Published online Jun 26, 2017. doi: 10.4330/wjc.v9.i6.547
Peer-review started: November 2, 2016
First decision: January 14, 2017
Revised: May 16, 2017
Accepted: May 22, 2017
Article in press: May 24, 2017
Published online: June 26, 2017
Processing time: 239 Days and 19.1 Hours
To describe the long-term follow-up of patients with complex congenital heart disease who underwent subcutaneous implantable cardiac defibrillator (S-ICD), focusing on local complications, appropriate and inappropriate shocks.
Patients with complex congenital heart disease underwent S-ICD implant in two centers with the conventional technique. Data at follow-up were retrieved from clinical notes and institutional database.
Eight patients were implanted in two centres between 2010 and 2016. Median age at implant was 37.5 years (range 13-57). All patients who were deemed suitable for S-ICD implant passed the pre-procedural screening. Three patients were previously implanted with a anti-bradycardia device, one of whom with CRT. In one patient the device was explanted due to local infection. During the total median follow-up of 874 d, one patient had an appropriate and one inappropriate shock triggered by fast atrial tachycardia. None of the patients had inappropriate shocks secondary to T wave oversensing or electrical interference with anti- bradycardia devices.
S-ICD appears to be effective and safe in patients with complex congenital heart disease.
Core tip: Implantation of subcutaneous implantable cardiac defibrillator in patients with complex congenital heart disease appears to be effective and reliable at long term follow-up. The high proportion of grossly abnormal baseline electrocardiogram and the significant incidence of atrial arrhythmias does not seem to affect the rate of inappropriate shocks.
- Citation: Ferrero P, Ali H, Barman P, Foresti S, Lupo P, D’Elia E, Cappato R, Stuart AG. Entirely subcutaneous defibrillator and complex congenital heart disease: Data on long-term clinical follow-up. World J Cardiol 2017; 9(6): 547-552
- URL: https://www.wjgnet.com/1949-8462/full/v9/i6/547.htm
- DOI: https://dx.doi.org/10.4330/wjc.v9.i6.547
Arrhythmic complications are common in patients with congenital heart disease (CHD) requiring implantation of an anti-tachycardic device[1]. Traditional endocardial approach can be particularly challenging in these patients due to limited vascular access, intracardiac shunts or abnormal cardiac chambers[2]. Furthermore the trans venous implantable cardiac defibrillator (ICD) implant carries a risk of long term lead related complication, which have been reported in up to 20% of cases at 10 years[3]. This figure is thought to be even higher in the pediatric and CHD population. Most of the long-term event in this clinical setting are lead related[4]. Alternative implant techniques have been devised in this population, including surgical epicardial or subcutaneous coils implant. Unfortunately, these non trans-venous approaches are associated with a higher procedural risk and have a significant failure rate during follow-up[5]. The entirely subcutaneous ICD (S-ICD) proved to be effective and safe in patients fulfilling both primary and secondary prevention indication[6]. This technology is particular appealing for CHD subjects, as it does not involve the implantation of lead within the cardiac chambers. Data about the performance of S-ICD in this subset of patients are still scant, particularly as far as the long-term outcomes are concerned. A major theoretical concern of the S-ICD in this population is related to the presence of significant ventricular hypertrophy causing profound anomalies of both depolarization and repolarization phase on the surface electrocardiogram (ECG) and the high incidence of atrial tachycardia[7]. In theory, both these issues could lead to an inappropriate shock in the long-term follow-up. Furthermore, a significant proportion of patient with CHD develop atrial-ventricular conduction impairment either as the result of their specific congenital lesion or after cardiac surgery requiring long term pacing. A recent sub analysis from a pooled analysis of the IDE and EFFORTLESS registry showed the safety of S-ICD in patients with CHD[8]. However, during the median follow-up of 567 d none of the patients had any appropriate shocks, consequently no observations about the efficacy of this technology could be performed[9].
In this article, we report medium and long term follow-up in eight patients with complex CHD implanted with a S-ICD, reflecting the experience of two adult CHD referral centres. The details of previous surgical history, the occurrence of either appropriate or inappropriate shocks, local complications, and interference with previously implanted anti-bradycardia and CRT devices are described.
Patients with CHD were implanted with a S-ICD in two centres between 2010 and 2016. All patients underwent pre-implant eligibility screening. Suitability for S-ICD implantation was pre-assessed by a standardized protocol recommended by the manufacture, as previously published. This included assessment of the QRS and T wave amplitude ratio in at least two different postures (supine and standing), and during exercise on a treadmill[10]. Implant was performed under general anaesthesia with the conventional technique. Briefly, the device and lead positioning was guided by standard chest anatomical landmarks and fluoroscopy to ensure proper vector configuration across the cardiac silhouette. All devices but one were programmed from the beginning with a shock conditional zone (180-240 beats per minute, bpm) and a shock only zone above 240 bpm. All patients underwent defibrillation testing by induction of ventricular fibrillation using 50 Hz current to assess accurate detection and effective termination of the arrhythmia. Clinical and surgical details were retrieved from institutional databases. All patients implanted underwent regular follow-up at 1 and 6 mo thereafter. Data about patient clinical status, occurrence of local complication, arrhythmic burden, inappropriate and appropriate shocks were recorded.
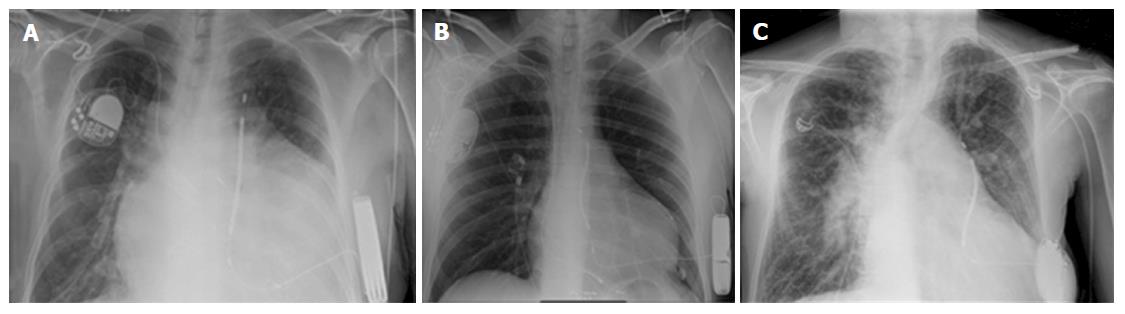
Eight patients were implanted with a S-ICD device between 2010 and 2016 in two centres. Median age at implant was 37.5 years (range 13-57). Seven patients had a secondary prevention indication while one patient was implanted after one episode of syncope and documented not-sustained ventricular tachycardia. The anatomical diagnoses were: Tetralogy of Fallot, repaired ventricular septal defect with subaortic obstruction, transposition of the great arteries that underwent Mustard repair, double inlet left ventricle with Eisenmenger physiology, atrial septal defect associated with cardiomyopathy. Three patients (37.5%) had previously undergone endocardial pacemaker implantation due to brady-tachyarrhythmic syndrome in two cases, and a CRT device in the other one (Figures 1A, B and 2). Four patients (50%) had a previous history of documented paroxysmal atrial tachyarrhythmia. The reasons for S-ICD vs conventional endocardial approach were limited or difficult access to the cardiac chambers in five patient, high infective risk in two patients and presence of right to left shunt in one patient (Table 1). In all patients, the option of conventional ICD was offered and discussed as part of the preoperative consent process.
| Anatomical diagnosis | Sex | Age at implant (yr) | Pre implant ECG | Indication | Reason for SICD | Prev implant | F.U (d) |
| TOF | F | 47 | RBBB + RVH | VT | Tricuspid mech valve | No | 360 |
| VSD subaortic obstr. | M | 52 | LVH | Out hospital card arrest | Previous endocarditis | No | 334 |
| TGA, Mustard | M | 36 | RBBB + RVH | VF | SVC baffle stenosis | Yes | 486 |
| DILV TGA Eisemenger | M | 57 | IVCD (aspecific) | Sustained VT | Right to left shunt | No | 1139 |
| ASD DCM | M | 39 | Paced LV endo | Syncope sustained VT | Previous leads implanted | Yes | 1827 |
| TGA, Mustard | M | 24 | Paced sub pulm vent. | Syncope NOT Sustained VT | SVC baffle occlusion | No | 1890 |
| HLVS Fontan | M | 13 | IVCD + RVH | Sustained VT | Extracardiac Fontan | No | 1499 |
| TGA VSD | M | 23 | RBBB + RVH | Sustained VT | TV repair | Yes | 90 |
Pre implant ECG showed right bundle branch block in 3 patients (37.5 %), non specific interventricular conduction delay in two patients (28%), narrow QRS with left ventricular (LV) hypertrophy in one patient (12.5%), and paced QRS in two patients. Overall the QRS duration ranged from 110 ms to 180 ms (Table 1). All patients clinically deemed as good candidate for S-ICD passed the pre-implant screening (100% success rate). We did not observe any difference in the QRS/T ratio between the left and right position of the electrodes.
Median procedural time was 65 min (range 58-90 min). The use of fluoroscopy was limited to check the proper position of the device and coil relative to the cardiac silhouette. In the patient with an epicardial pacing system, the coil was tunnelled in the usual way without creating any mechanical interference with the previously implanted pacing lead (Figure 2). In one patient with a systemic right ventricle following Mustard operation the defibrillation test was effective only at 80 J despite repositioning of the pulse generator. None of the patients had significant bleeding during the procedure.
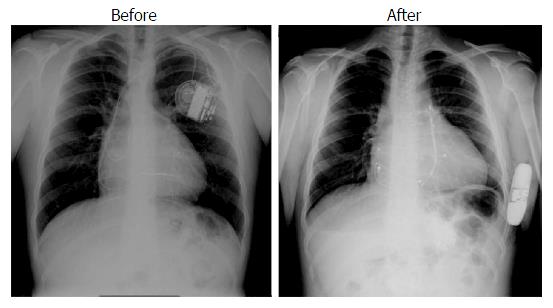
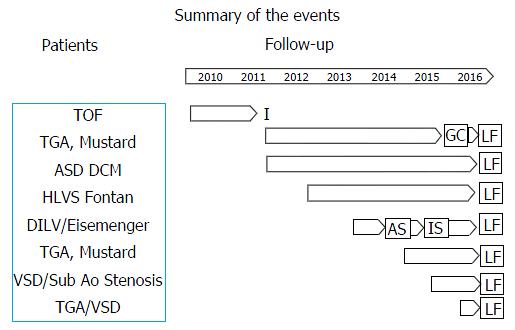
Median follow-up was 812 d (range 90-1890). In four patients (50%) a follow-up longer than three years was available. Figure 3 depicts a summary of the events occurred in each individual patient according to the background anatomy. During follow-up, one patient underwent device extraction due to local infection secondary to hematoma eight months after the implant. This patient did not develop any signs of systemic infection and underwent subsequent surgical epicardial implantation. Three patients had multiple episodes of documented sustained atrial tachycardia (four clinically relevant episodes requiring admission and arrhythmia termination), triggering an inappropriate shock in one patient 18 mo after the implant (inappropriate discharge overall rate 12.5%). These patients had a history of atrial tachycardia before the implant. The same patient had an effective, appropriate shock due to fast ventricular tachycardia that occurred after eight months (appropriate discharge overall rate 12.5%). None of the patients had inappropriate shocks related to over-sensing. One patient underwent uncomplicated pulse generator replacement owing to battery depletion. One patient with large atrial septal defect associated with cardiomyopathy developed progressive LV dysfunction and underwent upgrading to biventricular stimulation. The trans-coronary sinus left LV lead was then switched off as it was causing diaphragmatic capture in the bipolar configuration and interference with the S-ICD in the unipolar one. A new transeptal endocardial lead was implanted in the left ventricle and the atrial septal defect was thereafter successfully closed with an occlude device (Figure 1). In the remaining two patients with conventional endocardial pacing system we did not observe any electrical interference during follow-up.
Abnormal cardiac rhythm is the most common cause of hospital admission in adults with CHD. The absolute risk of sudden death and/or ventricular arrhythmias increases with prolonged follow-up, particularly in the subset of patients with reduced systemic ventricular function and evidence of extensive scar within the ventricular mass[11]. The risk of sudden death in the adult CHD population ranges from 0.1% and 0.5% per year[12,13]. However, there is no established guidance for ICD implantation in this group of patients apart from those fulfilling a secondary prevention indication. It is well recognized that ICD implantation in adult CHD might be particularly technically challenging due to vascular access issues or to the complex anatomy. Furthermore, complications at follow-up are significantly higher when compared to the general population of ICD recipients. These include endocarditis in patient with prosthetic valve or residual native valve disease, baffle obstruction in patients who underwent atrial switch, and thromboembolism in patient with intracardiac shunts. S-ICD represents an attractive alternative for this population and may offer an effective protection against sudden death in a higher proportion of patients with reduced morbidity.
Effective screening plays a pivotal role in selecting CHD subjects for the S-ICD. In congenital heart patients, a particular concern is the presence of a large percentage of T wave inversion or enlargement throughout the precordial leads, secondary to ventricular hypertrophy, right bundle branch block, or a paced QRS complex. These features, have been reported as a risk factor for ECG screening failure in the S-ICD[14,15]. Despite the fact that most of our patient had right bundle branch block and two had a paced ventricular complex, all passed the preimplant screening. Contrary to experience reported previously, all patients passed the screening with electrodes positioned on the left parasternal edge[16]. This finding should be interpreted cautiously owing to the small sample size, and might simply reflect the variability in ECG presentation in this population or the highly unpredictable vector configuration.
As expected, a significant proportion of patient (37%) had multiple episodes of sustained atrial tachycardia that were correctly discriminated by the device. Overall two patients had device related complications (an early local hematoma and an inappropriate shock). Only one patient had one appropriate and one inappropriate shock, accounting for a cumulative incidence of inappropriate shock over the follow-up of 1.5%. The device settings were optimized by activating the conditional shock zone. However, this percentage is consistent with the one reported in the IDE and EFFORTLESS, confirming the reliability of the S-ICD algorhythm in discriminating supraventricular from ventricular tachycardias, even in this clinical setting charachterized by a high incidence of atrial arrhythmias[17]. One patient had an appropriate shock during the entire follow-up (cumulative prevalence 12.5%). Although consistent with the EFFORTLESS registry data, this percentage was lower compared with the population of patients with endocardial ICD[18]. Interestingly, a significant number of self-terminating ventricular tachycardia episodes were detected at follow-up, underscoring the appropriateness of a deliberate high cut-off rate and long time to therapy setting in this subset of patient. Furthermore, the low percentage of shocks also reflects the overall lower arrhythmic risk in patients with CHD as compared with patients with cardiomyopathies or ischaemic substrates, as already observed[12].
A theoretical major limitation in the eligibility of CHD patient for S-ICD is the high likelihood of developing pacing dependency during follow-up. Data about the effect of a previously implanted endocardial pulse generator on the long term performance of the S-ICD are limited. In the EFFORTLESS registry, 2.8% of patients had a anti-bradycardic pacemaker implanted. In the CHD population, the experience of combined implantation of S-ICD and pacemaker did not raise concern regarding electrical interferences[19]. Although a significant proportion of patients had a pacemaker previously implanted, we did not reported clinical relevant electrical interferences in any patient but one in which the LV lead was temporarly programmed in the unipolar configuration. This finding suggest the long-term compatibility of the S-ICD with an endocardial anti-bradycardic device, provided that pacing configuration is bipolar. This safety issue, if consistently confirmed, may theoretically extend the indication to S-ICD in this particular subset of patients.
Our data suggest that S-ICD might represent a valid option for patient with complex CHD at high risk of sudden death. This technology may overcome some of the technical constrain and long-term risk related to the conventional transvenous ICD, eventually expanding the eligibility of this subset of patient for anti-tachycardic therapy.
A major limitation of this paper is the low number of patients, which reflect the experience of only two centres. Furthermore, we do not have a matched group of CHD patients who underwent transvenous ICD implant as control group. Systematic collection of prospective data is needed to support S-ICD as a routine alternative in this population.
Endocardial implant of devices in patients with congenital heart disease may pose a particular challenge owing to limited vascular access and complex anatomy. Furthermore conventional endocardial pacing in this population is associated with a higher risk of lead related complications.
Subcutaneous implantable cardiac defibrillator (S-ICD) has been proved to be effective and safe in patients with a wide range of cardiomyopathies and arrhythmogenic syndromes. Limited evidences from subgroup of patients enrolled in clinical trials support the extension of the use of this technology in patients with structural heart disease.
This paper report data relative to a uniquely long follow-up patients with congenital heart disease that underwent S-ICD implantation. Although the number of patients is low they represent a wide range of clinical settings, including single ventricle physiology.
ICD implantation in patients with congenital heart disease is still a matter of debate, particularly concerning primary prevention indication. The development and optimization of subcutaneous technology might be a suitable tool to provide and extend the protection against sudden death in this group of patients.
S-ICD is a relative new technology made of a pulse generator connected with a coil. The whole system is implanted subcutaneously and is able to provide effective termination of fast rhythm by DC shock and only limited back up pacing.
Very interesting and well written article. It gives an important overview of the topic in a subgroup of very complex patients.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: De Maria E, Iacoviello M, Kettering K, Zhang XQ, Zielinski TA S- Editor: Song XX L- Editor: A E- Editor: Li D
| 1. | Oechslin EN, Harrison DA, Connelly MS, Webb GD, Siu SC. Mode of death in adults with congenital heart disease. Am J Cardiol. 2000;86:1111-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 366] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 2. | Walsh EP. Practical aspects of implantable defibrillator therapy in patients with congenital heart disease. Pacing Clin Electrophysiol. 2008;31 Suppl 1:S38-S40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Borleffs CJ, van Erven L, van Bommel RJ, van der Velde ET, van der Wall EE, Bax JJ, Rosendaal FR, Schalij MJ. Risk of failure of transvenous implantable cardioverter-defibrillator leads. Circ Arrhythm Electrophysiol. 2009;2:411-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 4. | Fortescue EB, Berul CI, Cecchin F, Walsh EP, Triedman JK, Alexander ME. Patient, procedural, and hardware factors associated with pacemaker lead failures in pediatrics and congenital heart disease. Heart Rhythm. 2004;1:150-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 189] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 5. | Griksaitis MJ, Rosengarten JA, Gnanapragasam JP, Haw MP, Morgan JM. Implantable cardioverter defibrillator therapy in paediatric practice: a single-centre UK experience with focus on subcutaneous defibrillation. Europace. 2013;15:523-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Bardy GH, Smith WM, Hood MA, Crozier IG, Melton IC, Jordaens L, Theuns D, Park RE, Wright DJ, Connelly DT. An entirely subcutaneous implantable cardioverter-defibrillator. N Engl J Med. 2010;363:36-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 562] [Cited by in RCA: 567] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 7. | Bouchardy J, Therrien J, Pilote L, Ionescu-Ittu R, Martucci G, Bottega N, Marelli AJ. Atrial arrhythmias in adults with congenital heart disease. Circulation. 2009;120:1679-1686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 334] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 8. | Knops RE, Brouwer TF, Barr CS, Theuns DA, Boersma L, Weiss R, Neuzil P, Scholten M, Lambiase PD, Leon AR. The learning curve associated with the introduction of the subcutaneous implantable defibrillator. Europace. 2016;18:1010-1015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 9. | Bordachar P, Marquié C, Pospiech T, Pasquié JL, Jalal Z, Haissaguerre M, Thambo JB. Subcutaneous implantable cardioverter defibrillators in children, young adults and patients with congenital heart disease. Int J Cardiol. 2016;203:251-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Ali H, Lupo P, Cappato R. The Entirely Subcutaneous Defibrillator - A New Generation and Future Expectations. Arrhythm Electrophysiol Rev. 2015;4:116-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Khairy P, Van Hare GF, Balaji S, Berul CI, Cecchin F, Cohen MI, Daniels CJ, Deal BJ, Dearani JA, Groot Nd, Dubin AM, Harris L, Janousek J, Kanter RJ, Karpawich PP, Perry JC, Seslar SP, Shah MJ, Silka MJ, Triedman JK, Walsh EP, Warnes CA. PACES/HRS Expert Consensus Statement on the Recognition and Management of Arrhythmias in Adult Congenital Heart Disease: developed in partnership between the Pediatric and Congenital Electrophysiology Society (PACES) and the Heart Rhythm Society (HRS). Endorsed by the governing bodies of PACES, HRS, the American College of Cardiology (ACC), the American Heart Association (AHA), the European Heart Rhythm Association (EHRA), the Canadian Heart Rhythm Society (CHRS), and the International Society for Adult Congenital Heart Disease (ISACHD). Heart Rhythm. 2014;11:e102-e165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 408] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 12. | Gatzoulis MA, Balaji S, Webber SA, Siu SC, Hokanson JS, Poile C, Rosenthal M, Nakazawa M, Moller JH, Gillette PC. Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: a multicentre study. Lancet. 2000;356:975-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1288] [Cited by in RCA: 1199] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 13. | Silka MJ, Hardy BG, Menashe VD, Morris CD. A population-based prospective evaluation of risk of sudden cardiac death after operation for common congenital heart defects. J Am Coll Cardiol. 1998;32:245-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 369] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 14. | Okamura H, McLeod CJ, DeSimone CV, Webster TL, Bonnichsen CR, Grogan M, Phillips SD, Connolly HM, Ammash NM, Warnes CA. Right Parasternal Lead Placement Increases Eligibility for Subcutaneous Implantable Cardioverter Defibrillator Therapy in Adults With Congenital Heart Disease. Circ J. 2016;80:1328-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Groh CA, Sharma S, Pelchovitz DJ, Bhave PD, Rhyner J, Verma N, Arora R, Chicos AB, Kim SS, Lin AC. Use of an electrocardiographic screening tool to determine candidacy for a subcutaneous implantable cardioverter-defibrillator. Heart Rhythm. 2014;11:1361-1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 16. | Wilson DG, Zeb M, Veldtman G, Dimitrov BD, Morgan JM. Left and Right Parasternal Sensing for the S-ICD in Adult Congenital Heart Disease Patients and Normal Controls. Pacing Clin Electrophysiol. 2016;39:282-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Gold MR, Theuns DA, Knight BP, Sturdivant JL, Sanghera R, Ellenbogen KA, Wood MA, Burke MC. Head-to-head comparison of arrhythmia discrimination performance of subcutaneous and transvenous ICD arrhythmia detection algorithms: the START study. J Cardiovasc Electrophysiol. 2012;23:359-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Germano JJ, Reynolds M, Essebag V, Josephson ME. Frequency and causes of implantable cardioverter-defibrillator therapies: is device therapy proarrhythmic? Am J Cardiol. 2006;97:1255-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 169] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 19. | Hong PS, Callinan P, Amit G, Healey JS. Successful Implant of a Subcutaneous ICD System in a Patient with an Ipsilateral Epicardial Pacemaker. Indian Pacing Electrophysiol J. 2015;15:62-64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |