Published online Jul 26, 2017. doi: 10.4330/wjc.v9.i7.574
Peer-review started: February 17, 2017
First decision: March 8, 2017
Revised: May 8, 2017
Accepted: May 12, 2017
Article in press: May 15, 2017
Published online: July 26, 2017
Processing time: 160 Days and 0.6 Hours
Transcatheter aortic valve replacement (TAVR) is a relatively newer therapeutic modality which offers a promising alternative to surgical aortic valve replacement for patients with prohibitive, high and intermediate surgical risk. The increasing trend to pursue TAVR in these patients has also led to growing awareness of the associated potential vascular complications. The significant impact of these complications on eventual clinical outcome and mortality makes prompt recognition and timely management a critical factor in TAVR patients. We hereby present a concise review with emphasis on diverse vascular complications associated with TAVR and their effective management to improve overall clinical outcomes.
Core tip: Latest review of literature regarding vascular complications of transcatheter aortic valve replacement, optimum access techniques, key technical considerations and potential management strategies have been addressed.
- Citation: Chaudhry MA, Sardar MR. Vascular complications of transcatheter aortic valve replacement: A concise literature review. World J Cardiol 2017; 9(7): 574-582
- URL: https://www.wjgnet.com/1949-8462/full/v9/i7/574.htm
- DOI: https://dx.doi.org/10.4330/wjc.v9.i7.574
Transcatheter aortic valve replacement (TAVR) is an evolving percutaneous valve replacement procedure especially with the new improved low profile sheaths. The most widely used approach for TAVR is retrograde access through a common femoral artery (CFA). Although there is a striking decrease in all-cause mortality and cardiovascular outcomes between TAVR and standard therapy at 5 years in high risk patients[1], there is a significant component of associated major vascular complications such as annular rupture, vessel dissection, major bleeding (16.7%)[2]. Analysis of the PARTNER trial showed the rate of major and minor complications as 15.3% and 11.9% respectively which was associated with a higher rate of 30 d and 1 year mortality especially among cohort B[3]. In comparison to this historic trend, newer temporal data from the STS/ACC TVT registry has shown a significant decrease in the annual rate of vascular complications to as low as 4.2%[4]. Effective management of vascular complications to improve outcomes is of paramount importance as recent studies have shown TAVR to be non-inferior to surgical aortic valve replacemen in moderate risk patients[5] and that reflects a higher potential patient pool who could benefit from this procedure.
Risk factors associated with increased risk of vascular complications in TAVR include female gender, renal failure, peripheral vascular disease with significant calcification (especially when circumferential) and concomitant peripheral vascular disease. The sheath to femoral artery diameter ratio (SFAR) greater than 1.05 also compounds risk[6]. Newer delivery systems such as Edwards SAPIEN XT and S3 decrease the risk as does operator experience and skill[7]. The subclavian approach is comparable to transfemoral TAVR[8]. Most well-known complication of caval-aortic access is caval-aortic fistula but there is paucity of data regarding vascular complications as this approach is not used commonly.
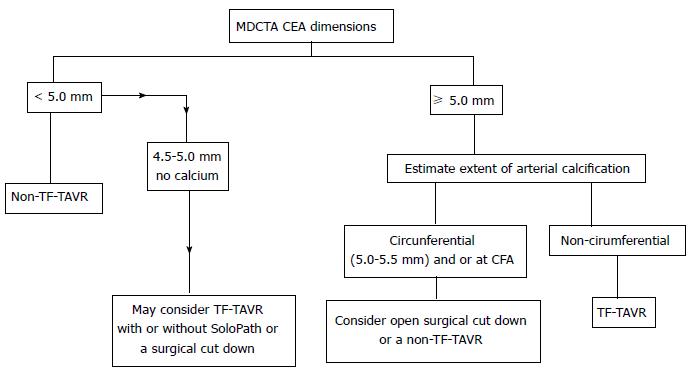
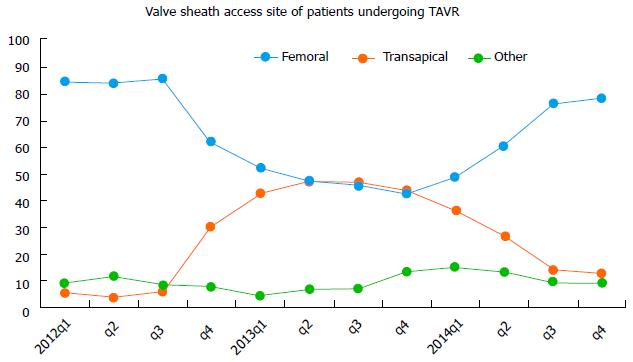
Pre-procedural multidetector computed tomography (MDCT) can help assess CFA calcification, determine the distance from skin to artery, and the vessel diameter, all of which can aid in selecting the optimal vessel entry site. A general schematic for femoral access based on MDCT is outlined in Figure 1. In recent times, there has been a significant surge in transfemoral approach as reflected by TVT registry results (Figure 2). In many centers, TF TAVR is performed using a micropuncture needle and 4 or 5 Fr sheath[9] but there is wide variability of approach dependent on an operator.
The micropuncture technique avoids potential large bore needle trauma at an unwanted CFA site (low, high or otherwise suboptimal), which may ultimately culminate in development of vascular complications. Fluoroscopy or ultrasound can be utilized to assist with identification of the appropriate site of entry, although the latter provides greater anatomic detail. A radio-opaque marker (e.g., a hemostat) can be placed on the groin to mark the femoral head, facilitating guidance of needle entry under fluoroscopy. The level of sheath entry relative to the femoral head can be confirmed using fluoroscopy in an antero-posterior (AP) projection and its relation to the superficial-profunda femoral bifurcation ascertained using angiography performed from an ipsilateral oblique projection (i.e., right or left anterior oblique for right- and left-sided access, respectively). B-mode ultrasound can be used as well and may result in less frequent vascular complications and higher first pass access success[10].
Finally, needle puncture may be guided using real time digital subtraction angiography (DSA) or “road mapping” by using contralateral common femoral access and placing a cross over sheath or catheter in the common or external iliac artery on the side to be accessed. Regardless of the method employed, after confirming appropriate CFA access, the micropuncture sheath can then be exchanged over guidewire to a larger sheath as required.
Subclavian access is an alternative option with a prerequisite vessel diameter of greater than 6 mm. Increased angulation, ectasia and calcification are all adverse risk factors. With history of coronary artery bypass grafting and a left internal mammary graft, subclavian vasculature diameter should be greater than 7 mm with no significant atherosclerotic disease and no ostial stenosis to consider it feasible for TAVR access. Surgical cut-down for proper visualization and ease of access is commonly used[11].
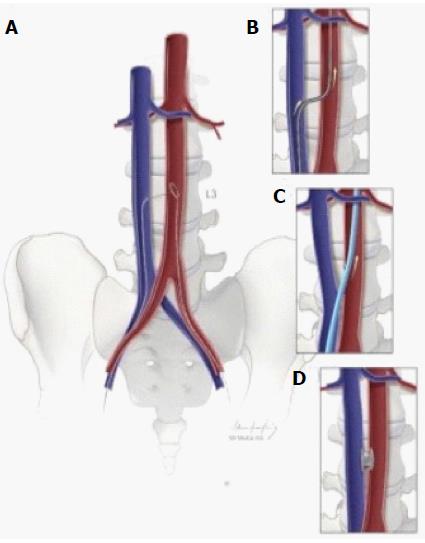
This is an option for TAVR in patients with significant peripheral vascular disease in the femoral and iliac systems thereby limiting arterial access. The caliber and pliability of venous system provides advancement of the delivery system through the femoral vein into the inferior vena cava followed by puncture of the descending aorta and sealing the pathway with a nitinol plug on completion of the procedure[12]. A stepwise illustration is provided in Figure 3.
Hemostasis following removal of the sheath is usually achieved with Prostar XL10F and Perclose ProGlide (Abbott Vascular Devices, Redwood City, CA, United States) in which the mechanism of action is a suture release and delivery. This method greatly decreases the incidence of access site complications[10].
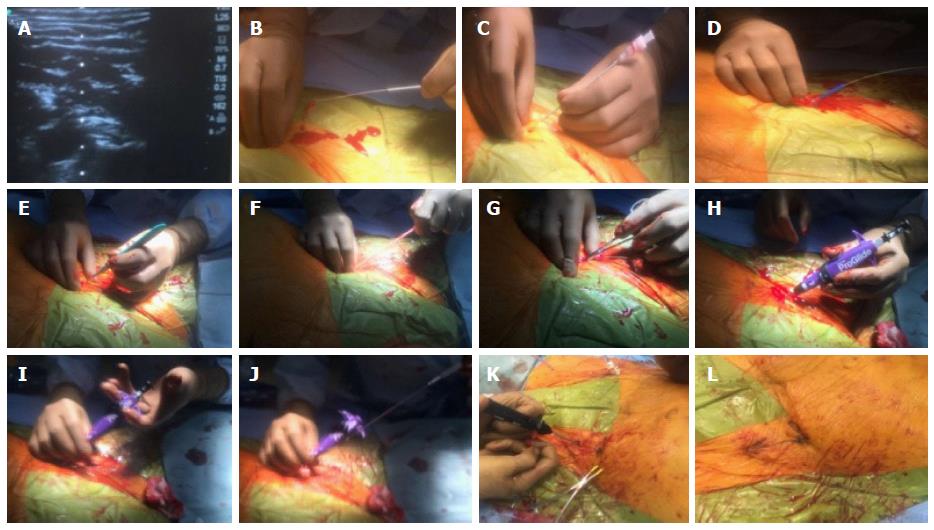
The Prostar XL and 6 Fr ProGlide are used for closure of upto 10F and 8F arteriotomies. If the devices are initially used at the time of initial access and sheath placement, hemostasis can be achieved in a more predictable and effective fashion. This is termed as the “Preclose” method and is illustrated in Figure 4. After TAVR completion, a 0.035” guidewire is always left in the artery while removing the sheath, to maintain continuous access in case an upstream perforation becomes apparent. Once sufficient hemostasis is achieved, the 0.035 wire can be removed and suture knots locked to ensure complete hemostasis. Occasionally a third Proglide or 8 Fr Angio-Seal (Abbott Vascular, Redwood City, California) can be used to adequately achieve hemostasis if required[13].
Cross over balloon technique (COBT) is an alternative method to achieve hemostasis. CBOT involves inflating a balloon above the access site prior to the retrieval of TAVR sheath and closure of arteriotomy. CBOT can be performed over 0.018” or 0.035” guidewires, and the hemostasis balloon can be inflated at low pressure to ensure safe deployment of the suture-mediated closure device[14].
There are different guidewires with varying degrees of stiffness and flexibility which can be employed during the TAVR procedure. Wires used for TAVR delivery are usually 0.035” diameter and have an inner core with a tapered distal tip that is easily shapeable to facilitate steerability. Length is usually in the range of 260 cm and some are coated to reduce resistance to minimize vessel trauma during catheter and device exchange in TAVR procedures. One should be wary of the fact that verbal description of guidewires does not co-relate with actual wire stiffness. Objective parameters such as “flexural modulus” co-relate well with wire stiffness and are more reliable as shown by Harrison et al in a retrospective analysis[15] rather than market terminology such as “super stiff” or “extra stiff”. If higher stiffness is required as in cases with significant vessel tortuosity, a pig tail curve is typically placed at the distal tip of the wire to prevent vascular or left ventricular trauma. Table 1 shows important characteristics of guidewires commonly used during TAVR.
| Guidewire | Core material and coating | Wire guide diameter inch | Wire guide length (cm) | Taper length (cm) | Floppy tip length (cm) | Stiffness | Preshaped curve | Use |
| Amplatz Extra Stiff wire (Cook Medical Inc.) | PTFE-coated stainless steel | 0.035 | 260 | 7 | 3 | Least stiff | 3 cm | TAVR device delivery Straightening of tortuous vessels Sheath insertion |
| Amplatz Super Stiff wire (Boston Scientific) | PTFE-Coated Stainless Steel | 0.035 | 260 | 6 | 3 | Stiffer than Extra stiff | 1 and 3 cm | Straightening of tortuous vessels Sheath insertion |
| Lunderquist Extra Stiff wire (Cook Medical Inc.) | PTFE-Coated Stainless Steel | 0.035 | 260 and 300 | 7.5 | 4 | Stiffer than Amplatz extrastiff and superstiff | 4 cm | Straightening of tortuous vessels Sheath insertion |
| Safari Guide wire (Boston Scientific) | LUBRIGREENTM PTFE-Coated Stainless Steel | 0.035 | 260 and 300 | Stiffness equal to Amplatz extrastiff | Small curve - 16 cm distal grind and 1.7 inch/4.25 cm curve Large curve - 18 cm distal grind and 1.9 inch/4.90 cm curve | TAVR device delivery |
Balloon-expandable Edwards SAPIEN S3 and Edwards SAPIEN XT(TM) valves (Edwards Lifesciences Inc., Irvine, CA) and the self-expanding Medtronic CoreValve® Evolut(TM) R System valves (Medtronic, Minneapolis, MN) are United States Food and Drug Administration (FDA)-approved TAVR devices which are being used nowadays. Core Valve and Edwards Sapien S3 have a range of 23-32 mm and 20-29 mm valve size respectively with sheath size either 14 or 16 French. Currently there are two CE mark approved TAVR devices, the Lotus™ valve system (Boston Scientific Corporation,) available in 23, 25 and 27 mm sizes and the Portico Valve (St. Jude Medical, Minneapolis, Minnesota), available in 27 and 29 mm sizes[16].
The risk of vascular trauma increases with bigger sheath sizes and has shown a downward trend with the new generation TAVR delivery systems[17].
Older generation Edwards SAPIEN and SAPIEN XT valves required up to 24 Fr and 20 Fr sheaths, respectively, while the first Medtronic CoreValve required up to a 25 Fr sheath. Newer generation valves require 14-16 Fr sheaths.
The SoloPath sheath (Terumo Medical Corporation, Irvine, CA, United States) is a balloon expandable and re-collapsible sheath, available in various internal diameters/outer diameters of 14/17, 16/19, 18/21, 19/22, 20/23 and 21/24 Fr, in working lengths of 25 and 35 cm, and balloon expandable lengths of 20 and 30 cm respectively. The sheath is inserted into the vessel in a folded state over a balloon-expandable dilator. Once the sheath is in the desired position, the dilator is inflated, the sheath expands, and later dilator is removed. Upon completion of the procedure, balloon is deflated and sheath returns to its original OD. The safety and efficacy of the 19F SoloPath sheath was investigated for TF-TAVR in a single arm study of 90 patients. When patients were dichotomized into those with a sheath to femoral artery ratio (SFAR) of ≤ 1.05 vs > 1.05, the 19 F Solopath sheath appeared feasible and safe even in patients with SFAR > 1.05 (a traditional indicator of increased vascular risk) and there was no difference in technical or procedural success, total vascular complications, or total bleeding rates between groups[18]. The safety of the SoloPath access sheath was confirmed in a recent multicenter study of patients with ≤ 5.0 mm ilio-femoral access undergoing TF-TAVR using the CoreValve device[19]. A detailed list of different access sheaths used during TAVR is given in Table 2.
| Manufacturer | Sheath | Sheath internal diameter (F) | Sheath outer diameter (mm)/prosthesis size | Minimum vessel diameter (mm) |
| Edwards Lifesciences | RetroFlex 3 introducer sheath | 22 | 8.4/23 Sapien | 7.0 |
| 24 | 9.2/26 Sapien | 8.0 | ||
| NovaFlex introducer sheath | 16 | 6.7/23 Sapien XT | 6.0 | |
| 18 | 7.2/26 Sapien XT | 6.5 | ||
| 20 | 8.0/29 Sapien XT | 7.0 | ||
| Expandable Sheath1 | 14 | 6.0/20 | 5.0 | |
| 14 | 6.0/23 | 5.5 | ||
| 14 | 6.0/26 | 5.5 | ||
| 16 | 6.7/29 | 6.0 | ||
| Medtronic | InLine Sheath for Evolut R System1 | 14 F equivalent2 | 6.0 | 5.0 |
| Gore medical | GORE® DrySeal Sheath13 | 14 | 5.5 | 5.0 |
| 16 | 6.2 | 5.5 | ||
| 18 | 6.8 | 6.0 | ||
| 20 | 7.5 | 6.0 | ||
| Terumo medical corporation | SoloPath sheath13 | 144 | 3.835/5.674 (11.5-17F) | |
| 164 | 5.05/6.334 (15-19F) | |||
| 184 | 5.05/7.04 (15-21F) | |||
| 194 | 5.05/7.34 (15-22F) |
There are variable reports of access site infection after TF-TAVR, ranging from 1.6% to 12.1% with 90% of access site infections encountered after surgical cut down with a 10% associated mortality[20]. In many practices, all patients undergoing TAVR are pretreated with broad spectrum antibiotics and the access site skin incision is closed with a topical skin adhesive. It provides a flexible microbial barrier with wound support that may prevent infection better than traditional wound dressings[21].
Hematomas at point of access can occur ranging from a few minutes to days following completion of TAVR procedure. Careful attention must be paid to the access site as the ideal point is the mid femoral head above the femoral bifurcation. Prolonged hospital stay as well as increased morbidity and mortality are potential associations[22]. The occurrence has continued to decrease due to enhanced center experience, operator skill and smaller delivery systems over the last few years. Manual pressure and anti-coagulation reversal is sufficient for successful management in majority of cases. In case of compressive symptoms or profound blood loss indicated by rapid drop in hemoglobin, contralateral femoral access with balloon tamponade is usually employed. Viabahn covered stent placement (W.L. Gore and Associates, Newark, DE) has been shown to co- relate with an efficacy of 98%[23].
Pseudoaneurysm (PSA) is a contained rupture can occur with arterial puncture below the femoral bifurcation involving superficial or deep femoral arteries or the iliac system[24]. The frequency of pseudoaneursym ranges from 2%-6%. PSA can thromobose spontaneously in upto 90% of cases at 3 wk if the size is less than 3 cm[25]. Size greater than 2 cm along with aggressive anticoagulation are potential predisposing risk factors and can lead to persistent discomfort and act as nidus of infection to increase risk of septic embolism[26]. Rupture can occur with devastating consequences with major bleeding and hypovolemic shock and diameter greater than 3 cm potentiates the possibility[27].
Palpation of a pulsatile mass at the access site or the auscultation of a systolic bruit are suggestive of a possible PSA. For diagnostic purposes, doppler ultrasound has a 94% sensitivity and 97% specificity for PSA[28].
Usually a 5-7 MHz frequency probe is used and the depth should be greater than 4 cm from the skin. Color Doppler shows a turbulent flow pattern in the PSA tract and pulse Doppler shows constant flow shift towards and away from the probe which is diagnostic. Ultrasound guided compression when used has a success rate of 30%-70%. Multiple attempts are mostly needed to obtain sustained compression and thrombosis with a mean time of 33 min[29,30]. Ultrasound guided thrombin injection is an effective treatment modality with a 97% success rate. It facilitates the conversion of fibrinogen to fibrin[31]. Injections are usually given in incremental doses of 0.2-0.4 mL till no flow is observed on the pulse Doppler.
Ileo-femoral dissection in transfemoral TAVR has an incidence of approximately 7% even in high volume centers. External iliac artery is the most commonly involved vessel and can occur during initial advancement of the delivery system or during sheath withdrawal.
Diagnosis can be made with careful review of DSA with either retrograde or contralateral antegrade contrast injections. Significant dissections can lead to lower limb ischemia which can be critical or subcritical depending on the spread of dissection and the vessel territory involved. This can predispose to thrombus formation and vessel occlusion or compression symptoms with blood extravasation.
Computed tomographic angiography (CTA) with distal runoff can be used to identify the focus of dissection.
Contained dissections can be managed by careful monitoring as mostly they resolve on their own with course of time. In case of extensive dissections, endovascular repair can be pursued. Ballooning alone with appropriate stent placement as needed is the preferred approach. Balloon inflation can tamponade the bleeding site and in addition maintenance stents are used for vessel patency. If the dissection is unable to be sealed with this modality, surgical repair is required. Follow up imaging with CTA as indicated can be used[32].
Ileo-femoral rupture is another feared complication of TAVR but the occurrence has decreased significantly with the use of smaller and compact delivery systems as compared to initial TAVR procedures when the frequency was roughly 4%. As long as the sheath is in place, pelvic vascular rupture is not evident but as soon as the sheath is withdrawn, the pressure seal is taken off and huge pelvic bleed can occur with rapid clinical deterioration and death[33].
During the sheath withdrawal, the patient’s clinical status should be monitored closely. Sudden hemodynamic compromise may be evident if there is a significant defect although small tears can extend and cause clinical instability within a few hours post procedure. Angiography prior to complete sheath removal is usually performed to assess for any focal dissection or to detect any extra-luminal contrast flow.
Management includes quick sheath reintroduction to seal the site of dissection while contralateral balloon delivery and inflation can be attempted. Massive fluid repletion, quick anticoagulation reversal and covered stent placement is required although in cases of complex dissections, surgical intervention is indicated[34].
In cases of extensive atheromatous and calcified vessels, vessel avulsion can rarely occur during sheath withdrawal. Urgent proximal occlusive balloon placement and prompt surgical intervention needed in his scenario[35].
CFA stenosis can occur following device closure of the arteriotomy site. If there is evidence of significant flow limitation, angioplasty can be done to reduce the stenosis. In cases of arterial thrombosis and occlusion, critical limb ischemia can occur and thrombectomy is needed to restore vessel patency.
Aortic dissection is an uncommon but potentially fatal complication of TAVR procedure, Incidence has been reported in the range of 0.6%-1.9%[36].
As the access approach varies from transfemoral and transapical to transaortic, any segment can be involved including the ascending or descending aorta. In a study of 412 patients reported by Lange et al[37] who were treated with transapical and transfemoral approach, annular and abdominal aortic rupture occurred in four patients. Generally, continuous transesophageal monitoring is done throughout the procedure, and Type A dissections can be diagnosed promptly. If aortic dissection is suspected post procedure, aortic angiography is pursued.
The clinical manifestation of aortic dissection may manifest at any time during or after he procedure. Symptoms vary from chest pain and abdominal pain to neurological deficits depending on the extent of involvement (mesenteric, renal, carotid arteries). Clinically, hypotension and pressure difference of greater than 20 mmHg between the arms can be suggestive. Imaging modalities such as computed tomography, magnetic resonance imaging and transesophageal echocardiography (TEE) can be used depending on availability and the complexity of the clinical scenario.
The management of aortic dissection depends on initial site of dissection, extent and vascular compromise. Strict blood pressure control with systolic less than 110 mmHg by using beta blocker with alpha blocking additive effect such as Labetalol or Carvedilol is preferred. Non-dihydropyridine calcium channel blockers can be used as well. In case of hypotension, volume repletion is done to maintain mean arterial pressure of greater than 70 mmHg. Type A dissections should be treated with prompt surgical repair while Type B dissections are medically managed and uncommonly endovascular repair is considered[38].
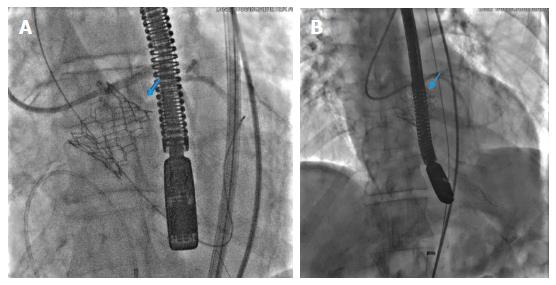
Aortic rupture is a dreaded, rare complication with extremely poor prognosis. It has an incidence of less than 1%. Commonly the presentation is acute with rapid hemodynamic instability and circulatory shock. The rupture extends quickly along tissue planes and hemorrhagic tamponade can be seen on TEE quite frequently. Infrequently, there is a subacute clinical picture as the initial aortic tear takes time to extend and manifest clinically. The mechanisms includes trauma by the device catheter if it overshoots the guidewire or forceful attempts made to manoeuver the catheter through vessels with steep angulation and tortuosity. It can be spotted on fluoroscopy at the time of valve deployment as shown in Figure 5. There should be an extremely low threshold of suspicion in TAVR patients with even mild hemodynamic instability as prompt intervention with open surgical or endovascular approach with covered stents is needed to stabilize or repair the ruptured focus. The overall prognosis is strikingly dismal even in skilled hands as rupture extension can take place exponentially with dramatic reduction in chances of recovery.
TAVR has certainly evolved exponentially since its initial days. Improved device profiles, equipment design and operator expertise are major factors which have significantly improved success rates by reducing possible procedure complications. Nonetheless continuing awareness, meticulous technique, timely management and availability of even better delivery systems in the future would be the key to better clinical outcomes.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Cosmi E, Kahlert P, Wang F S- Editor: Song XX L- Editor: A E- Editor: Wu HL
| 1. | Mack MJ, Leon MB, Smith CR, Miller DC, Moses JW, Tuzcu EM, Webb JG, Douglas PS, Anderson WN, Blackstone EH. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385:2477-2484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1153] [Cited by in RCA: 1340] [Article Influence: 134.0] [Reference Citation Analysis (0)] |
| 2. | Ducrocq G, Francis F, Serfaty JM, Himbert D, Maury JM, Pasi N, Marouene S, Provenchère S, Iung B, Castier Y. Vascular complications of transfemoral aortic valve implantation with the Edwards SAPIEN prosthesis: incidence and impact on outcome. EuroIntervention. 2010;5:666-672. [PubMed] |
| 3. | Thyregod HG, Steinbrüchel DA, Ihlemann N, Nissen H, Kjeldsen BJ, Petursson P, Chang Y, Franzen OW, Engstrøm T, Clemmensen P. Transcatheter Versus Surgical Aortic Valve Replacement in Patients With Severe Aortic Valve Stenosis: 1-Year Results From the All-Comers NOTION Randomized Clinical Trial. J Am Coll Cardiol. 2015;65:2184-2194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 566] [Cited by in RCA: 708] [Article Influence: 70.8] [Reference Citation Analysis (0)] |
| 4. | Holmes DR, Nishimura RA, Grover FL, Brindis RG, Carroll JD, Edwards FH, Peterson ED, Rumsfeld JS, Shahian DM, Thourani VH. Annual Outcomes With Transcatheter Valve Therapy: From the STS/ACC TVT Registry. J Am Coll Cardiol. 2015;66:2813-2823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 221] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 5. | Généreux P, Webb JG, Svensson LG, Kodali SK, Satler LF, Fearon WF, Davidson CJ, Eisenhauer AC, Makkar RR, Bergman GW. Vascular complications after transcatheter aortic valve replacement: insights from the PARTNER (Placement of AoRTic TraNscathetER Valve) trial. J Am Coll Cardiol. 2012;60:1043-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 411] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 6. | Hayashida K, Lefèvre T, Chevalier B, Hovasse T, Romano M, Garot P, Mylotte D, Uribe J, Farge A, Donzeau-Gouge P. Transfemoral aortic valve implantation new criteria to predict vascular complications. JACC Cardiovasc Interv. 2011;4:851-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 425] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 7. | Badheka AO, Patel NJ, Panaich SS, Patel SV, Jhamnani S, Singh V, Pant S, Patel N, Patel N, Arora S. Effect of Hospital Volume on Outcomes of Transcatheter Aortic Valve Implantation. Am J Cardiol. 2015;116:587-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 8. | Petronio AS, De Carlo M, Bedogni F, Maisano F, Ettori F, Klugmann S, Poli A, Marzocchi A, Santoro G, Napodano M. 2-year results of CoreValve implantation through the subclavian access: a propensity-matched comparison with the femoral access. J Am Coll Cardiol. 2012;60:502-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 129] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 9. | Schofer N, Deuschl F, Conradi L, Lubos E, Schirmer J, Reichenspurner H, Blankenberg S, Treede H, Schäfer U. Preferential short cut or alternative route: the transaxillary access for transcatheter aortic valve implantation. J Thorac Dis. 2015;7:1543-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 10. | Masson JB, Kovac J, Schuler G, Ye J, Cheung A, Kapadia S, Tuzcu ME, Kodali S, Leon MB, Webb JG. Transcatheter aortic valve implantation: review of the nature, management, and avoidance of procedural complications. JACC Cardiovasc Interv. 2009;2:811-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 302] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 11. | Nakamura M, Chakravarty T, Jilaihawi H, Doctor N, Dohad S, Fontana G, Cheng W, Makkar RR. Complete percutaneous approach for arterial access in transfemoral transcatheter aortic valve replacement: a comparison with surgical cut-down and closure. Catheter Cardiovasc Interv. 2014;84:293-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 12. | Reidy C, Sophocles A, Ramakrishna H, Ghadimi K, Patel PA, Augoustides JGT. Challenges after the first decade of transcatheter aortic valve replacement: focus on vascular complications, stroke, and paravalvular leak. J Cardiothorac Vasc Anesth. 2013;27:184-9. |
| 13. | Kiramijyan S, Magalhaes MA, Ben-Dor I, Koifman E, Escarcega RO, Baker NC, Torguson R, Okubagzi P, Bernardo NL, Satler LF. The adjunctive use of Angio-Seal in femoral vascular closure following percutaneous transcatheter aortic valve replacement. EuroIntervention. 2016;12:88-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Genereux P, Kodali S, Leon MB, Smith CR, Ben-Gal Y, Kirtane AJ, Daneault B, Reiss GR, Moses JW, Williams MR. Clinical outcomes using a new crossover balloon occlusion technique for percutaneous closure after transfemoral aortic valve implantation. JACC Cardiovasc Interv. 2011;4:861-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | Harrison GJ, How TV, Vallabhaneni SR, Brennan JA, Fisher RK, Naik JB, McWilliams RG. Guidewire stiffness: what’s in a name? J Endovasc Ther. 2011;18:797-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Commissioner O of the Press Announcements - FDA approves expanded indication for two transcatheter heart valves for patients at intermediate risk for death or complications associated with open-heart surgery [Internet]. [accessed 2016 Aug 25]. Available from: http: //www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm517281.htm. |
| 17. | Barbanti M, Binder RK, Freeman M, Wood DA, Leipsic J, Cheung A, Ye J, Tan J, Toggweiler S, Yang TH. Impact of low-profile sheaths on vascular complications during transfemoral transcatheter aortic valve replacement. EuroIntervention. 2013;9:929-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 18. | Van Mieghem NM, Tchetche D, Chieffo A, Dumonteil N, Messika-Zeitoun D, van der Boon RM, Vahdat O, Buchanan GL, Marcheix B, Himbert D. Incidence, predictors, and implications of access site complications with transfemoral transcatheter aortic valve implantation. Am J Cardiol. 2012;110:1361-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 203] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 19. | Seiffert M, Schnabel R, Conradi L, Diemert P, Schirmer J, Koschyk D, Linder M, Kersten JF, Grosser A, Wilde S. Predictors and outcomes after transcatheter aortic valve implantation using different approaches according to the valve academic research consortium definitions. Catheter Cardiovasc Interv. 2013;82:640-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Ndrepepa G, Berger PB, Mehilli J, Seyfarth M, Neumann FJ, Schömig A, Kastrati A. Periprocedural bleeding and 1-year outcome after percutaneous coronary interventions: appropriateness of including bleeding as a component of a quadruple end point. J Am Coll Cardiol. 2008;51:690-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 395] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 21. | Kinnaird TD, Stabile E, Mintz GS, Lee CW, Canos DA, Gevorkian N, Pinnow EE, Kent KM, Pichard AD, Satler LF. Incidence, predictors, and prognostic implications of bleeding and blood transfusion following percutaneous coronary interventions. Am J Cardiol. 2003;92:930-935. [PubMed] |
| 22. | Osten MD, Feindel C, Greutmann M, Chamberlain K, Meineri M, Rubin B, Mezody M, Ivanov J, Butany J, Horlick EM. Transcatheter aortic valve implantation for high risk patients with severe aortic stenosis using the Edwards Sapien balloon-expandable bioprosthesis: a single centre study with immediate and medium-term outcomes. Catheter Cardiovasc Interv. 2010;75:475-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | De Backer O, Arnous S, Sandholt B, Brooks M, Biasco L, Franzen O, Lönn L, Bech B, Søndergaard L. Safety and efficacy of using the Viabahn endoprosthesis for percutaneous treatment of vascular access complications after transfemoral aortic valve implantation. Am J Cardiol. 2015;115:1123-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Katzenschlager R, Ugurluoglu A, Ahmadi A, Hülsmann M, Koppensteiner R, Larch E, Maca T, Minar E, Stümpflen A, Ehringer H. Incidence of pseudoaneurysm after diagnostic and therapeutic angiography. Radiology. 1995;195:463-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 159] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 25. | Toursarkissian B, Allen BT, Petrinec D, Thompson RW, Rubin BG, Reilly JM, Anderson CB, Flye MW, Sicard GA. Spontaneous closure of selected iatrogenic pseudoaneurysms and arteriovenous fistulae. J Vasc Surg. 1997;25:803-808; discussion 803-808. [PubMed] |
| 26. | Oweida SW, Roubin GS, Smith RB, Salam AA. Postcatheterization vascular complications associated with percutaneous transluminal coronary angioplasty. J Vasc Surg. 1990;12:310-315. [PubMed] |
| 27. | Kresowik TF, Khoury MD, Miller BV, Winniford MD, Shamma AR, Sharp WJ, Blecha MB, Corson JD. A prospective study of the incidence and natural history of femoral vascular complications after percutaneous transluminal coronary angioplasty. J Vasc Surg. 1991;13:328-333; discussion 333-335. [PubMed] |
| 28. | Coughlin BF, Paushter DM. Peripheral pseudoaneurysms: evaluation with duplex US. Radiology. 1988;168:339-342. [PubMed] |
| 29. | Dean SM, Olin JW, Piedmonte M, Grubb M, Young JR. Ultrasound-guided compression closure of postcatheterization pseudoaneurysms during concurrent anticoagulation: a review of seventy-seven patients. J Vasc Surg. 1996;23:28-34, discussion 34-5. [PubMed] |
| 30. | Cox GS, Young JR, Gray BR, Grubb MW, Hertzer NR. Ultrasound-guided compression repair of postcatheterization pseudoaneurysms: results of treatment in one hundred cases. J Vasc Surg. 1994;19:683-686. [PubMed] |
| 31. | La Perna L, Olin JW, Goines D, Childs MB, Ouriel K. Ultrasound-guided thrombin injection for the treatment of postcatheterization pseudoaneurysms. Circulation. 2000;102:2391-2395. [PubMed] |
| 32. | Stortecky S, Wenaweser P, Diehm N, Pilgrim T, Huber C, Rosskopf AB, Khattab AA, Buellesfeld L, Gloekler S, Eberle B. Percutaneous management of vascular complications in patients undergoing transcatheter aortic valve implantation. JACC Cardiovasc Interv. 2012;5:515-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 33. | Dahdouh Z, Roule V, Grollier G. Life-threatening iliac artery rupture during transcatheter aortic valve implantation (TAVI): diagnosis and management. Heart. 2013;99:1217-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 34. | Mussardo M, Latib A, Chieffo A, Godino C, Ielasi A, Cioni M, Takagi K, Davidavicius G, Montorfano M, Maisano F. Periprocedural and short-term outcomes of transfemoral transcatheter aortic valve implantation with the Sapien XT as compared with the Edwards Sapien valve. JACC Cardiovasc Interv. 2011;4:743-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 35. | Laganà D, Carrafiello G, Mangini M, Giorgianni A, Lumia D, Cuffari S, Fugazzola C. Emergency percutaneous treatment of arterial iliac axis ruptures. Emerg Radiol. 2007;14:173-179. [PubMed] |
| 36. | Thomas M, Schymik G, Walther T, Himbert D, Lefèvre T, Treede H, Eggebrecht H, Rubino P, Michev I, Lange R. Thirty-day results of the SAPIEN aortic Bioprosthesis European Outcome (SOURCE) Registry: A European registry of transcatheter aortic valve implantation using the Edwards SAPIEN valve. Circulation. 2010;122:62-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 663] [Cited by in RCA: 656] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 37. | Lange R, Bleiziffer S, Piazza N, Mazzitelli D, Hutter A, Tassani-Prell P, Laborde JC, Bauernschmitt R. Incidence and treatment of procedural cardiovascular complications associated with trans-arterial and trans-apical interventional aortic valve implantation in 412 consecutive patients. Eur J Cardiothorac Surg. 2011;40:1105-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 38. | Dahdouh Z, Roule V, Lognoné T, Sabatier R, Grollier G. Aortic arch rupture: an uncommon but fatal complication during transcatheter aortic valve implantation. JACC Cardiovasc Interv. 2013;6:416-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 39. | Sardar MR, Goldsweig AM, Abbott JD, Sharaf BL, Gordon PC1, Ehsan A, Aronow HD. Vascular complications associated with transcatheter aortic valve replacement. Vasc Med. 2017;22:234-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |