |
| Medications | |
| ACEI/ARBs | 28 (59.6) |
| Hydralazine | 3 (6.4) |
| Beta-blocker | 39 (83.0) |
| Diuretics | |
| Oral furosemide | 39 (83.0) |
| Thiazide | 9 (19.1) |
| Spironolactone | 8 (17.0) |
| Zaroxolyn | 1 (2.1) |
| Furosemide dose per day (mg) | 128.2 ± 106.7 |
Before receiving HSS + F, six (12.8%) patients required non-invasive ventilation and three (6.4%) patients were intubated for respiratory failure, but no form of mechanical ventilation was initiated during treatment with HSS + F. In addition, eight (17%) patients had a thoracentesis during hospitalization (two during the HSS + F treatment period and six outside of the observation period) and four (8.5%) patients had a paracentesis (two during the standard treatment and none during the HSS + F treatment). Moreover, eight (17%) patients received an infusion of inotropes, but only three during the observation period (one during the experimental treatment, one throughout the two treatments studied and the remaining during the standard treatment only). Six (12.8%) patients underwent coronary angiography during hospitalization, but only two patients had it during the observation period (one during the standard treatment and the other had two coronary angiograms: One before and another during treatment with HSS + F).
Patients received a mean of 5.1 ± 2.0 doses of HSS + F for a mean duration of 2.3 ± 1.0 d. During the treatment period with HSS + F, patients lost 3.9 ± 3.8 kg. Interestingly, the treating physician reported a significant improvement in signs and symptoms of congestion with this experimental treatment on 38 (81%) patients. In addition, weight loss per day of treatment was significantly greater with HSS + F treatment than with the standard treatment (-1.4 ± 1.4 kg/d vs -0.4 ± 1.0 kg/d, mean difference of 0.8 ± 1.8 kg/d, P = 0.0168) (Table 2). The change in creatinine per day of treatment was not statistically different between treatments (Table 2). The mean serum Na increased by 2.4 mmol/L (95%CI: 1.6-3.1, P < 0.0001) and the mean serum K decreased by 0.2 mmol/L (95%CI: -0.4 to -0.1, P = 0.0001) with the experimental treatment compared to the standard treatment. The mean daily dose of intravenous furosemide given during the standard treatment period was 106 ± 67 mg. Four patients received an additional continuous infusion of furosemide for a mean duration of 2.3 d. Nine patients received mainly oral furosemide, with a correspondingly larger mean daily dose of 196 ± 165 mg. Seven doses of metolazone were given during the standard treatment and 7 doses during the experimental treatment.
| Variable | mean ± SD | 95%CI | P value |
| Weight loss (kg/d) | |||
| Standard treatment | -0.39 ± 1.02 | (-0.77, -0.03) | |
| Experimental treatment | -1.43 ± 1.43 | (-1.86, -1.02) | |
| Standard-experimental difference | 0.80 ± 1.77 | (0.15, 1.44) | 0.0168 |
| Change in creatinine (μmol/L per day) | |||
| Standard treatment | 3.48 ± 9.89 | (0.51, 6.68) | |
| Experimental treatment | -0.69 ± 9.62 | (-3.51, 2.00) | |
| Standard-experimental difference | 4.20 ± 14.25 | (-0.49, 8.88) | 0.331 |
The administration of HSS + F was well tolerated by all patients and no major adverse events were observed. It is noteworthy to be highlighted that there was no pulmonary congestion or neurological consequences due to HSS + F strategy. However, the HSS + F treatment was discontinued in 2 (4.3%) patients due to an excessive increase in serum Na (i.e., from 120 mmol/L to 128 mmol/L) in 1 patient and a significant decrease in blood pressure for the other.
A total of 7 (15%) patients died during the hospital stay. The median time between death and the end of treatment with HSS + F was 3 (IQR: 1-33) d. However, two of these deaths were attributed to a shift to end-of-life palliative care requested by the family (treatment with HSS + F originally scheduled for 48 h was discontinued). The average hospital stay was 20 ± 12 d with a median of 16 (IQR: 11-24) d.
Notably, in the impasse treatment subgroup (n = 15), consisting of patients selected because of their negative response to the standard treatment, in addition to a significant weight loss achieved with the experimental treatment (-1.2 kg/d ± 1.3 kg/d vs -0.3 kg/d ± 0.6 kg/d with the standard treatment, mean difference of 1.5 kg/d ± 1.7 kg/d, P = 0.0026), there was an increase in creatinine level with the standard therapy that was not seen with the experimental therapy; indeed, the mean creatinine difference was also statistically significant (11 ± 13 μmol/L per day, P = 0.008) (Table 3).
| Variable | mean ± SD | 95%CI | P value |
| Weight loss (kg/d) | |||
| Standard treatment | 0.25 ± 0.64 | (-0.04, 0.58) | |
| Experimental treatment | -1.20 ± 1.30 | (-1.89, -0.57) | |
| Standard-experimental difference | 1.45 ± 1.65 | (0.54, 2.36) | 0.0026 |
| Change in creatinine (μmol/L per day) | |||
| Standard treatment | 7.33 ± 8.65 | (3.01, 11.70) | |
| Experimental treatment | -3.79 ± 11.34 | (-10.41, 1.63) | |
| Standard-experimental difference | 11.13 ± 13.29 | (3.77, 18.49) | 0.008 |
In a population of patients admitted with ADHF, the administration of HSS + F led to a greater weight loss per day of treatment compared to the standard intravenous furosemide strategy; even if a considerable proportion of them presented HF with preserved LVEF and/or advanced renal failure.
The difference in weight loss achieved through treatment with HSS + F is comparable to that demonstrated in previous studies[16,18,19,21-23,29,30]. Among these studies, the difference between the average in weight loss in the group treated with HSS + F compared to the group treated with intravenous furosemide alone ranged from 0.3-5.6 kg[21,30]. Because patients generally had some weight loss with the treatment with intravenous furosemide alone before starting treatment with HSS + F, it is possible that we have underestimated the weight loss due to HSS + F that could have been achieved without the prior use of intravenous furosemide alone.
We were unable to demonstrate a statistically significant difference in terms of creatinine, although it tended to decrease with the experimental treatment while the trend was reversed with the standard treatment. It has been previously shown an increase in creatinine among those treated with intravenous furosemide alone, while there is either a decrease in creatinine in patients treated with HSS + F[16,18,22,23] or a mild increase that is less than furosemide alone[19,21,30]. It is noteworthy to be outlined that the lack of statistical significant in creatinine levels may be explained in part by the inclusion of patients with advanced renal failure. Indeed, Engelmeier et al[29] recruited patients with advanced renal failure (eGFR < 40 mL/min) and did not demonstrate a significant advantage of using HSS for the prevention of worsening renal function. Moreover, another study showed that HSS affords a protective role in the deterioration of renal function induced by loop diuretics, but does not exert a substantial protective effect in patients with ADHF who have pre-existing advanced renal failure and exhibiting a mean creatinine ≥ 194 µmol/L[15] However, in our study, the renal function of many patients worsened during treatment with intravenous furosemide alone, so the change in creatinine during treatment with HSS + F could be a reflection of the previous treatment.
Treatment with HSS + F was well tolerated and its safety profile was reassuring as demonstrated in previous studies[18,24]. Of note, although we did not adjust the Na concentration in the HSS depending on the patient’s serum Na as done in most studies, there were no severe electrolyte disturbances, except in one patient who had an increase in serum Na of 8 mmol/L within 24 h, but without any neurological symptoms or further consequences. Our results indicate that serum Na levels should be monitored, but adjusting the tonicity of the HSS based on the serum Na level may not be necessary. This facilitates the administration of HSS and reduces the risk of errors.
The result for the impasse subgroup, with few available treatment options, is of particular interest. Those patients, who increased their weight and creatinine while treated with intravenous furosemide alone, had benefited from the therapy in terms of weight loss and renal function.
The use of parenteral inotropes in a number of patients hospitalized for HF is potentially deleterious and requires tighter monitoring[31]. Moreover, the treatment of advanced ADHF by ultrafiltration or intravenous inotropes is not associated with a better prognosis and is limited by the cost and availability[11-14]. Therefore, according to some studies[19,30], simultaneous administration of appropriate doses of HSS during treatment with intravenous diuretics reduce diuretic resistance, which in fact, is the phenomenon of a decrease in the natriuretic response and thus, requires the use of further increasing doses of diuretics that often results in the deterioration of renal function[3]. Hence, the administration of small intravenous boluses of HSS associated with intravenous furosemide is a valid and inexpensive therapeutic option.
The main limitation of this study lies with the fact of a small sample size and even prospective, the non-randomized nature of the study. Therefore, the fact that certain clinical variables that appeared to account more frequently in some group but finally did not reach statistical significance were related to the small sample size. Patients were compared to themselves under the standard and experimental treatments, and the latter being influenced by the previous one. In addition, since the standard treatment was at the discretion of the clinician, the doses of furosemide, the doses of other drugs and the use of thoracentesis or paracentesis were not the same for both treatments. Thus, confirming these results in a larger series of randomized patients might have a high impact on patient selection and clinical decision-making in this high-risk group of patients.
In conclusion, the results of this study support the effectiveness of HSS + F on weight loss. The safety profile, particularly with regard to renal function, leads us to believe that HSS + F may be a valuable option for those patients presenting with ADHF who do not respond to conventional treatment with intravenous furosemide alone.
Compared to the administration of high doses of furosemide monotherapy, the concomitant use of hypertonic saline solution (HSS + F) has shown, in some single-centre studies, clinical benefits and a good safety profile in patients with acute decompensated heart failure (ADHF).
Patients can develop resistance to diuretics and congestive symptoms may persist despite treatment with high doses of furosemide.
This study supports the effectiveness of HSS + F on weight loss in patients with ADHF.
The safety profile, particularly regarding renal function, leads us to believe that HSS + F may be a valuable option for those patients presenting with ADHF who do not respond to conventional treatment with intravenous furosemide alone.
This study aims to test the safety and effectiveness of HSS + F as a strategy for weight loss and prevention of further deterioration of renal function.
This is a well-written manuscript about the treatment of severe acute heart failure.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: Canada
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Farand P, Said SAM S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ
| 1. | Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S, Lam CS, Sato N, Shah AN. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. 2014;63:1123-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1248] [Cited by in RCA: 1570] [Article Influence: 142.7] [Reference Citation Analysis (0)] |
| 2. | Guidelines for the evaluation and management of heart failure. Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Evaluation and Management of Heart Failure). J Am Coll Cardiol. 1995;26:1376-1398. [PubMed] |
| 3. | Liszkowski M, Nohria A. Rubbing salt into wounds: hypertonic saline to assist with volume removal in heart failure. Curr Heart Fail Rep. 2010;7:134-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, LeWinter MM, Deswal A, Rouleau JL, Ofili EO. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364:797-805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1359] [Cited by in RCA: 1226] [Article Influence: 87.6] [Reference Citation Analysis (0)] |
| 5. | Felker GM, O’Connor CM, Braunwald E; Heart Failure Clinical Research Network Investigators. Loop diuretics in acute decompensated heart failure: necessary? Evil? A necessary evil? Circ Heart Fail. 2009;2:56-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 224] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 6. | Dormans TP, Gerlag PG. Combination of high-dose furosemide and hydrochlorothiazide in the treatment of refractory congestive heart failure. Eur Heart J. 1996;17:1867-1874. [PubMed] |
| 7. | Ellison DH. The physiologic basis of diuretic synergism: its role in treating diuretic resistance. Ann Intern Med. 1991;114:886-894. [PubMed] |
| 8. | Capomolla S, Febo O, Opasich C, Guazzotti G, Caporotondi A, La Rovere MT, Gnemmi M, Mortara A, Vona M, Pinna GD. Chronic infusion of dobutamine and nitroprusside in patients with end-stage heart failure awaiting heart transplantation: safety and clinical outcome. Eur J Heart Fail. 2001;3:601-610. [PubMed] |
| 9. | Cusick DA, Pfeifer PB, Quigg RJ. Effects of intravenous milrinone followed by titration of high-dose oral vasodilator therapy on clinical outcome and rehospitalization rates in patients with severe heart failure. Am J Cardiol. 1998;82:1060-1065. [PubMed] |
| 10. | Unverferth DV, Magorien RD, Altschuld R, Kolibash AJ, Lewis RP, Leier CV. The hemodynamic and metabolic advantages gained by a three-day infusion of dobutamine in patients with congestive cardiomyopathy. Am Heart J. 1983;106:29-34. [PubMed] |
| 11. | Abraham WT, Adams KF, Fonarow GC, Costanzo MR, Berkowitz RL, LeJemtel TH, Cheng ML, Wynne J; ADHERE Scientific Advisory Committee and Investigators; ADHERE Study Group. In-hospital mortality in patients with acute decompensated heart failure requiring intravenous vasoactive medications: an analysis from the Acute Decompensated Heart Failure National Registry (ADHERE). J Am Coll Cardiol. 2005;46:57-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 566] [Cited by in RCA: 552] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 12. | Bart BA, Goldsmith SR, Lee KL, Givertz MM, O’Connor CM, Bull DA, Redfield MM, Deswal A, Rouleau JL, LeWinter MM. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med. 2012;367:2296-2304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 634] [Cited by in RCA: 740] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 13. | Bart BA, Goldsmith SR, Lee KL, Redfield MM, Felker GM, O’Connor CM, Chen HH, Rouleau JL, Givertz MM, Semigran MJ. Cardiorenal rescue study in acute decompensated heart failure: rationale and design of CARRESS-HF, for the Heart Failure Clinical Research Network. J Card Fail. 2012;18:176-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 128] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 14. | Elkayam U, Tasissa G, Binanay C, Stevenson LW, Gheorghiade M, Warnica JW, Young JB, Rayburn BK, Rogers JG, DeMarco T. Use and impact of inotropes and vasodilator therapy in hospitalized patients with severe heart failure. Am Heart J. 2007;153:98-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 214] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 15. | De Vecchis R, Ciccarelli A, Ariano C, Pucciarelli A, Cioppa C, Giasi A, Fusco A, Cantatrione S. [Renoprotective effect of small volumes of hypertonic saline solution in chronic heart failure patients with marked fluid retention: results of a case-control study]. Herz. 2011;36:12-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Licata G, Di Pasquale P, Parrinello G, Cardinale A, Scandurra A, Follone G, Argano C, Tuttolomondo A, Paterna S. Effects of high-dose furosemide and small-volume hypertonic saline solution infusion in comparison with a high dose of furosemide as bolus in refractory congestive heart failure: long-term effects. Am Heart J. 2003;145:459-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 183] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 17. | Paterna S, Fasullo S, Di Pasquale P. High-Dose Torasemide is Equivalent to High-Dose Furosemide with Hypertonic Saline in the Treatment of Refractory Congestive Heart Failure. Clin Drug Investig. 2005;25:165-173. [PubMed] |
| 18. | Paterna S, Di Pasquale P, Parrinello G, Amato P, Cardinale A, Follone G, Giubilato A, Licata G. Effects of high-dose furosemide and small-volume hypertonic saline solution infusion in comparison with a high dose of furosemide as a bolus, in refractory congestive heart failure. Eur J Heart Fail. 2000;2:305-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 90] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 19. | Paterna S, Di Pasquale P, Parrinello G, Fornaciari E, Di Gaudio F, Fasullo S, Giammanco M, Sarullo FM, Licata G. Changes in brain natriuretic peptide levels and bioelectrical impedance measurements after treatment with high-dose furosemide and hypertonic saline solution versus high-dose furosemide alone in refractory congestive heart failure: a double-blind study. J Am Coll Cardiol. 2005;45:1997-2003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 137] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 20. | Issa VS, Bacal F, Mangini S, Carneiro RM, Azevedo CH, Chizzola PR, Ferreira SM, Bocchi EA. Hypertonic saline solution for renal failure prevention in patients with decompensated heart failure. Arq Bras Cardiol. 2007;89:251-255. [PubMed] |
| 21. | Parrinello G, Di Pasquale P, Torres D, Cardillo M, Schimmenti C, Lupo U, Iatrino R, Petrantoni R, Montaina C, Giambanco S. Troponin I release after intravenous treatment with high furosemide doses plus hypertonic saline solution in decompensated heart failure trial (Tra-HSS-Fur). Am Heart J. 2012;164:351-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Parrinello G, Paterna S, Di Pasquale P, Torres D, Mezzero M, Cardillo M, Fasullo S, La Rocca G, Licata G. Changes in estimating echocardiography pulmonary capillary wedge pressure after hypersaline plus furosemide versus furosemide alone in decompensated heart failure. J Card Fail. 2011;17:331-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Paterna S, Fasullo S, Parrinello G, Cannizzaro S, Basile I, Vitrano G, Terrazzino G, Maringhini G, Ganci F, Scalzo S. Short-term effects of hypertonic saline solution in acute heart failure and long-term effects of a moderate sodium restriction in patients with compensated heart failure with New York Heart Association class III (Class C) (SMAC-HF Study). Am J Med Sci. 2011;342:27-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 24. | Paterna S, Parrinello G, Amato P, Dominguez L, Pinto A, Maniscalchi T, Cardinale A, Licata A, Amato V, Licata G. Tolerability and efficacy of high-dose furosemide and small-volume hypertonic saline solution in refractory congestive heart failure. Adv Ther. 1999;16:219-228. [PubMed] |
| 25. | McKelvie RS, Moe GW, Ezekowitz JA, Heckman GA, Costigan J, Ducharme A, Estrella-Holder E, Giannetti N, Grzeslo A, Harkness K. The 2012 Canadian Cardiovascular Society heart failure management guidelines update: focus on acute and chronic heart failure. Can J Cardiol. 2013;29:168-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 144] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 26. | Writing committee members, Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240-e327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 795] [Cited by in RCA: 1569] [Article Influence: 130.8] [Reference Citation Analysis (0)] |
| 27. | Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative workgroup. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204-R212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4448] [Cited by in RCA: 4702] [Article Influence: 223.9] [Reference Citation Analysis (0)] |
| 28. | Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A; Acute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4846] [Cited by in RCA: 4987] [Article Influence: 277.1] [Reference Citation Analysis (0)] |
| 29. | Engelmeier RS, Le TT, Kamalay SE, Utecht KN, Nikstad TP, Kaliebe JW, Olson K, Larrain G. Randomized trial of high dose furosemide-hypertonic saline in acute decompensated hearth failure with advanced renal desease. JACC. 2012;59:E958-E958. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Issa VS, Andrade L, Ayub-Ferreira SM, Bacal F, de Bragança AC, Guimarães GV, Marcondes-Braga FG, Cruz FD, Chizzola PR, Conceição-Souza GE. Hypertonic saline solution for prevention of renal dysfunction in patients with decompensated heart failure. Int J Cardiol. 2013;167:34-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 31. | Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147-e239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4116] [Cited by in RCA: 4654] [Article Influence: 387.8] [Reference Citation Analysis (1)] |