Published online Jul 26, 2017. doi: 10.4330/wjc.v9.i7.594
Peer-review started: March 14, 2017
First decision: April 14, 2017
Revised: May 19, 2017
Accepted: May 22, 2017
Article in press: May 24, 2017
Published online: July 26, 2017
Processing time: 135 Days and 3.4 Hours
The main stay pharmacotherapy for heart failure (HF) is targeted towards rennin-angiotensin-aldosterone (RAAS) and neprilysin pathways (NP). Both therapeutic strategies decreases morbidity and mortality but also carry considerable adverse effects. This review of the literature highlights the new generation of HF drug, sacubitril-valsartan (SV), trade name Entresto (researched as LCZ696, Novartis) which simultaneously blocks RAAS and NP. This dual action of angiotensin receptors blocker and neprilysin inhibitor (NPi) has improved HF prognosis and it is an evolution in the management of HF. Although the initial follow-up of patients treated with SV has yielded promising results, there are concerns regarding potential side effects especially an increase in the risk of Alzheimer’s disease (AD) and young onset of AD. NPi interferes with the breakdown and clearing of beta-amyloid peptides, the plaques seen in AD, raising concern for AD in SV patients. On the other hand, hypertension and cardiovascular diseases are established risk factors for AD which can be decreased by SV therapy. It is therefore essential that SV treated patients are followed up over an extended period of time to detect any adverse cognitive changes.
Core tip: We are discussing an innovative and exciting new treatment for heart failure (HF). This advance in pharmacotherapy has shown promising results and is rapidly incorporating into standard medical therapy for HF. There is, however, a theoretical concern for cognitive dysfunction and early onset Alzheimer’s disease particularly in the young. This review informs clinicians of the mechanism and potential for cognitive dysfunction, thereby increasing awareness and promoting informed prescribing.
- Citation: Patel N, Gluck J. Is Entresto good for the brain? World J Cardiol 2017; 9(7): 594-599
- URL: https://www.wjgnet.com/1949-8462/full/v9/i7/594.htm
- DOI: https://dx.doi.org/10.4330/wjc.v9.i7.594
Heart failure (HF) is typified by the reduced ability of the heart to deliver an adequate supply of blood and oxygen to the tissues. Its causes are numerous including ischemic heart disease, diabetes, hypertension, cigarette smoking, obesity and valvular heart disease[1]. Over 5 million individuals worldwide suffer from HF and its incidence is rising with 550000 new diagnoses annually[1,2]. With a steadily aging population, HF incidence is projected to increase to 46% by the year 2030[1,2]. HF is associated with increased morbidity, mortality and cost[1,3].
HF occurring due to depressed left ventricular function [ejection fraction (EF) ≤ 40%] is known as HF with reduced EF (HFrEF)[4]. Pharmacological intervention for HF largely depended on angiotensin inhibitors such as angiotensin receptor blockers (ARBs) and angiotensin converter enzyme inhibitors (ACEi). Recently, a new strategy using a Neprilysin inhibition (NIs) and recombinant natriuretic peptides was proven as a therapeutic option to target HF pathophysiology[5]. The new generation of HF pharmacotherapy entails the simultaneous inhibition of both the angiotensin and Neprilysin pathways, the latest version of which is Entresto® - the combination of sacubitril and valsartan (SV) (researched as LCZ696)[6,7]. In this concise review, we highlight the mechanisms of SV activity, the results of the successful clinical trial and the potential adverse effects, highlighting those on cognitive function.
The search for the relevant articles was conducted on Medline. The following terms “Entresto”, “neprilysin inhibitors”, “angiotensin inhibitors”, “dementia” and “Alzheimer’s Disease” “cognitive impairment” were searched in different combinations. The search was limited to articles in English language but no search filters were used for timeline and subjects.
Articles that met our following inclusion criteria were included in this review: (1) discussed pathophysiology of HF and target pharmacotherapy mechanism; (2) discussed pathophysiology of development of AD; (3) ongoing trials of Entresto; (4) reported link between neprilysin inhibitors and development of AD; and (5) articles that were published full and in English language.
