Published online Jul 26, 2017. doi: 10.4330/wjc.v9.i7.634
Peer-review started: October 23, 2016
First decision: January 14, 2017
Revised: January 29, 2017
Accepted: May 3, 2017
Article in press: May 5, 2017
Published online: July 26, 2017
Processing time: 276 Days and 4.6 Hours
We present a case report about percutaneous closure of a congenital Gerbode defect using Nit-Occlud® Lê VSD coil. The patient was referred to our hospital with a diagnosis of ventricular septal defect (VSD) and severe pulmonary arterial hypertension. But transthoracic echocardiography revealed a communication between the left ventricle (LV) and the right atrial (RA), called Gerbode defect. Catheterization confirmed the shunt from the LV to the RA. We successfully closed the defect with a VSD coil. After uneventful 6 mo follow-up, the patient was out of dyspnea, the symptom urged him to have medical attention. This case report is to discuss the diagnosis and percutaneous treatment approach for this rare congenital heart disease.
Core tip: Congenital Gerbode defect is rare, only accounts for about 0.08% among congenital heart diseases. The diagnosis is easily misinterpreted with others condition on clinical examination and echocardiography. The treatment of this disease is also lack of recommendation. There are several approaches can be applied for this kind of defect such as conservation, cardiac surgery, intravascular intervention or intraoperative device closure. There are several devices can be used for transcatheter closure such as ventricular septal occluder, atrial septal occluder, ADO I or ADO II. This is the first report using Nit-Occlud® Lê VSD coil to close Gerbode defect successfully.
- Citation: Phan QT, Kim SW, Nguyen HL. Percutaneous closure of congenital Gerbode defect using Nit-Occlud® Lê VSD coil. World J Cardiol 2017; 9(7): 634-639
- URL: https://www.wjgnet.com/1949-8462/full/v9/i7/634.htm
- DOI: https://dx.doi.org/10.4330/wjc.v9.i7.634
The left ventricular to right atrial (LV-RA) communication was first described by Frank Gerbode in 1958[1]. This defect can be either congenital or acquired. While congenital LV-RA shunt is very rare, acquired LV-RA shunt is reported more common, can be induced by endocarditis, trauma, valve replacement, myocardial infarction, etc[2]. There are several varieties of Gerbode defect[1]: Supravalvular (direct) type, subvalvular (indirect) type and a combination of these two lesions.
The diagnosis of congenital Gerbode defects is quite challenging: While the clinical symptoms may mimic ventricular septal defect (VSD), it can be misinterpreted on TTE with tricuspid regurgitation and pulmonary arterial hypertension. Further investigation by transesophageal echocardiography (TEE), magnetic resonance imaging (MRI), computed tomographic angiography (CTA), … may help to determine the right diagnosis.
A 31-year-old male who had dyspnea on exertion 3 mo before hospitalization, was referred to our hospital with a diagnosis of heart failure in patient with VSD and severe pulmonary hypertension. He had healthy active lifestyle, normal physical and mental development. The clinical examination showed a loud harsh holosystolic murmur (4/6 Levine scale) at 4th intercostal spaces along left sternal border, radiated downward and a systolic thrill could be palpated. His blood pressure was 125/85 mmHg. The ECG showed sinus rhythm. The BNP was slightly increased. Chest X-ray, ionogram, creatinine, glucose, blood count and clotting times were in normal range. The TTE revealed a shunt between the LV and the RA. The jet went into the RA looked similar to the tricuspid valve regurgitation flow with high velocity, about 4.8 m/s. The other findings included slight dilation of the right heart chambers, mild RV systolic dysfunction and Qp:Qs was 1.6.
Cardiac catheterization was performed. LV contrast injection illustrated quite large shunt from the LV to the RA. The defect was in long conical figuration with diameters of 9.5 mm at the biggest LV ampulla, 4.0 mm at the narrowest position and 8.5 mm length. It was quite far from the aortic valve and coronaries. The pulmonary arterial pressure was 56/22/35 mmHg.
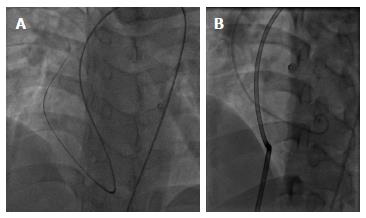
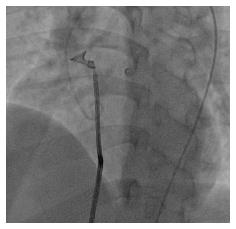
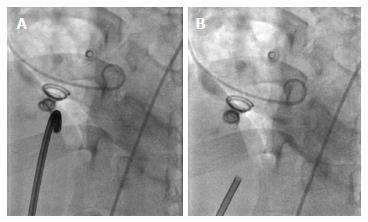
After getting across the defect with a Terumo wire from LV and snaring the wire at the superior vena cava for making the arteriovenous loop, an 8F delivery catheter was introduced to LV and aorta from RA through the defect (Figure 1). Then, a 12 mm × 6 mm Nit-Occlud® Lê VSD coil (Figure 2) was deployed to close the defect with aortic approach (Figure 3). The procedure was quite similar to perimembranous VSD occlusion with likely satisfied result (Figure 4). There was only small residual shunt from LV to RA on LV contrast injections and echocardiography, no aortic regurgitation, no cardiac arrhythmia on ECG and the hemodynamic was stable.
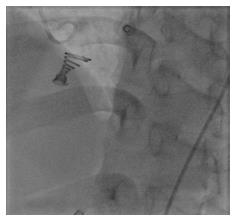
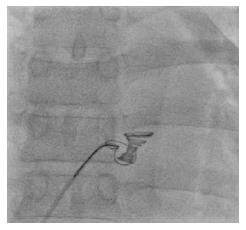
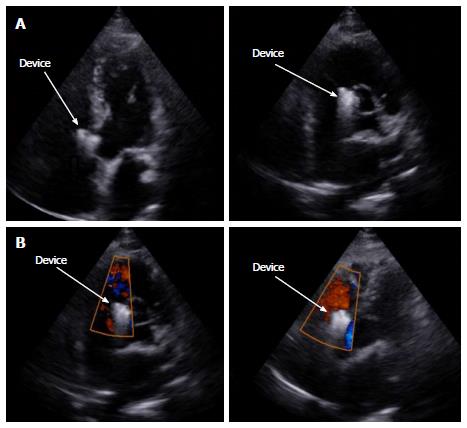
But while the patient was kept following on cathlab table for complications, mostly concerning bradycardia and heart block, we detected some free bizarre movement of device distal part (Video 1). The device was going to drift out of the defect at 15 minutes after coil release. We quickly retrieved the coil with multi-snare and 10F catheter from the RA through inferior vena cava (Figure 5). Another attempt to close the defect with a bigger 14 mm × 8 mm Nit-Occlud® Lê VSD coil was performed (Figure 6). The final result looked fine with mild residual LV-RA shunt, no aortic regurgitation, no arrhythmia. Six hours after the procedure, there was still a grade 2/6 murmur can be found on auscultation and mild shunt from LV to RA on echocardiography. After 24 h, both the murmur and residual shunt flow were gone. After 5 d of close following up uneventful, the patient was discharged in good physical and mental condition. We have been checking the patient at 1 mo, 3 mo and 6 mo after the procedure. Till now, the patient has no symptom on exertion, the device is in good position without any shunt on echocardiography and there is no murmur on heart auscultation.
Congenital Gerbode defect is rare, only accounts for about 0.08% among congenital heart diseases[1]. Patients may have symptoms or not depends on the defect size and the shunt from LV to RA. Heart auscultation can detect a systolic murmur with position and intensity similar to the VSD but slightly lower and radiating downward.
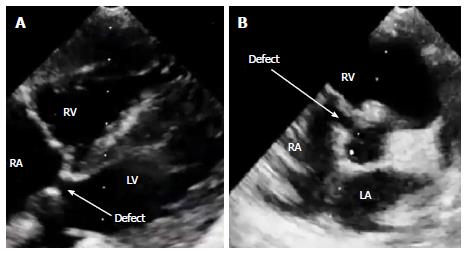
TTE is useful for diagnosis of Gerbode defect. In 2D mode, the LV-RA shunt may be detected in some views, such as parasternal short axis, four and five chambers or subcostal views (Figure 7). The image quality was better in subcostal view as there was no bone or lung tissue to obstruct the view. Pulsed wave Doppler was helpful in detecting the shunt by the high turbulent audio signal. Continuous wave Doppler helped detect and measure peak systolic velocities across the shunt. Color flow imaging was useful in localizing defect position and shunt flow. However, in Gerbode defect, the shunt flow, affected by the septal leaflet of the tricuspid valve, may change direction unexpectedly. So, it can mimic the direction of tricuspid regurgitation flow and made sonographers misrecognize as tricuspid regurgitation in the setting of severe pulmonary artery hypertension[3]. In this case, at first, the clinical physician thought about VSD and sent the patient to the sonographer for more detail. The conclusion they received was VSD with very high systolic pulmonary arterial pressure, about 115 mmHg. Both the clinical physician and sonographer were cheated by the shunt flow. So quick clinical examination and echocardiography in this kind of defect may easily lead to a misdiagnosis. The sonographer should meticulously look for Gerbode defect if physical examination suspects VSD but echocardiography can not detect any high velocity flow or aliasing in the right ventricle. The presence of normal diastolic pulmonary arterial pressure using pulmonic regurgitation jet is also very useful to distinguish the true pulmonary arterial hypertension from high velocity jet in the RA caused by Gerbode defect[4,5]. Actually, only about 2/3 of the LV-RA shunts of either congenital or acquired origin can be well diagnosed with TTE[6]. The 1/3 of others have to rely on other means like contrast echo, TEE, MRI, CTA or catheterization for accurate diagnosis.
Till now, there has been no clear official guidance for optimal treatment of Gerbode defect. Physicians may personally choose suitable therapy among conservation, cardiac surgery, intravascular intervention or intraoperative device closure. Patients without symptom and right ventricular volume overload, due to a small LV-RA shunt, may not need the treatment[7]. But if left untreated, the significant LV-RA shunt may lead to progressive congestive heart failure[8] and up to 8.7% of patients with LV-RA shunt developed infectious endocarditis in long-term follow-up[9]. So, some authors[10-14] suggested that correction of this type of defect with significant LV-RA shunt is necessary.
Even though the surgical closure is accepted as a treatment of choice[12], some successful transcatheter closures for Gerbode defect have been reported. There is a paucity of information in the existing literature on transcatheter therapy for this type of defect. Indications of intervention may be same as those in the left to right shunt lesion[14]. The devices used for occlusion this kind of defect varies from centers to centers. The first device reported used to close of an acquired Gerbode defect following VSD surgical correction was Amplatzer ventricular septal occluder[11]. Another device, amplatzer septal occluder (ASO) commonly used for atrial septal defect closure, also being used to close Gerbode defect complicated from mitral valve surgery with marked improvement in exercise tolerance[13]. The smallest patient reported, a 3-mo old baby with an acquired Gerbode shunt after VSD patch closure, was treated using an amplatzer duct occluder (ADO) with complete closure achieved 2 wk after device deployment and good long term follow-up[12]. Recently, a report of 12 Gerbode defect patients that were transcatheterly closed with ADO II showed satisfactory outcomes[14]. Until now, there is no specific device purposely manufactured for Gerbode defects closure. The devices used to occlude this kind of defects are borrowed from products created for other defect types such as atrial septal defect, VSD, patent ductus arteriosus, etc. In this case, giving the size and shape of the defect, a suitable device could be chosen a for transcatheter occlusion among ADO, ADO II, membranous or muscular VSD occluder, VSD Coil or ASO. With a quite big and long defect like that, heart block and injuring adjacent structures could likely happen when using ADO, VSD occluder or ASO. A good device might be ADO II, but it was not available in our center at that time. So, we finally decided to use the Nit-Occlud® Lê VSD coil (PFM Medical, Germany), which are commonly used for VSD closure. This device is made of Nitinol coils with securely attached polyester fibers and a cone-in-cone configuration (Figure 2). The bigger proximal cone is more flexible and will be partially deployed on the left ventricular side of the defect. The smaller distal cone will be deployed on the other side of the defect and its diameter should be at least twice the defect smallest diameter. The first device chosen might be undersized purposely, for minimizing surrounding structures injury. That could explain for device embolization after deployment. The second bigger device was appropriate for the defect with firmly sitting in the correct position. So, choosing a suitable device with correct size is very important to prevent device embolization in transcatheter Geporde defect closure.
After device successfully released, there was rising concern of hemolysis and hematuria because the contrast ejection and echocardiography showed small residual shunt. But after 24 h, the murmur on heart auscultation and residual shunt on echocardiography was completely gone. Six months following up also showed steady result with no heart murmur, good device position, no shunt on echocardiography (Figure 8) and the patient achieved almost normal life.
In conclusion, the diagnosis of congenital Gebode defect is quite challenging, can be easily misinterpreted. Percutaneous device occlusion offers a feasible, safe and effective therapy for this type of defect. Among devices used for transcatheter closure of Gerbode defects, the Nit-Occlud® Lê VSD coil may be a good candidate.
A 31-year-old male who had normal physical and mental development presented with dyspnea on exertion and a loud harsh holosystolic murmur at 4th intercostal spaces along the left sternal border with a systolic thrill could be palpated.
Ventricular septal defect.
Tricuspid regurgitation, mitral regurgitation, pulmonic stenosis, patent ductus arteriosus.
The BNP was slightly increased. Other labs were within normal limits.
Echocardiography showed a shunt from left ventricle to the right atrium.
Communication between the left ventricle and right atrium (congenital Gerbode defect).
The defect was transcatheterly closed using a Nit-Occlud® Lê VSD coil with no residual shunt at 6 mo follow-up.
Congenital Gebode defect is a rare congenital heart disease and can be easily misinterpreted. Percutaneous device occlusion offers a feasible, safe and effective therapy for this disease.
In congenital Geborde defect, careful review of echocardiography is an important key to avoid misdiagnosis and the transcatheter closure with an appropriate device is a crucial factor to ensure the procedural success.
The reported case is well described and interesting.
Manuscript source: Unsolicited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: Vietnam
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Said SAM, Teragawa H S- Editor: Kong JX L- Editor: A E- Editor: Wu HL
| 1. | Gerbode F, Hultgren H, Melrose D, Osborn J. Syndrome of left ventricular-right atrial shunt; successful surgical repair of defect in five cases, with observation of bradycardia on closure. Ann Surg. 1958;148:433-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 195] [Article Influence: 7.8] [Reference Citation Analysis (1)] |
| 2. | Demirkol S, Gurkan Yesil F, Bozlar U, Balta S, Sahin MA, Guler A. Multimodality imaging of a congenital Gerbode defect. Kardiol Pol. 2013;71:104. [PubMed] |
| 3. | Pillai V, Menon S, Kottayil B, Karunakaran J. Tricuspid endocarditis with indirect Gerbode: septal translocation of posterior leaflet. Heart Lung Circ. 2011;20:362-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Tehrani F, Movahed MR. How to prevent echocardiographic misinterpretation of Gerbode type defect as pulmonary arterial hypertension. Eur J Echocardiogr. 2007;8:494-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Primus C, Grabscheit G, Ng CK, Auer J. Unusual cause of dyspnoea: a case presentation of an echocardiographic pitfall. J Cardiothorac Surg. 2013;8:230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Yuan SM. Left ventricular to right atrial shunt (Gerbode defect): congenital versus acquired. Postepy Kardiol Interwencyjnej. 2014;10:185-194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Toprak C, Kahveci G, Akpinar S, Tabakçi MM, Güler Y. Concomitant Gerbode-like defect and anterior mitral leaflet perforation after aortic valve replacement for endocarditis. Echocardiography. 2013;30:E231-E235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Lax D, Bhatt RD, Klewer SE, Sorrell VL. Are all ventricular septal defects created equal? J Am Soc Echocardiogr. 2010;23:791.e5-791.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Wu MH, Wang JK, Lin MT, Wu ET, Lu FL, Chiu SN, Lue HC. Ventricular septal defect with secondary left ventricular-to-right atrial shunt is associated with a higher risk for infective endocarditis and a lower late chance of closure. Pediatrics. 2006;117:e262-e267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Cabalka AK, Hagler DJ, Mookadam F, Chandrasekaran K, Wright RS. Percutaneous closure of left ventricular-to-right atrial fistula after prosthetic mitral valve rereplacement using the Amplatzer duct occluder. Catheter Cardiovasc Interv. 2005;64:522-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Trehan V, Ramakrishnan S, Goyal NK. Successful device closure of an acquired Gerbode defect. Catheter Cardiovasc Interv. 2006;68:942-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Lee SY, Song JY, Baek JS. Percutaneous closure of the acquired gerbode shunt using the amplatzer duct occluder in a 3-month old patient. Korean Circ J. 2013;43:429-431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Rothman A, Galindo A, Channick R, Blanchard D. Amplatzer device closure of a tortuous Gerbode (left ventricle-to-right atrium) defect complicated by transient hemolysis in an octogenarian. J Invasive Cardiol. 2008;20:E273-E276. [PubMed] |
| 14. | Vijayalakshmi IB, Natraj Setty HS, Chitra N, Manjunath CN. Amplatzer duct occluder II for closure of congenital Gerbode defects. Catheter Cardiovasc Interv. 2015;86:1057-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |