Published online Jun 26, 2017. doi: 10.4330/wjc.v9.i6.481
Peer-review started: October 23, 2016
First decision: February 17, 2017
Revised: April 10, 2017
Accepted: May 18, 2017
Article in press: May 19, 2017
Published online: June 26, 2017
Processing time: 250 Days and 21 Hours
Aortic valve disease [aortic stenosis (AS) and aortic regurgitation (AR)] represents an important global health problem; when severe, aortic valve disease carries poor prognosis. For AS, aortic valve replacement, either surgical or interventional, may provide definite treatment in carefully selected patients. For AR, valve surgery (either replacement or - in selected cases - aortic valve repair) remains the gold standard of care. To properly identify those patients who are candidates for surgery, the clinician has to carefully assess the severity of valve disease with an understanding of the potential pitfalls involved in these assessments. This review focuses on the practical issues concerning the evaluation of patients with AS and AR from a general cardiologist’s perspective. The most important issues regarding the documentation of the severity of AS and AR are summarized. More specific issues, such as the role of stress echocardiography, other imaging techniques and details regarding the treatment options (medical, surgical, or interventional), are mentioned briefly.
Core tip: Aortic stenosis (AS) and aortic regurgitation (AR) represent important health problems world-wide. This review focuses on the practical issues concerning the evaluation of patients with AS and AR from a general cardiologist’s perspective. The most important issues regarding the documentation of the severity of AS and AR are summarized, and potential pitfalls are highlighted. More specific issues, such as the role of stress echocardiography, other imaging techniques and details regarding the treatment options (medical, surgical, or interventional), are mentioned briefly.
- Citation: Mǎrgulescu AD. Assessment of aortic valve disease - a clinician oriented review. World J Cardiol 2017; 9(6): 481-495
- URL: https://www.wjgnet.com/1949-8462/full/v9/i6/481.htm
- DOI: https://dx.doi.org/10.4330/wjc.v9.i6.481
Aortic valve disease [aortic stenosis (AS) and aortic regurgitation (AR)] represents an important global health problem. The data on the exact prevalence of AS and AR in the general population are lacking, but studies performed in Western populations estimate that 3% to 4% of the adult population suffers from moderate or severe aortic valve disease. The prevalence of AS and AR increases with age; it is estimated that 1% of persons aged < 55 years and 6% of persons aged > 75 years suffer from moderate or severe AS/AR[1,2].
This review focuses on the practical issues concerning the evaluation of patients with AS and AR from a general cardiologist’s perspective. The most important issues regarding the documentation of the severity of AS and AR using echocardiography are summarized. More specific issues, such as the role of stress echocardiography, other imaging techniques and details regarding the treatment options (medical, surgical, or interventional), are mentioned briefly. For more detailed information on these topics, the reader is referred to several recent excellent reviews, mostly regarding AS[3-11].
AS is defined as a narrowing of the surface area of the aortic orifice [aortic valve area (AVA)] below the normal value (approximately 3 cm2). AS becomes significant [i.e., determines a significant increase in left ventricular (LV) afterload] only after the AVA decreases by more than half. In general, the accepted criteria for the definition of severe AS is an AVA ≤ 1 cm2 (or ≤ 0.6 cm2/m2 of body surface area). These cut-off values have been used in clinical studies but are patient-dependent and do not completely overlap with other indices that are also used to define severe AS (e.g., transaortic pressure gradients - see below).
In Western countries, AS has the following two major causes: Degenerative (calcific) and congenital. Calcific AS is predominant in the elderly population, shares common pathological features and is commonly associated with atherosclerosis. Congenital AS [> 90% represented by bicuspid aortic valve (BAV)] manifests clinically 10 to 20 years earlier than calcific AS. Contemporary data from 932 isolated aortic valves excised from adults aged 26 to 91 years between 1993 and 2004 suggest that 54% of these cases were congenital in origin[12].
AS is a slowly progressive disease. Almost 50 years ago, Ross and Braunwald highlighted that the appearance of symptoms marks a sharp decline in survival with nearly universal death within 5 years[13]. The types of symptoms are important, as follows: The mean survival after the appearance of angina was 5 years, 3 years after syncope and 2 years after the appearance of heart failure. When these data were published, the predominant etiology was rheumatic heart disease, and the mean patient age was 63 years old[14]. Thus, the contemporary application of these data is limited. Recent data suggest that the presence of AS is associated with a 68% increased risk of coronary events, a 27% increased risk of cerebrovascular events, and a 36% increased risk of mortality[15]. The data from the PARTNER study in elderly patients with severe calcific AS suggest an annual mortality of 50% with conservative treatment[16].
The evaluation of patients with AS must define the following 2 issues: (1) identification of patients with severe AS; and (2) in patients with severe AS, identification of patients whose prognosis will be improved by aortic valve replacement (AVR) (surgical or interventional).
Identifying patients with severe AS: The clinical (presence of symptoms, grade ≥ 4/6 ejection murmur, and “tardus et parvus” peripheral pulse), electrocardiographical (left ventricular hypertrophy) or radiological (valve calcification) criteria for severity have high sensitivity but low specificity in identifying patients with severe AS. Therefore, objective assessment of AS severity is needed.
Historically, invasive direct measurement of transaortic pressure gradients was performed, and the aortic valve area was calculated using the Gorlin formula. This practice was abandoned because of the following important drawbacks: (1) invasively measured pressure gradients (mean transaortic pressure gradient and the difference between peak aortic pressure and peak LV systolic pressure) do not overlap with the Doppler estimation of transaortic pressure gradients. This is because Doppler echocardiography measures instantaneous velocities and through the use of Bernoulli equation estimates instantaneous pressure gradients, whereas peak LV pressure occurs before peak aortic pressure (the invasively measured peak-to-peak transaortic pressure difference is not instantaneous); and (2) the risk of atherosclerotic cerebral embolism during the transaortic passage of the pressure catheter may reach 20%[17]. Thus, today, objective assessment of AS severity almost completely relies on proper performance and interpretation of Doppler echocardiography.
The currently used criteria for the definition of severe AS by echocardiography are listed in Table 1[18]. These criteria have advantages and disadvantages.
| Criteria | Severe AS | Advantages | Disadvantages |
| Aortic surface area | ≤ 1.0 cm2 | Measures effective AVA. However, this may also constitute a disadvantage because it does not measure anatomical AVA | Very sensitive to measurement errors |
| Less flow-dependent compared with other measurements | |||
| Indexed AVA to body surface area | ≤ 0.6 cm2/m2 | Useful for extreme heights/weights | Very sensitive to measurement errors |
| Mean transaortic pressure gradient | ≥ 40 mmHg | Flow-dependent | |
| Requires correct alignment of Doppler signal with the flow direction | |||
| Peak transaortic flow velocity | ≥ 4.0 m/s | Measures instantaneous velocity | Flow-dependent |
| Best predictor of adverse events | Requires correct alignment of Doppler signal with the flow direction | ||
| Ratio between peak transaortic flow velocity and peak LVOT velocity | ≤ 1/4 | Good reproducibility (compared with AVA calculation) | Limited data on prognostic utility |
It is of critical importance that the echocardiographic evaluation of AS is based on correctly performed measurements, using an integrative approach, because the echocardiographic criteria for the definition of severe AS are not interchangeable, and the criteria based on pressure gradients and velocities are highly dependent on blood flow.
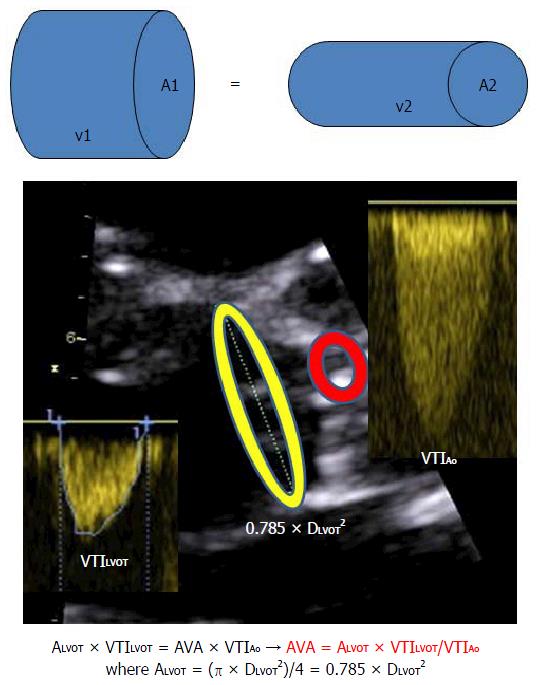
The most robust and reproducible estimation of AVA is based on the continuity equation (Figure 1).
To calculate the AVA, it is essential to perform correct measurements, especially for the left ventricular ejection tract diameter (DLVOT) and velocities.
Any error in the measurements of DLVOT will be squared when calculating AVA using the continuity equation. Thus, for a correct measurement of DLVOT, the following technical requirements are suggested: (1) use of the “zoom” function on the echocardiograph to focus and enlarge the LVOT; (2) decrease the grey-scale gain towards the minimum; (3) DLVOT measurement is performed from the inner anterior edge to the inner posterior edge of the LVOT in mid-systole (“inner-edge to inner-edge”), which is immediately under the aortic valve. The maximal visualized DLVOT is considered, using an echocardiographic section that passes through the center of the LVOT and is not excentric because an excentric slice will underestimate the true maximal diameter. The measured DLVOT should be compatible with the patient’s height and weight. A DLVOT < 16 mm is extremely rarely seen in adults and should raise suspicion of measurement errors[19].
It is important to realize that the true shape of the LVOT is not circular but oval; thus, echocardiographically determined AVA will always be an estimation and not a true measurement.
The correct measurement of transaortic velocities and gradients requires the use of the following multiple echo windows: Modified apical 5 chamber view towards the axilla; apical long axis view; 4th right intercostal space; and suprasternal window. Given these views, the following precautions should be applied: (1) the full envelope of the Doppler signal should be measured to avoid noise and/or aliasing; (2) measurements should not be made on post-extrasystolic beats; (3) correct measurement of the LVOT velocity-time-integral (VTILVOT) should be made. The sample area should be placed immediately under the aortic valve in the middle of the LVOT where the velocity is maximal. In this location, the signal should record the clear click of the aortic valve closure without the click of the aortic valve opening; and (4) for the correct assessment of AVA using the simplified Bernoulli equation (automatically given by the echocardiograph), the measured VTILVOT should be < 1.5 m/s. When the VTILVOT is ≥ 1.5 m/s (e.g., increased LVOT flow due to severe AR, etc.), the simplified Bernoulli equation cannot be used because it will overestimate the transaortic pressure gradient and AS severity based on the continuity equation and calculated AVA.
In conclusion, a correct and complete echocardiographic assessment of AS severity should report on the overall context of the cardiac pathology, LV volumes and LVEF, stroke volume (based on Doppler, not volumetric measurements), grade of calcification of the aortic valve (is it compatible with the measured severity?), associated abnormalities, estimation of pulmonary pressures, dimensions of right heart chamber, and estimation of right ventricular function.
The echocardiographic criteria for the definition of severe AS are not interchangeable. For example, a recent study on the correlation between mean transaortic pressure gradient and AVA in patients with AS and normal LVEF proved that for a mean pressure gradient of 40 mmHg, the corresponding AVA was 0.8 cm2 and not 1 cm2 as is the standard definition of severe AS[20]. Similarly, it is important to understand that a simple documentation of an AVA ≤ 0.8 cm2 does not prove the presence of severe AS because AVA is calculated using pressure gradients that are highly dependent on flow. Thus, when the transaortic flow is low, any valve (including normal ones) will appear “stenotic” because the orifice will not be fully opened. It has been proven that at transaortic flow rates < 125 mL/s (corresponding to a cardiac output of approximately 3 L/min) the effective orifice area of any aortic valve, from mild anatomic AS to severe anatomic AS, will be ≤ 1 cm2. Similarly, the mean transaortic pressure gradient will be ≤ 40 mmHg for any AS severity (from mild to severe, based on anatomical AVA) when the transaortic flow is < 175 mL/min[21].
Thus, the major problem in assessing AS severity rests with low-flow states. The prevalence of “low-flow, low-gradient” severe AS is approximately 25% of all severe AS cases. A low flow state is defined as an indexed stroke volume < 35 mL/m2 of the body surface area. A low-flow/low-gradient state can appear in patients with both reduced LVEF due to myocardial systolic dysfunction or preserved LVEF due to small LV cavity size[22]. These 2 conditions will be detailed below.
Low-flow, low-gradient, low-LVEF, severe AS: Low-flow, low-gradient, low-LVEF, severe AS (“classical” low-flow, low-gradient severe AS) was described for the first time by Carabello et al[23] in 1980. It is defined as severe AS in the presence of systolic LV dysfunction (LV ejection fraction < 40%) with a mean transaortic pressure gradient < 40 mmHg if estimated by echocardiography or < 30 mmHg if measured invasively.
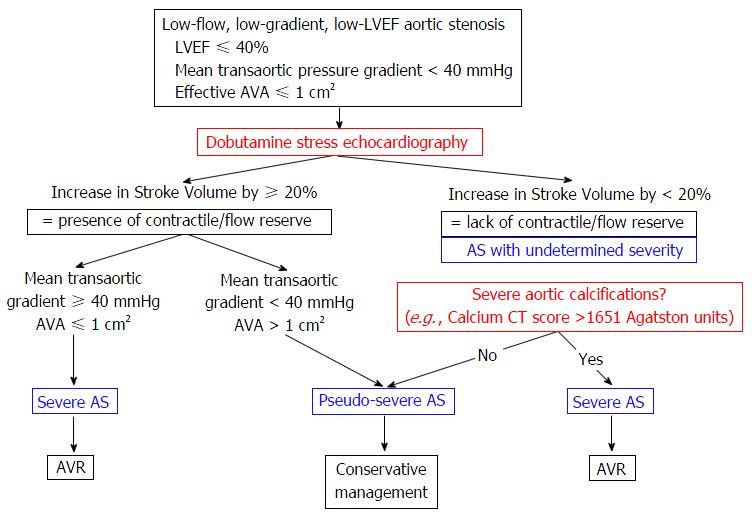
When the calculated AVA is ≤ 1 cm2 in low flow states, one should differentiate whether this is primarily due to the low flow (pseudo severe AS, where the anatomical AVA is > 1 cm2) or if there is true severe AS (AVA remains fixed and ≤ 1 cm2 regardless of flow). Dobutamine stress echocardiography (DSE) is typically performed to differentiate between the two conditions because it evaluates the response of AVA to increased transaortic flow. Figure 2 exemplifies the role of DSE in diagnosing low-flow, low-gradient, low-EF severe AS[24].
Thus, when the calculated AVA is ≤ 1 cm2, the mean transaortic pressure gradient is < 40 mmHg and the LVEF is < 40%, the DSE will help define the following parameters: (1) the severity of AS; and (2) the presence or absence of LV flow/contractile reserve, which is defined as an increase in LV stroke volume > 20% compared with baseline at maximal dobutamine dose. Thus, the following 2 responses to dobutamine and 3 conditions associated with AS are seen when using DSE.
Low-flow, low-gradient, low-EF severe AS is diagnosed when there is increased flow/contractile reserve with a subsequent increase in transaortic pressure gradient to > 40 mmHg while AVA remains ≤ 1 cm2. AVR is indicated in these patients.
Pseudo-severe AS is diagnosed when there is flow (contractile) reserve, but the AVA increases in parallel with the flow to > 1 cm2. AVR is not indicated in these patients.
AS with undetermined severity is defined by the lack of flow/contractile reserve[25]. Even in this situation, identifying severe AS is important because the prognosis without AVR is grim, although surgical mortality is high. Identifying low-flow, low-gradient, severe AS without flow (contractile) reserve is based on the following: (1) statistical data - approximately 95% of low-flow, low-gradient, low-EF AS with undetermined severity have truly severe AS; and (2) objective data - evidence of severe aortic valve calcification (using echocardiogram, plain radiology or computed tomography) is highly specific for severe AS[26]. In this situation, a calcium score of ≥ 1651 Agatston units on computed tomography has an 82% sensitivity, 80% specificity, 88% negative predictive value and 70% positive predictive value for severe AS[27]. Of note, for the same hemodynamic severity of AS, women have lower aortic calcium load compared with men, so the thresholds should probably be lower in women compared with men[28].
Low-flow, low-gradient severe AS with preserved LVEF: Approximately 10% of patients with anatomically severe AS have low-flow/low-gradient characteristics despite preserved LVEF (“paradoxical” low-flow, low-gradient severe AS). This form of severe AS was first described by Hachicha et al[29] in 2007 and is characterized by the following features: (1) concentrically remodeled LV with preserved LVEF, severe diastolic dysfunction, impaired LV filling and low cardiac output (stroke volume < 35 mL/m2); and (2) increased LV afterload generated by the AS and increased peripheral vascular resistance due to the rigid arterial system and frequent severe arterial hypertension in these patients.
Estimation of the global afterload faced by the LV (defined as the ventriculo-arterial impedance, Zva) is important because it is an independent negative prognostic factor and correlates with the appearance of symptoms in these patients[30]. Zva is calculated according to the following formula:
Zva = [Systolic blood pressure (mmHg) + mean transaortic pressure gradient (mmHg)]/[indexed stroke volume (mL/m2)].
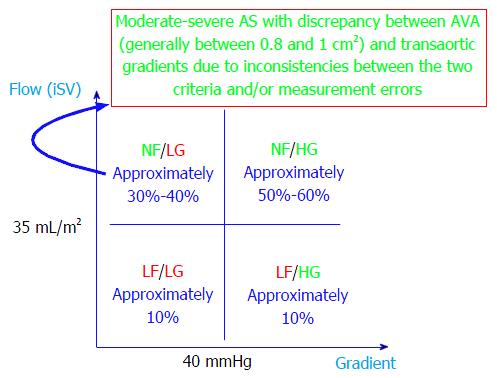
Taking into account the transaortic flow and pressure gradients, severe AS with preserved LVEF has been recently classified into the following 4 forms[31]: (1) normal flow, low-gradient (NFLG) - representing approximately 1/4 of patients; (2) normal flow, high-gradient: Representing approximately 2/3 of patients; (3) low-flow, low-gradient (LFLG) - also known as “paradoxical” low-flow/low-gradient - representing 10% of patients; and (4) low-flow, high-gradient - representing the remaining 10% of patients.
The principle of this classification scheme can be extended to all forms of AS, regardless of LVEF[11].
This classification has prognostic importance in patients with severe AS with preserved LVEF. The best prognosis [major adverse cardiovascular event (MACE) rate, 35% at 3 years] is carried by NFLG, and the most severe prognosis (MACE rate, 90% at 3 years) is carried by LFLG severe AS with preserved LVEF. The “high-gradient” forms have similar prognoses because they are intermediate forms between NFLG and LFLG[31]. However, this classification is limited by the fact that the existence of NFLG severe anatomical AS is counterintuitive. Indeed, the prognosis of these patients is similar to that for patients with moderate AS and is better than that for any other form of severe AS[32]. Thus, it is highly likely that what is known as NFLG severe AS with preserved LVEF is in fact moderate AS where the discrepancy between calculated AVA (which usually rests between 0.8 and 1 cm2 in these cases) and transaortic gradients is a consequence of the inconsistency of the criteria used to define severe AS (see above) and/or measurement errors (Figure 3). Thus, when one is faced with a discrepancy between the calculated AVA and measured gradients, the following elements should be taken into account: (1) measurement errors, especially of the DLVOT diameter and VTILVOT (underestimating flow); (2) extremes in body surface areas (very small or large individuals) - always use indexed measurements; and (3) inconsistency between the cut-off values used to define severe AS: An AVA of 1 cm2 corresponds better to a transaortic pressure gradient of 30-35 mmHg and not 40 mmHg (see comments above for NFLG “severe” AS with preserved LVEF).
To establish a diagnosis of LFLG severe AS with preserved LVEF the following 3 criteria are recommended. First, confirmation of low-flow states by and indexed stroke volume < 35 mL/m2. Second, confirmation of increased global LV afterload (ventriculo-arterial impedance) by Zva ≥ 4.5 mmHg/mL per square meter. Third, confirmation of concentric LV remodeling by the following: (1) relative wall thickness (RWT) ≥ 0.45. RWT is calculated using the following formula: RWT = (IVS + LVPW)/LVEDD, where IVS is the end-diastolic ventricular septal thickness; LVPW is the end-diastolic LV posterior wall thickness; and LVEDD is the end-diastolic LV diameter; (2) end-diastolic LV diameter < 47 mm; and (3) indexed end-diastolic LV volume < 55 mL/m2.
Identification of patients with severe AS who are candidates for aortic valve replacement: After establishing the diagnosis of severe AS, the next step is to identify those patients who will benefit from AVR. The European Society of Cardiology/European Association for Cardio-Thoracic Surgery (ESC/EACTS) and the American Heart Association/American College of Cardiology (AHA/ACC) guidelines establish clear indications for AVR in patients with symptomatic severe AS (class I for normal flow/normal LVEF and for patients with normal flow/low LVEF and class IIa for patients with low-flow/low-gradient/low LVEF with true severe AS) and asymptomatic severe AS with LV systolic dysfunction or symptoms unmasked at stress tests (Table 2)[33,34].
| Criteria | Level of recommendation | Differences between guidelines | |
| ESC/EACTS | AHA/ACC | ||
| Severe AS with any symptoms clearly due to AS, based on history or unmasked by stress test | I | I | "High-gradient" in AHA/ACC guidelines |
| Asymptomatic severe AS with LVEF < 50% | I | I | |
| Severe AS and another indication for surgery (CABG, thoracic aorta, another valve) | I | I | |
| Asymptomatic severe AS where the systolic blood pressure does not increase by > 20 mmHg or drops compared with baseline during the treadmill test | IIa | IIa | AHA/ACC guidelines acknowledge the presence of fatigability during stress test as an indication for AVR |
| Moderate AS and another indication for surgery (CABG, thoracic aorta, another valve) | IIa | IIa | |
| Low-flow/low-gradient/low-LVEF severe AS with proof of contractile reserve presence | IIa | IIa | |
| Symptomatic low-flow/low-gradient/preserved LVEF severe AS after careful confirmation of severity | IIa | IIa | |
| Truly asymptomatic severe AS (no symptoms during treadmill test, no risk criteria) with preserved LVEF if the surgical risk is deemed low and the following criteria are also satisfied: Very severe AS (maximal velocity ≥ 5.5 m/s); Severe valvular calcification and increased maximal velocity by ≥ 0.3 m/s per year | IIa | IIa for velocity ≥ 5 m/s (see text) | AHA/ACC guideline: Velocity ≥ 5 m/s or mean gradient ≥ 60 mmHg AND severe calcifications; velocity 4 to 4.9 m/s or mean gradient 40 to 59 mmHg AND severe valvular calcification AND stress test demonstrating reduced tolerance or drop in blood pressure |
| IIb for maximal velocity increase by ≥ 0.3 m/s per year | |||
| Truly asymptomatic severe AS (no symptoms during treadmill test, no risk criteria) with preserved LVEF if the surgical risk is deemed low and 1 or more of the following criteria are also satisfied: Severely increased BNP/Nt-ProBNP levels at serial determinations and without an alternative explanation; increased transaortic pressure gradient at stress echocardiography by > 20 mmHg; excessive LV hypertrophy without an alternative explanation | IIb | - | This indication is not covered in the AHA/ACC guidelines |
| Low-flow/low-gradient/low-LVEF severe AS without contractile/flow reserve | IIb | - | This indication is not covered in the AHA/ACC guidelines |
Establishing the presence or absence of symptoms can be difficult because many older patients (the majority of patients with AS) deny the presence of symptoms due to lifestyle adaptations to lower functional needs. Also, older patients refer symptoms that can be vague (e.g., fatigue), related to AS or to other comorbidities related with advanced age but not caused by AS. In these patients, unmasking the presence of symptoms (by treadmill or bicycle stress test) and/or LV systolic dysfunction (by stress echocardiography) establishes the indication for AVR[35]. The prognosis of patients with asymptomatic severe AS by positive stress test is identical to that for patients with symptomatic severe AS[36,37].
The indication for AVR in patients with asymptomatic severe AS with preserved LVEF is highly controversial[38,39]. For a detailed discussion and extensive review of the literature on this highly important topic, the reader is referred to the excellent article by Généreux et al[10]. The 1- and 5-year mortality rates for asymptomatic severe AS with preserved LVEF are 3% and 26.4%, respectively; also, 46% of initially asymptomatic patients develop symptoms during the next 5 years, and 20% develop heart failure[40]. Among patients with asymptomatic severe AS with preserved LVEF, those with very severe AS (defined as having a maximal transaortic velocity of ≥ 5.5 m/s) have twice the rate of MACE compared with that of patients with severe AS and a maximal transaortic velocity of 4 to 5 m/s (96% vs 39% at 4 years)[41]. Almost all patients (97%) with severe AS and a maximal velocity of ≥ 5 m/s suffered a MACE within 6 years of follow-up[41]. A recent registry study on patients with asymptomatic very severe AS, which compared 102 patients who had surgical AVR with 95 patients who were treated conservatively, showed that surgical AVR was associated with an 86% reduction in mortality compared with the conservatively managed group after 6 years of follow-up (2% vs 32%; HR = 0.14, 95%CI: 0.03-0.6, P = 0.008)[42]. Based on these non-randomized, single-center data, current guidelines provide a class IIa (“is reasonable”; “should be considered”) for AVR in patients with asymptomatic very severe AS with preserved LVEF (defined as having a maximal velocity of ≥ 5.5 m/s in the ESC/EACTS guidelines or ≥ 5 m/s in the AHA/ACC guidelines) only if the estimated perioperative mortality in that center is low[32,34]. Current guidelines also give a class IIa indication for AVR in patients with severe low-flow AS with preserved LVEF if the symptoms are judged to be secondary to AS only.
Observational and retrospective data suggest that several risk factors for MACE and poor prognosis may be useful to take into account in these cases (Table 3). However, it should be noted that the sensitivity and specificity of these parameters for the identification of patients with a good post-operative prognosis are only approximately 80%. Thus, implementation of these parameters to wide clinical practice cannot be recommended at present but can they can be useful for individual decision making in patients proposed for AVR. Among these parameters, the most widely studied are the prognostic role of aortic valvular calcifications and the hemodynamic response at stress echocardiography.
| Test | High risk criteria |
| Electrocardiogram | Presence of LV hypertrophy with secondary ST segment deviation ("LV strain") |
| Blood tests | Highly increased BNP/Nt-ProBNP levels |
| Stress test | Unmasked symptoms: Fatigability/dyspnea at < 75 W, syncope/near syncope; angina |
| Lack of increase in systolic blood pressure by > 20 mmHg (or decrease) with exercise | |
| Inducible myocardial ischemia (ST segment depression ≥ 2 mm) | |
| Severe ventricular arrhythmias (sustained VT, polymorphic VT, VF) | |
| Conventional Doppler echocardiography | Very severe AS (AVA ≤ 0.6 cm; maximal velocity ≥ 5 m/s) |
| LVEF < 50% | |
| Severe LV hypertrophy (≥ 15 mm)? | |
| Reduced LV longitudinal strain | |
| Zva ≥ 4.5 mmHg/mL per square meters | |
| Dobutamine stress echocardiography (in low-flow, low-gradient, low LVEF) | Lack of contractile reserve |
| Exercise echocardiography (ergometric bicycle) - any severe AS | Increase in transvalvular pressure gradient by > 20 mmHg during exercise |
| Inducible pulmonary hypertension during exercise (systolic pulmonary pressure ≥ 60 mmHg) | |
| Documentation of valvular calcification | Presence of severe valvular calcifications: Qualitatively (radiology, conventional echocardiography); quantitatively (computed tomography): Calcium score ≥ 1651 Agatston units (lower in women vs men) |
Eighty percent of patients with asymptomatic severe AS with preserved LVEF who have moderate or severe valvular calcifications develop MACE within the next 4 years, compared with only 20% of patients without moderate or severe calcifications[26,43].
The response of transaortic pressure gradient to exercise has also been suggested to have prognostic importance. Thus, MACE event rate is highest (100% at 2 years) in patients with high resting transaortic pressure gradient (> 35 mmHg) that increases by > 20 mmHg during exercise, intermediate in patients where the transaortic pressure gradient increases by < 20% during exercise (50% at 2 years for patients with high resting transaortic pressure gradient, and 20% at 2 years for patients with low transaortic pressure gradient), and lowest (10% at 2 years) in patients with low transaortic pressure gradient (≤ 35 mmHg) that increases by < 20% during exercise[44].
Another study that evaluated 105 patients with asymptomatic severe AS with preserved LVEF showed that the inducibility of pulmonary hypertension during exercise (defined as an echocardiographically estimated systolic pulmonary arterial pressure ≥ 60 mmHg) was associated with twice the risk of MACE within 3 years of follow-up compared with patients with asymptomatic severe AS with preserved LVEF who did not develop pulmonary hypertension during exercise (22% vs 55%, P = 0.014)[45]. However, the incidence of MACE in both of these groups was very high. In addition, a recent meta-analysis of 4 observational studies with a total of 2486 patients reporting on the utility of AVR (21% of patients) vs watchful waiting (until development of symptoms for a class I indication of AVR) (79% of patients) found that patients who were treated medically had a 3.5-fold increase in mortality compared with those who underwent AVR, suggesting the benefit of early AVR in this population[10]. However, in these observational studies, patients who were medically treated were older and sicker, and up to 50% of them developed a class I indication for AVR during follow-up but were refused for various reasons - suggesting they were too sick to undergo either surgical or interventional AVR[40]. Thus, there is urgent need for a randomized trial to directly compare the two strategies[46].
Although the ESC/EACTS guidelines for valvular heart disease suggest the use of natriuretic peptide levels (Nt-ProBNP) for decisions regarding the need for AVR in patients with asymptomatic severe AS with preserved LVEF[33], a recent study found that the discriminating value of Nt-ProBNP in identifying patients who need AVR is suboptimal (area under the curve, AUC 0.73)[47]. Further research is needed to establish the use of natriuretic peptides in these patients.
In patients proposed for AVR, estimation of operative risk is essential. Currently, two risk scores are widely used. The EuroSCORE II (http://www.euroscore.org/calc.html) includes 12 predictors identified from a retrospective population of 14799 patients who underwent different cardiovascular surgical interventions (mainly coronary artery bypass graft) in Europe, in 1995. The STS score (Society of Thoracic Surgeons, http://riskcalc.sts.org) includes 24 predictors identified from a population of 64292 patients who underwent surgical intervention only for AS in the United States between 2002 and 2006. The STS score is widely used in the United States for evaluating surgical risk for AS.
Both the EuroSCORE II and the STS score are quite precise in identifying patients with low surgical risk, but they tend to overestimate the risk of patients with high surgical risk (EuroSCORE II more than STS). For example, a patient with a logistic EuroCORE II > 20 has an estimated surgical mortality of 39%, which much higher than the real-world mortality of 11%[43]. Importantly, both the EuroSCORE II and the STS score can be used in practice in surgical institutions where the operative mortality lies within 1 standard deviation from the mean calculated mortality for the respective surgical procedure. None of the scores include frailty, which is a major limitation. The AHA/ACC guidelines recommend that the overall surgical risk should be divided into 4 groups (low, intermediate, high, and prohibitive) based on the overall assessment of surgical risk (STS score), patient frailty (Katz score)[48], presence of major co-morbidities (e.g., severe LV systolic dysfunction, fixed pulmonary hypertension, severe chronic renal failure, respiratory failure, cerebral dysfunction, cancer, and liver cirrhosis), and anticipated difficulties for surgical intervention (e.g., porcelain aorta, thoracic deformities, previous radiotherapy, internal mammary artery crossing the mid-line, and arterial bypass grafts that adhere to the posterior thoracic wall)[34]. The ESC/EACTS guidelines do not have similar recommendations[33].
Importantly, the overall decision regarding the relative risks vs benefits for AVR and the most appropriate type of AVR in individual patients should be made by a multidisciplinary heart team, consisting of a general cardiologist, an interventional cardiologist, a cardiac and vascular surgeon, imaging specialists (echocardiography, computed tomography), and an intensive care specialist with expertise in cardiac anesthesia.
Currently, the most effective treatment for AS is AVR. Simple valvuloplasty has no role in the treatment of severe AS except as a short-term palliation or as a bridge to more definite treatments (e.g., patients with very severe AS who also have abdominal surgical emergencies). Surgical AVR remains the main treatment option, and either a mechanical valve (in younger patients or patients with other indications for long-term anticoagulant therapy) or a bioprosthesis (in older patients due to durability issues or patients with contraindications to life-long anticoagulant therapy) can be used[49]. For a detailed discussion regarding the choice of surgical prosthesis, the reader is referred to recent reviews[50,51]. The newer alternative of percutaneous transcatheter AVR (TAVR) is given a class I indication for patients who have an indication for AVR but are not candidates for surgery (e.g., porcelain aorta, severe frailty) and a class IIa indication for patients with high surgical risk scores[33,34,52]. The morbidity and mortality associated with TAVR have significantly decreased recently as the technique has matured and experience increased; thus, TAVR is currently being investigated for possible expansion to lower risk patients with an indication for a bioprosthesis because recent trials have suggested that TAVR compared favorably to SAVR in these groups[53]. For a detailed discussion regarding the selection of TAVR candidates, the reader is referred to excellent recent reviews[7,8].
AR is defined as the presence of diastolic incompetence of the aortic valve with the subsequent regurgitation of blood back from the aorta into the LV. The generally accepted criteria for the definition of severe AR is a regurgitant volume > 60 mL/cardiac cycle or an effective regurgitant orifice area (EROA) > 0.3 cm2. However, these parameters are very difficult to measure; therefore, numerous alternative parameters are used to define AR severity. One should be careful when using these parameters because the cut-off values are not interchangeable, and their sensitivity and specificity are suboptimal. Similarly to any other valvular heart disease, the echocardiographic assessment has to use an integrative, complete and correct approach.
The prevalence of AR is much lower compared with that for AS, and thus, far fewer studies are available for AR diagnosis and management. AR can be acute or chronic. Acute AR appears primarily as a result of aortic dissection or infective endocarditis. The heart cannot adapt by compensatory dilatation; as a result, the clinical picture is dominated by signs of low cardiac output (due to reduced effective circulating volume) and pulmonary edema (due to high LV filling pressures secondary to large regurgitant volume). The classical signs of severe chronic AR (diastolic murmur, peripheral signs due to wide pulse pressure) are absent in severe acute AR because the diastolic pressure gradient between the aorta and the LV quickly equalizes. For the same reason, some echocardiographic signs of severe AR may be absent (such as the Doppler signal aliasing in the LVOT); in these situations, documenting diastolic reversal flow in the descending aorta prevents missing the diagnosis of severe AR. The presence of severe acute AR should be considered in the differential diagnosis of any patient presenting with acute severe heart failure or cardiogenic shock in the absence of obvious causes (such as myocardial infarction)[54].
Chronic AR is mostly due to BAV or aortic root dilatation. Degenerative aortic valve disease is also important, whereas other etiologies are rare. Patients remain asymptomatic for a long time, but irreversible LV dysfunction may appear before symptom onset.
Bicuspid aortic valve (BAV) is the most frequent congenital heart disease in humans (prevalence: 2% of the general population)[55]. Congenital abnormalities of the aortic valve, of which > 90% are represented by BAV, are at the base of > 50% of so-called “calcific” severe AS in adults with an indication for AVR[12]. BAV is probably a disease of the entire aortic root characterized by fragmentation of elastin fibers, alteration of the media and increased collagen deposition in the ascending aorta[56]. These alterations are frequently seen in patients with ascending aortic dilatation and increased risk for aortic dissection.
BAV is characterized by fusion of one of the aortic commissures, which results in two functional aortic cusps of different dimensions. The terminology used to classify BAV may be confusing. Depending on the commissure that is fused, the orientation of the abnormal orifice can be anterior-posterior (by fusion of the right with the left coronary cusps - encountered in 56% of cases) or right-left (by fusion of the non-coronary with the right coronary cusp - encountered in 44% of cases). Less than 2% of cases are characterized by fusion of the non-coronary with the left coronary cusp. Thus, the morphology of the BAV can be described by the orientation of the opening orifice (anterior-posterior, AP/right-left, RL)[57] or by the cusps that fuse (right - left coronary, RL/right coronary - non-coronary, RN)[58].
Recently, in a study that used 4-dimension flow magnetic resonance imaging, Mahadevia et al[58] suggested that the type of BAV determines the pattern of dilatation of the ascending aorta through the direction of the systolic transaortic jet and subsequent differential pressures on the various regions of the ascending aortic walls. Thus, BAV type AP/RL is associated with an excentric systolic jet and increased parietal pressures on the anterior and right ascending aortic wall and is frequently (87%) associated with dilatation of the root or the entire ascending thoracic aorta. Conversely, BAV type RL/RN determines increased parietal pressures on the right and posterior ascending aortic wall and is rarely associated with aortic dilatation. The role of hemodynamic vs genetic factors in stratifying the risk associated with BAV and aortic root disease is unclear[59].
The evaluation of AR severity follows the same principles as that for the other valvular heart disease and is primarily based on echocardiography. For AR, the following goals are to be achieved by the echocardiographic evaluation: (1) identification of patients with severe AR; (2) identification of patients with an indication for AVR (surgical); and (3) identification of patients with dilated ascending aorta (with or without aortic bicuspid valves).
Identifying patients with severe AR: The most important echocardiographic criteria for identification of severe AR are listed in Table 4. For AR patients, the impact of different flow states (normal vs low) has not been investigated and is not applicable for routine clinical practice. Studies that validated the echocardiographic criteria for AR severity have used angiography as the comparator[60,61]. Only one study has prospectively evaluated the role of echocardiographic AR severity criteria in relation to the long-term prognosis of patients with severe, asym–ptomatic AR[62].
| Mild AR | Moderate AR | Severe AR | |
| Ratio between the AR jet diameter and the LVOT diameter | < 25% | 25%-64% | ≥ 65% |
| Vena contracta (mm) | < 3 | 3-5.9 | ≥ 6 |
| Regurgitant volume (mL/beat) | < 30 | 30-59 | ≥ 60 |
| Regurgitant fraction | < 30% | 30%-49% | ≥ 50% |
| EROA (cm2) | < 0.1 | 0.1-0.29 | ≥ 0.3 |
| Diastolic backflow in the descending thoracic and/or abdominal aorta | Minimal | Less than holodiastolic | Holodiastolic (especially for backflow documented in the abdominal aorta) |
| Angiographic | 1+ | 2+ | 3-4+ |
| LV dilatation | No | No | Yes (mandatory for chronic severe AR) |
Identifying patients who are candidates for AVR: SAVR remains the gold-standard treatment for AR. In a few experienced centers, surgical aortic valve reconstruction may be an alternative for patients with favorable anatomy (e.g., dilated aortic root, prolapsed aortic cusp)[63,64]. TAVR has a very limited role in treating AR and has only been used anecdotally in these patients[65].
Unlike AS, the current recommendations for AR evaluation and management are based on far fewer data. Additionally, most of the data on the prognosis of AR come from studies published more than 2 decades ago, which used outdated evaluation techniques.
Severe acute AR is a surgical emergency. The current indications for surgery for chronic severe AR are summarized in Table 5[33,34]. Regarding patients with dilatation of the ascending aorta, there are considerable differences between different guidelines, and a summary is provided in Table 6[33,34,66]. This summary does not cover patients with connective tissue disorders (e.g., Marfan syndrome). In these patients, a recent AHA/ACC statement tried to clarify the differences between the 2 guidelines published in 2014 by the AHA/ACC (the “2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease”), and in 2010 by other collaborating societies (the “2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the Diagnosis and Management of Patients With Thoracic Aortic Disease”)[67].
| Criteria | Class of indication | Differences between guidelines | |
| ESC/EACTS | AHA/ACC | ||
| Symptomatic severe AR (any LVEF) | I | I | |
| Asymptomatic severe AR with depressed LV function (LVEF < 50%) | I | I | |
| Severe AR in patients with another indication for cardiac surgery (e.g., CABG, thoracic aorta, another valve) | I | I | |
| Asymptomatic severe AR with normal LVEF (> 50%) but with severe LV dilatation | IIa | IIa | Definition of severe LV dilatation: ESC/EACTS guideline: End-diastolic LV diameter > 70 mm, or end-systolic LV diameter > 50 mm (or > 25 mm/m2); AHA/ACC guidelines: End-systolic LV diameter > 50 mm |
| Moderate AR in patients with another indication for cardiac surgery (e.g., coronary bypass, thoracic aorta, another valve) | - | IIa | This indication is not covered in the ESC/EACTS guidelines |
| Severe AR with normal LVEF (> 50%) but with progressive LV dilatation (end-diastolic LV diameter > 65 mm) if the surgical risk is low | - | IIb | This indication is not covered in the ESC/EACTS guidelines |
| Class of indication | Guideline | Differences between guidelines | |
| ESC/ EACTS 2012 | AHA/ACC 2016 Consensus on AHA/ACC 2014, and ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM 2010 Guidelines | ||
| I | - | Asymptomatic bicuspid aortic valve with dilatation of Valsalva sinuses or the ascending thoracic aortic diameter > 55 mm | No class I indications in the 2012 ESC/EACTS guidelines |
| IIa | Bicuspid aortic valve with an ascending thoracic aortic diameter > 50 mm if the patient also has at least one of the followings: Family history of aortic dissection; documented increase in the aortic diameter > 2 mm/yr (assessed using the same imaging method, at the same level, and with comparative images available); arterial hypertension; coarctation of the aorta | Bicuspid aortic valve AND dilatation of the Valsalva sinuses or of the ascending thoracic aorta (> 50 mm) AND at least one of the following | |
| Family history of aortic dissection | |||
| Documented increase in aortic diameter > 5 mm/yr | |||
| OR low surgical risk in an expert center | |||
| - | Replacement of the ascending aorta if the patient also has an indication for surgery for AS/AR, and the ascending aortic/Valsalva sinus diameter is > 45 mm | Not covered by the 2012 ESC guidelines | |
The indications for surgery in AR are based on only a few small-medium sized prospective studies all of which were observational and published before 2000. Bonow et al[68] evaluated the long-term prognosis (mean follow-up, 8 years) of 104 patients with severe AR and preserved LVEF recruited between 1973 and 1988. In these patients, the independent prognostic factors were age, the initial end-systolic LV diameter, and modification in time of the LVEF. In this study, patients with an end-systolic LV diameter > 50 mm and an end-diastolic LV diameter > 70 mm had a > 10%/year risk for death, development of symptoms or development of LV systolic dysfunction. The LV diameters were measured using simple, M-mode echocardiography, which has major limitations and is completely outdated today.
A second prospective observational study evaluated the prognosis of 104 patients with severe AR recruited beginning in 1979 and followed-up for an average of 7.3 years[69]. In this study, the most powerful prognostic factor was the rate of decline of LVEF (normalized to wall stress). Tornos et al[70] evaluated 101 patients with asymptomatic severe AR and normal LVEF, and followed-up these patients for up to 10 years. The rate of AVR was 12% at 5 years and 24% at 10 years. The independent prognostic factors required for AVR were an end-systolic LV diameter > 50 mm and an LVEF < 60% (determined by radionuclide cardiac scan); patients who needed AVR more frequently had progressive LV systolic dysfunction. Dujardin et al[71] evaluated 246 patients with severe AR included between 1985 and 1994, and followed-up these patients for an average of 10 years. The incidence of MACE during this period was very high (83%) as follows: 34% deceased; 47% developed heart failure and 62% received AVR[71]. In this study, the following were the independent predictors of survival: Age, New York Heart Association functional class, presence of co-morbidities, presence of atrial fibrillation, end-systolic LV indexed diameter > 25 mm/m2, and the LVEF. In a retrospective cohort study of 166 patients with asymptomatic severe AR and severe systolic dysfunction (LVEF < 35%), Kamath et al[72] showed that those who underwent surgery had much better prognosis compared with that of patients treated medically (HR = 0.59, 95%CI: 0.42-0.98, P = 0.04).
Importantly, AR severity in these studies was determined by angiographic and not echocardiographic criteria. Only one study evaluated the utility of echocardiographic indices in AR severity by identifying patients who will need surgery. Detaint et al[62] evaluated 251 patients with asymptomatic severe AR with preserved LVEF (> 50%) recruited between 1991 and 2003 (a relatively contemporary population in comparison with previous studies). The independent prognostic factors required for AVR were severe AR as determined by quantitative echocardiographic indices and an end-systolic LV indexed volume > 45 mL/m2 (as measured by the Simpson bi-plane method). Patients with severe AR and an end-systolic LV indexed volume > 45 mL/m2 had an 87% risk of MACE at 10 years compared with only 40% of patients with severe AR and an end-systolic LV volume < 45 mL/m2. This study also showed that quantitative echocardiographic indices of AR severity had superior prognostic value compared with that of qualitative echocardiographic indices[62].
These studies also suggested that patients with severe AR may have severe prognosis even before the appearance of symptoms or LV dysfunction. The mortality of patients with asymptomatic severe AR with preserved LVEF may reach 35% at 10 years[62,71]. However, there is insufficient prognostic data that can be used to identify patients at risk. The role of stress echocardiography in stratifying the risk of patients with severe AR has been much less studied compared with for patients with AS, but it may be used to evaluate the presence of contractile reserve[73].
The role of myocardial deformation imaging in the selection of patients who may need AVR is also under investigation. A study of 64 patients with moderate or severe AR (regardless of symptoms and LVEF) showed that patients for whom AVR was eventually performed (n = 29) had lower values of LV strain, LVEF and higher LV volumes compared with patients who did not need surgery. However, the reported cut-off values for identifying patients who will need surgery had sensitivities and specificities that make them poorly applicable in clinical practice (area under the curve < 0.77)[74].
B-type natriuretic peptide (BNP) levels may also play a role in predicting outcomes in patients with severe AR. Pizarro et al[75] studied 294 patients with severe asymptomatic AR and LVEF > 55%, and found that a BNP level > 130 pg/mL had 77% sensitivity and 94% specificity for predicting LV dysfunction symptoms or death after 38 ± 9 mo of follow-up. BNP level had additive prognostic value to echocardiographic prognostic indices[75]. Further studies are needed to establish the role of BNP levels for indication of surgery in patients with AR.
A recent study of 159 patients with moderate or severe AR without a formal indication for surgery according to current guidelines (LVEF > 50%, end-diastolic LV diameter ≤ 70 mm, end-systolic LV diameter ≤ 70 mm or ≤ 25 mm/m2) showed that 31% of these patients needed AVR within 30 ± 21 mo of follow-up. The independent prognostic factors for early surgery were as follows: Global longitudinal LV strain, right ventricular longitudinal strain, and tricuspid annular peak systolic excursion (TAPSE); the combination of these 3 factors had a higher discriminating power compared with each one taken individually (χ2 = 64.4, P < 0.001)[76]. However, the individual variability of these indices was high, and their utility for clinical practice must be validated in prospective clinical studies. This study confirmed that patients with significant AR without an initial formal indication but who eventually needed AVR, developed progressive LV dilatation and LVEF decline during follow-up, despite similar degrees of LV dilatation and LVEF at baseline, when compared to patients who did not need AVR during follow-up[76].
Cardiac magnetic resonance imaging (CMRI) is highly accurate in quantifying cardiac chamber volumes, aortic regurgitant volume and EROA. CMRI is recommended for patients with suboptimal echocardiography for whom the exact determination of AR severity is important and has therapeutic consequences (class IIa indication, according to ACC/AHA guidelines)[34].
A recent study of 113 patients with moderate and severe AR (as determined by echocardiography) followed-up for up to 9 years suggested that a regurgitant fraction > 33% as determined by CMRI had a high positive predictive value (93%) in identifying patients who will need AVR. Additionally, an end-diastolic LV volume > 246 mL was also useful in identifying these patients (positive predictive value for AVR, 88%)[77]. However, contrary to previous data, in this study, the CMRI-measured LVEF was not useful in identifying patients with asymptomatic severe AR who needed AVR. More studies are needed to establish the exact role of all these parameters in selecting patients with asymptomatic severe AR who will need AVR.
AS and AR represent important health problems world-wide; when severe, they carry poor prognoses. For AS, both SAVR and TAVR may provide definite treatment in carefully selected patients. For AR, valve surgery (either SAVR or - in selected cases - aortic valve repair) remains the gold standard of care. To properly identify those patients who are candidates for surgery, the clinician has to carefully assess the severity of valve disease with an understanding of the potential pitfalls involved in these assessments. Thus, evaluation of aortic valve disease requires “a global view and a global understanding”[4].
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: Romania
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Barili F, Falconi M, Kirali K S- Editor: Kong JX L- Editor: A E- Editor: Li D
| 1. | Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3079] [Cited by in RCA: 3332] [Article Influence: 175.4] [Reference Citation Analysis (0)] |
| 2. | Osnabrugge RL, Mylotte D, Head SJ, Van Mieghem NM, Nkomo VT, LeReun CM, Bogers AJ, Piazza N, Kappetein AP. Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta-analysis and modeling study. J Am Coll Cardiol. 2013;62:1002-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 652] [Cited by in RCA: 944] [Article Influence: 78.7] [Reference Citation Analysis (0)] |
| 3. | Bonow RO, Leon MB, Doshi D, Moat N. Management strategies and future challenges for aortic valve disease. Lancet. 2016;387:1312-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 4. | Pibarot P, Dumesnil JG. Aortic stenosis: look globally, think globally. JACC Cardiovasc Imaging. 2009;2:400-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Lindman BR, Bonow RO, Otto CM. Current management of calcific aortic stenosis. Circ Res. 2013;113:223-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 124] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 6. | Magne J, Lancellotti P, Piérard LA. Exercise testing in asymptomatic severe aortic stenosis. JACC Cardiovasc Imaging. 2014;7:188-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Zamorano JL, Gonçalves A, Lang R. Imaging to select and guide transcatheter aortic valve implantation. Eur Heart J. 2014;35:1578-1587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Bax JJ, Delgado V, Bapat V, Baumgartner H, Collet JP, Erbel R, Hamm C, Kappetein AP, Leipsic J, Leon MB. Open issues in transcatheter aortic valve implantation. Part 1: patient selection and treatment strategy for transcatheter aortic valve implantation. Eur Heart J. 2014;35:2627-2638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 9. | Clavel MA, Magne J, Pibarot P. Low-gradient aortic stenosis. Eur Heart J. 2016;37:2645-2657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 244] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 10. | Généreux P, Stone GW, O’Gara PT, Marquis-Gravel G, Redfors B, Giustino G, Pibarot P, Bax JJ, Bonow RO, Leon MB. Natural History, Diagnostic Approaches, and Therapeutic Strategies for Patients With Asymptomatic Severe Aortic Stenosis. J Am Coll Cardiol. 2016;67:2263-2288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 198] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 11. | Pierard LA, Dulgheru R. Evaluation of aortic stenosis: an update--including low-flow States, myocardial mechanics, and stress testing. Curr Cardiol Rep. 2015;17:42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Ross J, Braunwald E. Aortic stenosis. Circulation. 1968;38:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 480] [Cited by in RCA: 552] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 13. | Roberts WC, Ko JM. Frequency by decades of unicuspid, bicuspid, and tricuspid aortic valves in adults having isolated aortic valve replacement for aortic stenosis, with or without associated aortic regurgitation. Circulation. 2005;111:920-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 656] [Cited by in RCA: 654] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 14. | Morrow AG, Roberts WC, Ross J, Fisher RD, Behrendt DM, Mason DT, Braunwald E. Obstruction to left ventricular outflow. Current concepts of management and operative treatment. Ann Intern Med. 1968;69:1255-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Coffey S, Cox B, Williams MJ. The prevalence, incidence, progression, and risks of aortic valve sclerosis: a systematic review and meta-analysis. J Am Coll Cardiol. 2014;63:2852-2861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 182] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 16. | Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597-1607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5086] [Cited by in RCA: 5503] [Article Influence: 366.9] [Reference Citation Analysis (1)] |
| 17. | Omran H, Schmidt H, Hackenbroch M, Illien S, Bernhardt P, von der Recke G, Fimmers R, Flacke S, Layer G, Pohl C. Silent and apparent cerebral embolism after retrograde catheterisation of the aortic valve in valvular stenosis: a prospective, randomised study. Lancet. 2003;361:1241-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 285] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 18. | Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, Iung B, Otto CM, Pellikka PA, Quiñones M. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. Eur J Echocardiogr. 2009;10:1-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 741] [Cited by in RCA: 731] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 19. | Leye M, Brochet E, Lepage L, Cueff C, Boutron I, Detaint D, Hyafil F, Iung B, Vahanian A, Messika-Zeitoun D. Size-adjusted left ventricular outflow tract diameter reference values: a safeguard for the evaluation of the severity of aortic stenosis. J Am Soc Echocardiogr. 2009;22:445-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Minners J, Allgeier M, Gohlke-Baerwolf C, Kienzle RP, Neumann FJ, Jander N. Inconsistencies of echocardiographic criteria for the grading of aortic valve stenosis. Eur Heart J. 2008;29:1043-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 290] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 21. | Grayburn PA. Assessment of low-gradient aortic stenosis with dobutamine. Circulation. 2006;113:604-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Awtry E, Davidoff R. Low-flow/low-gradient aortic stenosis. Circulation. 2011;124:e739-e741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Carabello BA, Green LH, Grossman W, Cohn LH, Koster JK, Collins JJ. Hemodynamic determinants of prognosis of aortic valve replacement in critical aortic stenosis and advanced congestive heart failure. Circulation. 1980;62:42-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 252] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 24. | Pibarot P, Dumesnil JG. Low-flow, low-gradient aortic stenosis with normal and depressed left ventricular ejection fraction. J Am Coll Cardiol. 2012;60:1845-1853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 323] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 25. | Tribouilloy C, Lévy F, Rusinaru D, Guéret P, Petit-Eisenmann H, Baleynaud S, Jobic Y, Adams C, Lelong B, Pasquet A. Outcome after aortic valve replacement for low-flow/low-gradient aortic stenosis without contractile reserve on dobutamine stress echocardiography. J Am Coll Cardiol. 2009;53:1865-1873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 261] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 26. | Rosenhek R, Binder T, Porenta G, Lang I, Christ G, Schemper M, Maurer G, Baumgartner H. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med. 2000;343:611-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 958] [Cited by in RCA: 908] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 27. | Cueff C, Serfaty JM, Cimadevilla C, Laissy JP, Himbert D, Tubach F, Duval X, Iung B, Enriquez-Sarano M, Vahanian A. Measurement of aortic valve calcification using multislice computed tomography: correlation with haemodynamic severity of aortic stenosis and clinical implication for patients with low ejection fraction. Heart. 2011;97:721-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 283] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 28. | Clavel MA, Messika-Zeitoun D, Pibarot P, Aggarwal SR, Malouf J, Araoz PA, Michelena HI, Cueff C, Larose E, Capoulade R. The complex nature of discordant severe calcified aortic valve disease grading: new insights from combined Doppler echocardiographic and computed tomographic study. J Am Coll Cardiol. 2013;62:2329-2338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 416] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 29. | Hachicha Z, Dumesnil JG, Bogaty P, Pibarot P. Paradoxical low-flow, low-gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation. 2007;115:2856-2864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 698] [Cited by in RCA: 712] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 30. | Dulgheru R, Magne J, Capoulade R, Davin L, Vinereanu D, Pierard LA, Pibarot P, Lancellotti P. Impact of global hemodynamic load on exercise capacity in aortic stenosis. Int J Cardiol. 2013;168:2272-2277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Lancellotti P, Magne J, Donal E, Davin L, O’Connor K, Rosca M, Szymanski C, Cosyns B, Piérard LA. Clinical outcome in asymptomatic severe aortic stenosis: insights from the new proposed aortic stenosis grading classification. J Am Coll Cardiol. 2012;59:235-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 294] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 32. | Jander N, Minners J, Holme I, Gerdts E, Boman K, Brudi P, Chambers JB, Egstrup K, Kesäniemi YA, Malbecq W. Outcome of patients with low-gradient “severe” aortic stenosis and preserved ejection fraction. Circulation. 2011;123:887-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 244] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 33. | Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Barón-Esquivias G, Baumgartner H, Borger MA, Carrel TP, De Bonis M, Evangelista A. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J. 2012;33:2451-2496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2682] [Cited by in RCA: 2649] [Article Influence: 203.8] [Reference Citation Analysis (0)] |
| 34. | Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Guyton RA, O’Gara PT, Ruiz CE, Skubas NJ, Sorajja P. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2438-2488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1367] [Cited by in RCA: 1379] [Article Influence: 125.4] [Reference Citation Analysis (1)] |
| 35. | Lancellotti P, Lebois F, Simon M, Tombeux C, Chauvel C, Pierard LA. Prognostic importance of quantitative exercise Doppler echocardiography in asymptomatic valvular aortic stenosis. Circulation. 2005;112:I377-I382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 168] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 36. | Schwarz F, Baumann P, Manthey J, Hoffmann M, Schuler G, Mehmel HC, Schmitz W, Kübler W. The effect of aortic valve replacement on survival. Circulation. 1982;66:1105-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 379] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 37. | Amato MC, Moffa PJ, Werner KE, Ramires JA. Treatment decision in asymptomatic aortic valve stenosis: role of exercise testing. Heart. 2001;86:381-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 200] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 38. | Carabello BA. Should severe aortic stenosis be operated on before symptom onset? Aortic valve replacement should be operated on before symptom onset. Circulation. 2012;126:112-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 39. | Shah PK. Should severe aortic stenosis be operated on before symptom onset? Severe aortic stenosis should not be operated on before symptom onset. Circulation. 2012;126:118-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 40. | Taniguchi T, Morimoto T, Shiomi H, Ando K, Kanamori N, Murata K, Kitai T, Kawase Y, Izumi C, Miyake M. Initial Surgical Versus Conservative Strategies in Patients With Asymptomatic Severe Aortic Stenosis. J Am Coll Cardiol. 2015;66:2827-2838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 249] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 41. | Rosenhek R, Zilberszac R, Schemper M, Czerny M, Mundigler G, Graf S, Bergler-Klein J, Grimm M, Gabriel H, Maurer G. Natural history of very severe aortic stenosis. Circulation. 2010;121:151-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 339] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 42. | Kang DH, Park SJ, Rim JH, Yun SC, Kim DH, Song JM, Choo SJ, Park SW, Song JK, Lee JW. Early surgery versus conventional treatment in asymptomatic very severe aortic stenosis. Circulation. 2010;121:1502-1509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 211] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 43. | Kalavrouziotis D, Li D, Buth KJ, Légaré JF. The European System for Cardiac Operative Risk Evaluation (EuroSCORE) is not appropriate for withholding surgery in high-risk patients with aortic stenosis: a retrospective cohort study. J Cardiothorac Surg. 2009;4:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 44. | Maréchaux S, Hachicha Z, Bellouin A, Dumesnil JG, Meimoun P, Pasquet A, Bergeron S, Arsenault M, Le Tourneau T, Ennezat PV. Usefulness of exercise-stress echocardiography for risk stratification of true asymptomatic patients with aortic valve stenosis. Eur Heart J. 2010;31:1390-1397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 193] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 45. | Lancellotti P, Magne J, Donal E, O’Connor K, Dulgheru R, Rosca M, Pierard LA. Determinants and prognostic significance of exercise pulmonary hypertension in asymptomatic severe aortic stenosis. Circulation. 2012;126:851-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 153] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 46. | Bonow RO. Exercise hemodynamics and risk assessment in asymptomatic aortic stenosis. Circulation. 2012;126:803-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 47. | Cimadevilla C, Cueff C, Hekimian G, Dehoux M, Lepage L, Iung B, Duval X, Huart V, Tubach F, Vahanian A. Prognostic value of B-type natriuretic peptide in elderly patients with aortic valve stenosis: the COFRASA-GENERAC study. Heart. 2013;99:461-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 48. | Katz S, Downs TD, Cash HR, Grotz RC. Progress in development of the index of ADL. Gerontologist. 1970;10:20-30. [PubMed] |
| 49. | van Geldorp MW, Eric Jamieson WR, Kappetein AP, Ye J, Fradet GJ, Eijkemans MJ, Grunkemeier GL, Bogers AJ, Takkenberg JJ. Patient outcome after aortic valve replacement with a mechanical or biological prosthesis: weighing lifetime anticoagulant-related event risk against reoperation risk. J Thorac Cardiovasc Surg. 2009;137:881-886, 886e1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 50. | Rahimtoola SH. Choice of prosthetic heart valve in adults an update. J Am Coll Cardiol. 2010;55:2413-2426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 243] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 51. | Pibarot P, Dumesnil JG. Prosthetic heart valves: selection of the optimal prosthesis and long-term management. Circulation. 2009;119:1034-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 469] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 52. | Holmes DR, Mack MJ, Kaul S, Agnihotri A, Alexander KP, Bailey SR, Calhoon JH, Carabello BA, Desai MY, Edwards FH. 2012 ACCF/AATS/SCAI/STS expert consensus document on transcatheter aortic valve replacement. J Am Coll Cardiol. 2012;59:1200-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 566] [Cited by in RCA: 563] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 53. | Siontis GC, Praz F, Pilgrim T, Mavridis D, Verma S, Salanti G, Søndergaard L, Jüni P, Windecker S. Transcatheter aortic valve implantation vs. surgical aortic valve replacement for treatment of severe aortic stenosis: a meta-analysis of randomized trials. Eur Heart J. 2016;37:3503-3512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 227] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 54. | Hamirani YS, Dietl CA, Voyles W, Peralta M, Begay D, Raizada V. Acute aortic regurgitation. Circulation. 2012;126:1121-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 55. | Siu SC, Silversides CK. Bicuspid aortic valve disease. J Am Coll Cardiol. 2010;55:2789-2800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 620] [Cited by in RCA: 661] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
| 56. | Braverman AC, Güven H, Beardslee MA, Makan M, Kates AM, Moon MR. The bicuspid aortic valve. Curr Probl Cardiol. 2005;30:470-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 272] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 57. | Kang JW, Song HG, Yang DH, Baek S, Kim DH, Song JM, Kang DH, Lim TH, Song JK. Association between bicuspid aortic valve phenotype and patterns of valvular dysfunction and bicuspid aortopathy: comprehensive evaluation using MDCT and echocardiography. JACC Cardiovasc Imaging. 2013;6:150-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 175] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 58. | Mahadevia R, Barker AJ, Schnell S, Entezari P, Kansal P, Fedak PW, Malaisrie SC, McCarthy P, Collins J, Carr J. Bicuspid aortic cusp fusion morphology alters aortic three-dimensional outflow patterns, wall shear stress, and expression of aortopathy. Circulation. 2014;129:673-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 321] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 59. | Uretsky S, Gillam LD. Nature versus nurture in bicuspid aortic valve aortopathy: more evidence that altered hemodynamics may play a role. Circulation. 2014;129:622-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 60. | Borrás X, Carreras F, Augé JM, Pons-Lladó G. Prospective validation of detection and quantitative assessment of chronic aortic regurgitation by a combined echocardiographic and Doppler method. J Am Soc Echocardiogr. 1988;1:422-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 61. | Chen M, Luo H, Miyamoto T, Atar S, Kobal S, Rahban M, Brasch AV, Makkar R, Neuman Y, Naqvi TZ. Correlation of echo-Doppler aortic valve regurgitation index with angiographic aortic regurgitation severity. Am J Cardiol. 2003;92:634-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 62. | Detaint D, Messika-Zeitoun D, Maalouf J, Tribouilloy C, Mahoney DW, Tajik AJ, Enriquez-Sarano M. Quantitative echocardiographic determinants of clinical outcome in asymptomatic patients with aortic regurgitation: a prospective study. JACC Cardiovasc Imaging. 2008;1:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 118] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 63. | David TE, Feindel CM, David CM, Manlhiot C. A quarter of a century of experience with aortic valve-sparing operations. J Thorac Cardiovasc Surg. 2014;148:872-879; discussion 879-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 64. | Bisleri G. Aortic valve repair. Curr Opin Cardiol. 2016;31:581-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 65. | Cerillo AG, Griese D, Berti S. Successful percutaneous implantation of symetis ACURATE neo transcatheter aortic bioprosthesis for the treatment of pure aortic regurgitation. Catheter Cardiovasc Interv. 2016;88:319-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 66. | Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE, Eagle KA, Hermann LK, Isselbacher EM, Kazerooni EA. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010;121:e266-e369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 952] [Cited by in RCA: 1210] [Article Influence: 80.7] [Reference Citation Analysis (1)] |
| 67. | Hiratzka LF, Creager MA, Isselbacher EM, Svensson LG, Nishimura RA, Bonow RO, Guyton RA, Sundt TM. Surgery for Aortic Dilatation in Patients With Bicuspid Aortic Valves: A Statement of Clarification From the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2016;67:724-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 106] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 68. | Bonow RO, Lakatos E, Maron BJ, Epstein SE. Serial long-term assessment of the natural history of asymptomatic patients with chronic aortic regurgitation and normal left ventricular systolic function. Circulation. 1991;84:1625-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 313] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 69. | Borer JS, Hochreiter C, Herrold EM, Supino P, Aschermann M, Wencker D, Devereux RB, Roman MJ, Szulc M, Kligfield P. Prediction of indications for valve replacement among asymptomatic or minimally symptomatic patients with chronic aortic regurgitation and normal left ventricular performance. Circulation. 1998;97:525-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 151] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 70. | Tornos MP, Olona M, Permanyer-Miralda G, Herrejon MP, Camprecios M, Evangelista A, Garcia del Castillo H, Candell J, Soler-Soler J. Clinical outcome of severe asymptomatic chronic aortic regurgitation: a long-term prospective follow-up study. Am Heart J. 1995;130:333-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 106] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 71. | Dujardin KS, Enriquez-Sarano M, Schaff HV, Bailey KR, Seward JB, Tajik AJ. Mortality and morbidity of aortic regurgitation in clinical practice. A long-term follow-up study. Circulation. 1999;99:1851-1857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 304] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 72. | Kamath AR, Varadarajan P, Turk R, Sampat U, Patel R, Khandhar S, Pai RG. Survival in patients with severe aortic regurgitation and severe left ventricular dysfunction is improved by aortic valve replacement: results from a cohort of 166 patients with an ejection fraction & lt; or =35%. Circulation. 2009;120:S134-S138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 73. | Lancellotti P, Magne J. Stress echocardiography in regurgitant valve disease. Circ Cardiovasc Imaging. 2013;6:840-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 74. | Olsen NT, Sogaard P, Larsson HB, Goetze JP, Jons C, Mogelvang R, Nielsen OW, Fritz-Hansen T. Speckle-tracking echocardiography for predicting outcome in chronic aortic regurgitation during conservative management and after surgery. JACC Cardiovasc Imaging. 2011;4:223-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 75. | Pizarro R, Bazzino OO, Oberti PF, Falconi ML, Arias AM, Krauss JG, Cagide AM. Prospective validation of the prognostic usefulness of B-type natriuretic peptide in asymptomatic patients with chronic severe aortic regurgitation. J Am Coll Cardiol. 2011;58:1705-1714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 76. | Kusunose K, Agarwal S, Marwick TH, Griffin BP, Popović ZB. Decision making in asymptomatic aortic regurgitation in the era of guidelines: incremental values of resting and exercise cardiac dysfunction. Circ Cardiovasc Imaging. 2014;7:352-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 77. | Myerson SG, d’Arcy J, Mohiaddin R, Greenwood JP, Karamitsos TD, Francis JM, Banning AP, Christiansen JP, Neubauer S. Aortic regurgitation quantification using cardiovascular magnetic resonance: association with clinical outcome. Circulation. 2012;126:1452-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 160] [Article Influence: 12.3] [Reference Citation Analysis (0)] |