The pathophysiology of HFrEF results mainly from the activation of the renin-angiotensin-aldosterone (RAAS) neuro-hormonal compensatory mechanism. Although the peripheral vasoconstriction initiated by the RAAS mechanism maintains blood pressure and cardiac output for a short time, sustained activation of RAAS leads to ventricular hypertrophy, hypertension and angioedema, ultimately worsening myocardial dysfunction[8,9]. A second compensatory mechanism, the natriuretic peptide (NP) system, counteracts the vasoconstrictive and sodium/water retentive effects of the RAAS system[10].
The initial HF pharmacotherapy targeted the RAAS circuit using ARBs[11], ACEi[12], beta-blockers[13], diuretics[14] and aldosterone inhibitors[15]. All of these drugs have proven to be effective in lowering the morbidity and mortality in HFrEF. The NP system consists of four related peptides (Atrial, Brain, C-Type, and Dendroaspis NP) and a membrane bound peptidase called Neprilysin that degrades these vasoactive peptides[16]. NP system targeting drugs have included a recombinant form of BNP (Nesiritide)[17] as well as NIs, e.g., candoxatril, rececodotril, etc[18,19]. HF pharmacotherapy targeting the NP system and the respective clinical trials are summarized in Table 1[20-32]. Although strategies blocking either of these two pathways have reduced mortality and morbidity in HF[12,28,32], the prognosis still remains poor due to long term ineffectiveness of the drugs as well as adverse physiological effects[5].
| Drug | Mechanism | Clinical trials and year | Results on HF symptoms |
| Nestiride | Increasing natriuretic peptide activity | VMAC, 2000[20] PRECEDENT, 2002[21] ASCENT-HF, 2009[22] | Improved BP and dyspnea Less cardiac arrhythmias More hypotension |
| Candoxatril | NPi | Single-centered investigation with only a limited number of patients[23-25] | More exercise tolerance Increase in vascular resistance |
| Omapatrilat | NPi | IMPRESS, 2000[26] OVERTURE, 2002[27] OCTAVE, 2004[28] | More exercise tolerance Increase in angioedema Reduction in BP Lower mortality |
| LCZ696 | NPi | PARAMOUNT, 2012[29] PARADIGM, 2014[30,31] PARAGON, ongoing[32] | Reduction in BP Improved ejection fraction Lower mortality No change in angioedema |
The newest strategy in HFrEF pharmaco-intervention is the combination of ARB and NI (ARNI) that causes a dual inhibition of the RAAS pathway and Neprilysin: The prototype drug was LCZ696[6,7] which is made up of 1:1 ratio of the ARB valsartan[33] and the NI sacubitril (AHU 377)[34]. The action of SV is multimodal. Sacubitril is a pro-drug which is activates to Sacubitrilat (LBQ657), the active metabolite that inhibits NP while valsartan simultaneously blocks the angiotensin receptor. The dual action of Sacubitril and valsartan augment the beneficial actions of the NPs and inhibits the deleterious effects of the RAAS system[7]. The PARADIGM-HF trial was conducted by McMurray et al[31] to determine the efficacy of SV compared to the ACE inhibitor Enalapril[35,36], which improves mortality and morbidity. The median follow-up duration was 27 mo and SV reduced HF related symptoms and overall survival by 20%[31]. Additionally, the ARNI approach avoids the common side-effects of ACEi such as cough and angioedema that result from impaired degradation and elevated levels of bradykinin[37]. In the ONTARGET trial, ARBs were documented to result in a lower rate of cough and angioedema compared to ACEi: Therefore, combination therapy prefers ARBs over ACEi[38].
The United States Food and Drug Administration had approved SV for clinical use and at present it is produced under the name of Entresto® by Novartis[39]. The recommended dose of Entresto is 49 mg sacubitril/51 mg valsartan twice daily increased to 97 mg sacubitril/103 mg valsartan after 2-4 wk. It is contraindicated in patients with history of angioedema, hypotension, hyperkalemia or renal dysfunction and in pregnant women due to fetal toxicity[40]. In the PARADIGM-HF trial, 10.7% of the patients reported at least one of the following adverse effects hypotension, renal failure, hyperkalemia, fatigue and dizziness[31].
In clinical practice, approximatley 50% of the HF patients have a preserved left ventricular ejection fraction (HFpEF) and present with similar morbidity and mortality as seen in patients with HFrEF[2-4]. Sacubitril/valsartan is validated in HFrEF but is being evaluated for HFpEF in the PARAMOUNT-HF (The Prospective comparison of ARNI with ARB on Management of Heart Failure with Preserved Ejection Fraction) trial. Patients who treated with sacubitril/valsartan showed a reduction in NYHA class and left atrial volumes[29]. At present, the PARAGON-HF trial is ongoing compareing the effects of sacubitril/valsartan versus valsartan in the HFpEF patients[32].
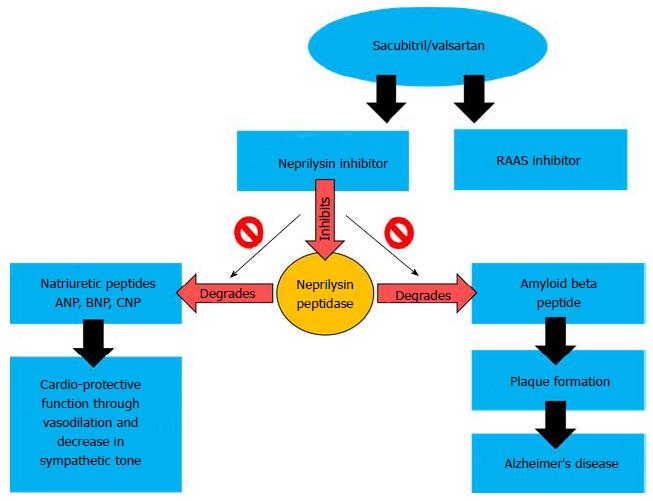
An interesting facet of the use of NIs in the treatment of cardiovascular diseases is their potential role in the development or progression of Alzheimer’s disease (AD), as there is considerable overlap between the populations suffering from HF and AD[41]. The hallmark of AD is the accumulation of beta amyloid (βA) peptide in the brain causing neurotoxic plaques that are supposedly responsible for the pathology of AD[42]. Under normal physiological conditions, the βA peptide is degraded by proteases such as ACE, NP and insulin degrading enzyme[43]. NP has a broad range of substrates apart from the NPs such as bradykinin, enkephalins as well as the βA peptide[44]. Additionally, patients with AD have lower expression of NP compared to healthy subjects[45], and NP deficient mice develop the murine form of AD[46]. This possible correlation was further highlighted when intracerebral infusion of NPi lead to the development of AD-like lesions in rabbits[47]. Lastly, certain polymorphisms in the NP gene (NEP) were associated with a higher propensity for AD in a Finnish cohort[48]. Therefore, NP is as much a pharmaceutical target for the treatment of AD as for HF, except that the strategies are opposite for both pathologies (Figure 1). Indeed, NP centered therapies have been developed independently for AD and tested at the pre-clinical levels. CNS targeted recombinant human NP was able to reduce βA peptide toxicity in the mouse model of AD[49,50].
Clinicians should be aware of the possible inhibitory action of SV in the clearing of βA peptide while considering it for HF treatment. In patients who are at the risk of developing AD, whether due to age or genetic predisposition, the chronic exposure to SV may accelerate the clinical onset of the disease. Critical to this hypothesis is the ability of SV to cross the blood brain barrier (BBB) in order to block brain NP. There is evidence that certain NIs like S-acetlythiorphan can cross the BBB[51]) while some like candoxatril cannot[52]. Both Sacubitril and its active metabolite LBQ657[7] are under the threshold size of 400 kD which makes them fit to cross the BBB[53,54]. It is noteworthy that the PARADIGM-HF trial[31] had excluded patients with AD and did not include any cognitive function tests to evaluate drug safety. McMurray et al[55] have confirmed some correlation between EN treatment and βA peptide levels in a recent review article. While cynomolgus monkeys treated with SV had increased levels of βA peptide in the CSF, the healthy volunteers treated with EN for two weeks had no change in βA peptide levels. McMurray et al[31] showed that the dementia and cognitive defects were not increased in the EN treated patients during the trial. However, it should be noted that the earliest symptoms of AD can take as long as 8-10 years to manifest[56]. If there is a correlation between EN therapy and AD, one would predict an earlier onset of symptoms.
It is therefore imperative that patients on SV are followed up for cognitive abilities and potentially evaluated for AD. One can consider cerebrospinal fluid (CSF) analysis for βA peptide levels and amyloid plaques through PET scans if early signs of dementia ensue[57]. In the ongoing PARAGON-HF[32] trial, AD patients have not been excluded and serial cognitive tests have also been included as part of initial follow-up.
Another concern is that the proportion of HF patients younger than 40 years old is increasing[58]. Younger patient’s receiving SV have the potential for a longer term exposure and the consequent potential for increased risk of young onset Alzheimer’s disease (YOAD) is noteworthy. YOAD is described in subjects less than 65 years of age and has a more rapid progression than the typical late onset Alzheimer’s[59].
Interestingly, one can also describe SV as having protective effect against AD since hypertension and cardiovascular diseases are established risk factors for AD[60], is decreased by SV therapy. ACEi or ARBs have also been shown to decrease in dementia and other symptoms of AD through reducing hypertension and cardiovascular disease[61]. It will be interesting to follow the neuro-cognitive outcomes from PARAGON-HF trial.
Clinicians should be aware of the potential adverse effects of SV and make informed decisions in prescribing SV, particularly to patients with existing neurodegenerative diseases or the very young. As there are no definitive answers yet about the long term effects of SV, we await the results from PARAGON-HF and reports to follow with interest. Patients who are currently receiving SV treatment should be well monitored for potential adverse events with particular attention to dementia. A low threshold for testing for AD if/when dementia symptoms occur seems warranted. More study on the implications for young HF patients is warranted.
Manuscript source: Unsolicited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Barili F, Joseph J, Wang Y S- Editor: Song XX L- Editor: A E- Editor: Wu HL
| 1. | American Heart Association. Classes of heart failure ([updated 2015 Apr 23]). Available from: http:// www.heart.org/HEARTORG/Conditions/HeartFailure/AboutHeartFailure/Classes-of-Heart-Failure_UCM_306328_Article.jsp. |
| 2. | Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després JP, Fullerton HJ, Howard VJ. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131:e29-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3462] [Cited by in RCA: 4527] [Article Influence: 411.5] [Reference Citation Analysis (1)] |
| 3. | Mayo Clinic staff. Diseases and conditions: heart failure ([accessed 2015 Jan 17]). Available from: http:// www.mayoclinic.org/diseases/conditions/heart-failure/basics/definition/con-20029801. |
| 4. | Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:1810-1852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2367] [Cited by in RCA: 2376] [Article Influence: 198.0] [Reference Citation Analysis (0)] |
| 5. | Singh JS, Lang CC. Angiotensin receptor-neprilysin inhibitors: clinical potential in heart failure and beyond. Vasc Health Risk Manag. 2015;11:283-295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Gu J, Noe A, Chandra P, Al-Fayoumi S, Ligueros-Saylan M, Sarangapani R, Maahs S, Ksander G, Rigel DF, Jeng AY. Pharmacokinetics and pharmacodynamics of LCZ696, a novel dual-acting angiotensin receptor-neprilysin inhibitor (ARNi). J Clin Pharmacol. 2010;50:401-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 393] [Article Influence: 24.6] [Reference Citation Analysis (1)] |
| 7. | Langenickel TH, Dole WP. Angiotensin receptor-neprilysin inhibition with LCZ696: a novel approach for the treatment of heart failure. Drug Discov Today Ther Strateg. 2012;9:e131-e139. [RCA] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, Kim HS, Smithies O, Le TH, Coffman TM. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci USA. 2006;103:17985-17990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 589] [Cited by in RCA: 543] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 9. | Holtz J. Pathophysiology of heart failure and the renin-angiotensin-system. Basic Res Cardiol. 1993;88 Suppl 1:183-201. [PubMed] |
| 10. | Bayes-Genis A, Morant-Talamante N, Lupón J. Neprilysin and Natriuretic Peptide Regulation in Heart Failure. Curr Heart Fail Rep. 2016;13:151-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Karnik SS, Unal H, Kemp JR, Tirupula KC, Eguchi S, Vanderheyden PM, Thomas WG. International Union of Basic and Clinical Pharmacology. XCIX. Angiotensin Receptors: Interpreters of Pathophysiological Angiotensinergic Stimuli [corrected]. Pharmacol Rev. 2015;67:754-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 229] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 12. | Pilote L, Abrahamowicz M, Eisenberg M, Humphries K, Behlouli H, Tu JV. Effect of different angiotensin-converting-enzyme inhibitors on mortality among elderly patients with congestive heart failure. CMAJ. 2008;178:1303-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Rienstra M, Damman K, Mulder BA, Van Gelder IC, McMurray JJ, Van Veldhuisen DJ. Beta-blockers and outcome in heart failure and atrial fibrillation: a meta-analysis. JACC Heart Fail. 2013;1:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 14. | Brandimarte F, Mureddu GF, Boccanelli A, Cacciatore G, Brandimarte C, Fedele F, Gheorghiade M. Diuretic therapy in heart failure: current controversies and new approaches for fluid removal. J Cardiovasc Med (Hagerstown). 2010;11:563-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Nappi JM, Sieg A. Aldosterone and aldosterone receptor antagonists in patients with chronic heart failure. Vasc Health Risk Manag. 2011;7:353-363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Jhund PS, McMurray JJ. The neprilysin pathway in heart failure: a review and guide on the use of sacubitril/valsartan. Heart. 2016;102:1342-1347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 113] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 17. | Colucci WS, Elkayam U, Horton DP, Abraham WT, Bourge RC, Johnson AD, Wagoner LE, Givertz MM, Liang CS, Neibaur M. Intravenous nesiritide, a natriuretic peptide, in the treatment of decompensated congestive heart failure. Nesiritide Study Group. N Engl J Med. 2000;343:246-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 728] [Cited by in RCA: 695] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 18. | Gros C, Souque A, Schwartz JC, Duchier J, Cournot A, Baumer P, Lecomte JM. Protection of atrial natriuretic factor against degradation: diuretic and natriuretic responses after in vivo inhibition of enkephalinase (EC 3.4.24.11) by acetorphan. Proc Natl Acad Sci USA. 1989;86:7580-7584. [PubMed] |
| 19. | Northridge DB, Jardine AG, Alabaster CT, Barclay PL, Connell JM, Dargie HJ, Dilly SG, Findlay IN, Lever AF, Samuels GM. Effects of UK 69 578: a novel atriopeptidase inhibitor. Lancet. 1989;2:591-593. [PubMed] |
| 20. | Young JB, Abraham WT, Stevenson LW, Horton DP. Results of the VMAC trial: vasodilation in the management of acute congestive heart failure. Circulation. 2000;102:2794. [DOI] [Full Text] |
| 21. | Burger AJ, Horton DP, LeJemtel T, Ghali JK, Torre G, Dennish G, Koren M, Dinerman J, Silver M, Cheng ML. Effect of nesiritide (B-type natriuretic peptide) and dobutamine on ventricular arrhythmias in the treatment of patients with acutely decompensated congestive heart failure: the PRECEDENT study. Am Heart J. 2002;144:1102-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 204] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 22. | Hernandez AF, O’Connor CM, Starling RC, Reist CJ, Armstrong PW, Dickstein K, Lorenz TJ, Gibler WB, Hasselblad V, Komajda M. Rationale and design of the Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure Trial (ASCEND-HF). Am Heart J. 2009;157:271-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 123] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 23. | Northridge DB, Currie PF, Newby DE, McMurray JJ, Ford M, Boon NA, Dargie HJ. Placebo-controlled comparison of candoxatril, an orally active neutral endopeptidase inhibitor, and captopril in patients with chronic heart failure. Eur J Heart Fail. 1999;1:67-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Westheim AS, Bostrøm P, Christensen CC, Parikka H, Rykke EO, Toivonen L. Hemodynamic and neuroendocrine effects for candoxatril and frusemide in mild stable chronic heart failure. J Am Coll Cardiol. 1999;34:1794-1801. [PubMed] |
| 25. | Kentsch M, Otter W, Drummer C, Nötges A, Gerzer R, Müller-Esch G. Neutral endopeptidase 24.11 inhibition may not exhibit beneficial haemodynamic effects in patients with congestive heart failure. Eur J Clin Pharmacol. 1996;51:269-272. [PubMed] |
| 26. | Rouleau JL, Pfeffer MA, Stewart DJ, Isaac D, Sestier F, Kerut EK, Porter CB, Proulx G, Qian C, Block AJ. Comparison of vasopeptidase inhibitor, omapatrilat, and lisinopril on exercise tolerance and morbidity in patients with heart failure: IMPRESS randomised trial. Lancet. 2000;356:615-620. [PubMed] |
| 27. | Packer M, Califf RM, Konstam MA, Krum H, McMurray JJ, Rouleau JL, Swedberg K. Comparison of omapatrilat and enalapril in patients with chronic heart failure: the Omapatrilat Versus Enalapril Randomized Trial of Utility in Reducing Events (OVERTURE). Circulation. 2002;106:920-926. [PubMed] |
| 28. | Kostis JB, Packer M, Black HR, Schmieder R, Henry D, Levy E. Omapatrilat and enalapril in patients with hypertension: the Omapatrilat Cardiovascular Treatment vs. Enalapril (OCTAVE) trial. Am J Hypertens. 2004;17:103-111. [PubMed] |
| 29. | Solomon SD, Zile M, Pieske B, Voors A, Shah A, Kraigher-Krainer E, Shi V, Bransford T, Takeuchi M, Gong J, Lefkowitz M, Packer M, McMurray JJ; Prospective comparison of ARNI with ARB on Management Of heart failUre with preserved ejectioN fracTion (PARAMOUNT) Investigators. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet. 2012;380:1387-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 831] [Cited by in RCA: 890] [Article Influence: 68.5] [Reference Citation Analysis (0)] |
| 30. | McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau J, Shi VC, Solomon SD, Swedberg K. Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin-converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF). Eur J Heart Fail. 2013;15:1062-1073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 334] [Cited by in RCA: 351] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 31. | McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau J, Shi VC, Solomon SD, Swedberg K. Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin-converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF). Eur J Heart Fail. 2013;15:1062-1073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 334] [Cited by in RCA: 351] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 33. | Mistry NB, Westheim AS, Kjeldsen SE. The angiotensin receptor antagonist valsartan: a review of the literature with a focus on clinical trials. Expert Opin Pharmacother. 2006;7:575-581. [PubMed] |
| 34. | Ksander GM, Ghai RD, deJesus R, Diefenbacher CG, Yuan A, Berry C, Sakane Y, Trapani A. Dicarboxylic acid dipeptide neutral endopeptidase inhibitors. J Med Chem. 1995;38:1689-1700. [PubMed] |
| 35. | CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med. 1987;316:1429-1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4505] [Cited by in RCA: 4106] [Article Influence: 108.1] [Reference Citation Analysis (0)] |
| 36. | Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5265] [Cited by in RCA: 4960] [Article Influence: 145.9] [Reference Citation Analysis (0)] |
| 37. | Byrd JB, Adam A, Brown NJ. Angiotensin-converting enzyme inhibitor-associated angioedema. Immunol Allergy Clin North Am. 2006;26:725-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 107] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 38. | Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, Dagenais G, Sleight P, Anderson C. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547-1559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2526] [Cited by in RCA: 2388] [Article Influence: 140.5] [Reference Citation Analysis (0)] |
| 40. | Entresto (sacubitril and valsartan) tablets [prescribing information]. East Hanover, NJ: Novartis; July 2015. Available from: https://www.entrestohcp.com/dosing. |
| 41. | Cermakova P, Eriksdotter M, Lund LH, Winblad B, Religa P, Religa D. Heart failure and Alzheimer’s disease. J Intern Med. 2015;277:406-425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 122] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 42. | Ghiso J, Frangione B. Amyloidosis and Alzheimer’s disease. Adv Drug Deliv Rev. 2002;54:1539-1551. [PubMed] |
| 43. | Iwata N, Higuchi M, Saido TC. Metabolism of amyloid-beta peptide and Alzheimer’s disease. Pharmacol Ther. 2005;108:129-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 166] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 44. | Webster CI, Burrell M, Olsson LL, Fowler SB, Digby S, Sandercock A, Snijder A, Tebbe J, Haupts U, Grudzinska J. Engineering neprilysin activity and specificity to create a novel therapeutic for Alzheimer’s disease. PLoS One. 2014;9:e104001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 45. | Yasojima K, Akiyama H, McGeer EG, McGeer PL. Reduced neprilysin in high plaque areas of Alzheimer brain: a possible relationship to deficient degradation of beta-amyloid peptide. Neurosci Lett. 2001;297:97-100. [PubMed] |
| 46. | Iwata N, Tsubuki S, Takaki Y, Shirotani K, Lu B, Gerard NP, Gerard C, Hama E, Lee HJ, Saido TC. Metabolic regulation of brain Abeta by neprilysin. Science. 2001;292:1550-1552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 717] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 47. | Newell AJ, Sue LI, Scott S, Rauschkolb PK, Walker DG, Potter PE, Beach TG. Thiorphan-induced neprilysin inhibition raises amyloid beta levels in rabbit cortex and cerebrospinal fluid. Neurosci Lett. 2003;350:178-180. [PubMed] |
| 48. | Vepsäläinen S, Helisalmi S, Mannermaa A, Pirttilä T, Soininen H, Hiltunen M. Combined risk effects of IDE and NEP gene variants on Alzheimer disease. J Neurol Neurosurg Psychiatry. 2009;80:1268-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 49. | Marr RA, Rockenstein E, Mukherjee A, Kindy MS, Hersh LB, Gage FH, Verma IM, Masliah E. Neprilysin gene transfer reduces human amyloid pathology in transgenic mice. J Neurosci. 2003;23:1992-1996. [PubMed] |
| 50. | Spencer B, Marr RA, Gindi R, Potkar R, Michael S, Adame A, Rockenstein E, Verma IM, Masliah E. Peripheral delivery of a CNS targeted, metalo-protease reduces aβ toxicity in a mouse model of Alzheimer’s disease. PLoS One. 2011;6:e16575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 51. | Lecomte JM, Costentin J, Vlaiculescu A, Chaillet P, Marcais-Collado H, Llorens-Cortes C, Leboyer M, Schwartz JC. Pharmacological properties of acetorphan, a parenterally active “enkephalinase” inhibitor. J Pharmacol Exp Ther. 1986;237:937-944. [PubMed] |
| 52. | Becker M, Siems WE, Kluge R, Gembardt F, Schultheiss HP, Schirner M, Walther T. New function for an old enzyme: NEP deficient mice develop late-onset obesity. PLoS One. 2010;5:pii: e12793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 53. | Pardridge WM. Drug transport across the blood-brain barrier. J Cereb Blood Flow Metab. 2012;32:1959-1972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1049] [Cited by in RCA: 1267] [Article Influence: 97.5] [Reference Citation Analysis (0)] |
| 54. | Bell RD, Zlokovic BV. Neurovascular mechanisms and blood-brain barrier disorder in Alzheimer’s disease. Acta Neuropathol. 2009;118:103-113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 679] [Cited by in RCA: 646] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 55. | McMurray JJ, Packer M, Solomon SD. Neprilysin inhibition for heart failure. N Engl J Med. 2014;371:2336-2337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 56. | Bäckman L, Jones S, Berger AK, Laukka EJ, Small BJ. Multiple cognitive deficits during the transition to Alzheimer’s disease. J Intern Med. 2004;256:195-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 197] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 57. | Vodovar N, Paquet C, Mebazaa A, Launay JM, Hugon J, Cohen-Solal A. Neprilysin, cardiovascular, and Alzheimer’s diseases: the therapeutic split? Eur Heart J. 2015;36:902-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 58. | Wong CM, Hawkins NM, Petrie MC, Jhund PS, Gardner RS, Ariti CA, Poppe KK, Earle N, Whalley GA, Squire IB. Heart failure in younger patients: the Meta-analysis Global Group in Chronic Heart Failure (MAGGIC). Eur Heart J. 2014;35:2714-2721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 59. | Stanley K, Walker Z. Do patients with young onset Alzheimer’s disease deteriorate faster than those with late onset Alzheimer’s disease? A review of the literature. Int Psychogeriatr. 2014;26:1945-1953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 60. | Thorin E. Hypertension and Alzheimer disease: another brick in the wall of awareness. Hypertension. 2015;65:36-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 61. | Villapol S, Saavedra JM. Neuroprotective effects of angiotensin receptor blockers. Am J Hypertens. 2015;28:289-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |