Published online May 26, 2017. doi: 10.4330/wjc.v9.i5.448
Peer-review started: November 25, 2016
First decision: January 16, 2017
Revised: March 3, 2017
Accepted: April 6, 2017
Article in press: April 10, 2017
Published online: May 26, 2017
Processing time: 177 Days and 6.2 Hours
To evaluate novel risk factors and biomarkers of cardiovascular disease in celiac disease (CD) patients compared with healthy controls.
Twenty adult patients with recent diagnosis of CD and 20 sex, age and body mass index-matched healthy controls were recruited during a period of 12 mo. Indicators of carbohydrate metabolism, hematological parameters and high sensitive C reactive protein were determined. Moreover, lipoprotein metabolism was also explored through evaluation of the lipid profile and the activity of cholesteryl ester transfer protein and lipoprotein associated phospholipase A2, which is also considered a specific marker of vascular inflammation. The protocol was approved by the Ethic Committee from School of Pharmacy and Biochemistry, University of Buenos Aires and from Buenos Aires Italian Hospital, Buenos Aires, Argentina.
Regarding the indicators of insulin resistance, CD patients showed higher plasma insulin levels [7.2 (5.0-11.3) mU/L vs 4.6 (2.6-6.7) mU/L, P < 0.05], increased Homeostasis Model Assessment-Insulin Resistance [1.45 (1.04-2.24) vs 1.00 (0.51-1.45), P < 0.05] and lower Quantitative Sensitive Check index [0.33 (0.28-0.40) vs 0.42 (0.34-0.65), P < 0.05] indexes. Folic acid concentration [5.4 (4.4-7.9) ng/mL vs 12.2 (8.0-14.2) ng/mL, P < 0.01] resulted to be lower and High-sensitivity C reactive protein levels higher (4.21 ± 6.47 mg/L vs 0.98 ± 1.13 mg/L, P < 0.01) in the patient group. With respect to the lipoprotein profile, CD patients showed lower high density lipoprotein-cholesterol (HDL-C) (45 ± 15 mg/dL vs 57 ± 17 mg/dL, P < 0.05) and apo A-I (130 ± 31 mg/dL vs 155 ± 29 mg/dL, P < 0.05) levels, as well as higher total cholesterol/HDL-C [4.19 (3.11-5.00) vs 3.52 (2.84-4.08), P < 0.05] and apo B/apo A-I (0.75 ± 0.25 vs 0.55 ± 0.16, P < 0.05) ratios in comparison with control subjects. No statistically significant differences were detected in lipoprotein-associated lipid transfer protein and enzymes.
The presence and interaction of the detected alterations in patients with CD, would constitute a risk factor for the development of atherosclerotic cardiovascular disease.
Core tip: Given that data about the presence of metabolic alterations and atherogenic risk factors in celiac disease are scarce and contradictory, we aimed to investigate carbohydrate metabolism, lipoprotein profile and inflammatory status in patients with celiac disease (CD). Patients presented higher insulin levels, Homeostasis Model Assessment-Insulin Resistance index, apo B/apo A-I ratio and High-sensitivity C reactive protein concentration, as well as lower Quantitative Sensitive Check index index, high density lipoprotein-cholesterol and apo A-I levels in comparison with sex and aged-matched healthy controls. Persistence of these alterations through long periods of time in a chronic pathologic condition, as it is the case with CD, would constitute a high risk of developing atherosclerotic cardiovascular disease.
- Citation: Tetzlaff WF, Meroño T, Menafra M, Martin M, Botta E, Matoso MD, Sorroche P, De Paula JA, Boero LE, Brites F. Markers of inflammation and cardiovascular disease in recently diagnosed celiac disease patients. World J Cardiol 2017; 9(5): 448-456
- URL: https://www.wjgnet.com/1949-8462/full/v9/i5/448.htm
- DOI: https://dx.doi.org/10.4330/wjc.v9.i5.448
Celiac disease (CD) is a multisystemic disease which mainly affects the digestive system, though not exclusively. Its main trait is chronic and diffuse inflammation of the mucosa of the small intestine and it can present a wide variety of clinical symptoms[1]. Thus far, the only available therapy for CD consists of the implementation of a gluten free diet (GFD), whose efficacy depends on strict adherence.
It is remarkable that most cases of CD lack typical gastrointestinal symptoms and are, instead, very frequently associated with presentations known as atypical or extra-intestinal. Thus, its diagnosis represents one of the main challenges for health professionals[2].
Commonly, CD has been associated with certain physiopathological conditions (type 1 diabetes, Hashimoto thyroiditis, etc.) which are not directly related to gluten ingestion[3]. Among these conditions, it is worth noting that the evidence linking CD and atherosclerotic cardiovascular disease (CVD) is scarce. It is well known that CD patients do not show classical CVD risk factors. In fact, hypertension and hypercholesterolemia are less frequent in CD patients than in the general population[4]. However, previous studies have failed to show lower CVD risk in CD patients than in healthy subjects[4,5]. Furthermore, an important study carried out in Sweden in approximately 14000 hospitalized CD patients showed higher risk of acute myocardial infarction, chest angina, cardiac insufficiency, brain hemorrhage and ischemic stroke when compared to sex and age-paired healthy controls[6].
These facts suggest that CD would be associated with novel atherogenic risk factors or even with other non-identified risk factors. In fact, inflammation and anemia, among other signs that characterize CD, could represent a link between this pathology and CVD[7,8].
Atherosclerosis is presently understood as a chronic inflammatory disease in which endothelial dysfunction and biomarkers of inflammation are present since the early stages of the pathology[9]. So far, the inflammatory process typical of CD has not been described in relation to increased risk of CVD.
The aim of the present study was to evaluate novel risk factors and biomarkers of CVD in CD patients in comparison to sex, age and body mass index (BMI)-matched healthy controls. In addition, the metabolic differences between patients with typical and atypical presentations of the disease were also analyzed.
Twenty patients with CD were consecutively recruited from the service of gastroenterology, Buenos Aires Italian Hospital, during a period of 12 mo. The inclusion criteria were adult age and recent diagnosis of CD (< 3 mo) based on histopathological findings and serological markers (anti-gliadin IgG and IgA and anti-transglutaminase IgA). Patients were not treated and they had not still started a GFD. All individuals presenting any other intestinal inflammatory disease, IgA deficiency, malignant diseases, chronic infections, pregnancy, thyroid, renal or hepatic alterations, history of CVD, smoking, alcohol consumption > 40 g/d, and treatment with drugs known to affect lipid and/or carbohydrate metabolism were excluded. Patients were classified according to the presence of gastrointestinal (typical presentation) or extra-digestive symptoms (atypical presentation)[2]. Gastrointestinal manifestations analyzed were: Diarrhea, abdominal distention, weight loss, and malabsorption syndrome. Extra-digestive alterations considered were: Anemia, mouth ulcers, osteoporosis, and modifications of liver function tests. Employing these criteria, 11 out of the 20 CD patients showed gastrointestinal symptomatology and 9 showed only extradigestive symptoms. The group of CD patients was compared with a sex, age and BMI-matched group of healthy volunteers (n = 20). Weight, height and waist circumference were registered in all subjects and an exhaustive anamnesis was performed. All participants in the study signed an informed consent. The protocol was approved by the Ethical Committees from School of Pharmacy and Biochemistry, University of Buenos Aires and from Buenos Aires Italian Hospital. Buenos Aires, Argentina.
Blood samples were obtained from the antecubital vein after 12 h of fasting. Serum and EDTA plasma (final EDTA concentration 1 mg/mL) were prepared from venous blood collected into sterile, evacuated tubes. The former was centrifuged at 1500 g for 15 min at 4 °C. Serum was isolated and stored at 4 °C and -70 °C.
Plasma concentrations of glucose, urea, uric acid, total bilirubin, folic acid and vitamin B12, as well as aspartate aminotransferase (ASAT), alanin aminotransferase and alkaline phosphatase activities, and hemogram were determined by standardized methods. Insulin levels were measured by radioimmunoassay (Diagnostics Products Corp., Los Angeles CA, United States). Homeostasis Model Assessment-Insulin Resistance (HOMA-IR) was calculated using the formula [glucose (mmol/L) insulin (uU/mL)]/22.5 and Quantitative Sensitive Check index (QUICKI) using the formula 1/[ln Glucose (mmol/L) + ln Insulin (mU/L)][10,11]. High-sensitivity C reactive protein (hsCRP) levels were determined by immunoturbidimetry (Roche, Mannheim, Germany) in a Hitachi 917 autoanalyzer (Tokyo, Japan).
Plasma levels of total cholesterol (TC) and triglycerides were quantified by standardized methods (Roche, Manheim, Germany) in a Hitachi 917 autoanalyzer (Tokyo, Japan). High density lipoproteins (HDL) were isolated from the supernatant obtained after selective precipitation of apolipoprotein (apo) B-containing lipoproteins using 0.44 mmol/L phosphotungstic acid in presence of magnesium ions[12]. Cholesterol (C) of low density lipoprotein (LDL) was estimated as the difference between TC and the cholesterol contained in the supernatant obtained after selective precipitation of LDL with 10 g/L polivinil sulfate in poliethileneglicol (600 Da; 2.5 w/v; pH = 6.7)[13]. Non HDL-C was calculated as the difference between TC and HDL-C. Very low density lipoprotein cholesterol (VLDL-C) was calculated as the difference between the supernatants of the LDL-C and HDL-C precipitations. Apo B and apo A-I levels were quantified by immunoturbidimetry (Roche, Manheim, Germany) in a Hitachi 917 autoanalyzer (Tokyo, Japan). Results were expressed as mg/dL. The following ratios were calculated: TG/HDL-C, TC/HDL-C and apo B/apo A-I.
Cholesteryl ester transfer protein (CETP) activity was evaluated in serum samples following the radiometric method previously described with minor modifications[14]. Briefly, the capacity of the serum to promote the transfer of tritiated esterified cholesterol (EC) from the biosynthetically marked HDL3 subfraction (3H-EC-HDL3) (NEN Life science products, Boston, United States) to apo B containing lipoproteins present in the serum. Serum samples were incubated with 3H-CE-HDL3 (50 µmol/L cholesterol) with iodoacetate (1.5 mmol/L) in TBS buffer (pH = 7.4) during 3 h at 37 °C. After incubation, apo B-containing lipoproteins were separated from HDL by selective precipitation with phosphotungstic acid (0.44 mmol/L) in the presence of magnesium ions. Radioactivity was measured in the reaction cocktail and in the supernatant containing the HDL subfraction in a liquid scintillation counter (Packard 210 TR, Packard Instruments, Meridians, CT, United States). Results were expressed as the percentage of tritiated EC transferred from HDL3 to apo B-containing lipoproteins, per ml, per hour. All samples were processed in the same assay.
Lipoprotein associated phospholipase A2 activity (Lp-PLA2) was evaluated employing the radiometric assay described by Blank et al[15] with minor modifications. The extraction of the marked acetate was performed using chloroform and the radioactivity of the aqueous phase was measured in a liquid scintillation counter (Packard 210 TR, Packard Instruments, Meridians, CT, United States). The radioactivity of the reaction buffer was also measured. Results were expressed as µmol of acetate liberated, per millilitre, per hour. All samples were processed in the same assay.
The sample size was calculated based on previous studies carried out in our laboratory. The outcome variables chosen to perform the sample size calculation for this study were HDL-C, CETP and Lp-PLA2. Having defined a 0.8 power, an effect size of 1.0 and a significance level of 0.05, the number of patients to be included in the present study was at least 17. Data distribution was analyzed with the Shapiro-Wilks test and data was expressed as mean ± SD, if distribution was found to be parametric, or as median (interquartile range) if distribution was non-parametric. To assess differences between groups, both parametric and non-parametric methods were employed. Correlation analyses were performed using Spearman or Pearson tests depending on variable distribution. When partial correlations, linear regressions or adjusted group differences were performed, all non-parametric variables were normalized prior to be included in the analysis. Statistical significance was defined as P < 0.05. A statistical review of the study was performed by a biomedical statistician. For the statistical analysis, the programs Infostat (Universidad Nacional de Cordoba, Argentina) and SPSS 19.0 (IBM, Chicago, United States) were used.
As expected, CD patients and control subjects did not show any difference in age, sex distribution and BMI (Table 1). Nevertheless, CD patients had significantly higher insulin levels and HOMA-IR, as well as lower QUICKI (Table 1). Both urea and creatinine concentrations were lower in the patient group, though individual results were comprised within the reference values. Additionally, ASAT activity was significantly increased in patients compared to controls.
| Patients with celiac disease (n = 20) | Control subjects (n = 20) | P | |
| Age (yr) | 50 (25-58) | 47 (28-60) | ns |
| Men/woman | 5/15 | 5/15 | ns |
| BMI (kg/m2) | 22.8 (20.4-26.2) | 23.0 (21.0-24.7) | ns |
| Glucose (mg/dL) | 87 ± 11 | 86 ± 12 | ns |
| Insulin (mU/L) | 7.2 (5.0-11.3) | 4.6 (2.6-6.7) | < 0.05 |
| HOMA-IR | 1.45 (1.04-2.24) | 1.00 (0.51-1.45) | < 0.05 |
| QUICKI | 0.33 (0.28-0.40) | 0.42 (0.34-0.65) | < 0.05 |
| Urea (mg/dL) | 27 (21-34) | 35 (34-39) | < 0.01 |
| Creatinine (mg/dL) | 0.74 (0.63-0.88) | 0.80 (0.75-1.10) | < 0.05 |
| Uric acid (mg/dL) | 5.0 ± 1.2 | 4.3 ± 1.6 | ns |
| Bilirubin (mg/dL) | 0.7 (0.5-0.8) | 0.6 (0.6-0.8) | ns |
| ASAT (U/L) | 26 (20-35) | 14 (12-20) | < 0.01 |
| ALAT (U/L) | 22 (17-39) | 18 (16-22) | ns |
| ALP (U/L) | 80 (59-102) | 124 (67-219) | ns |
The evaluation of hematological parameters showed no significant decrease in hemoglobin concentration in patients. Furthermore, only one woman met the criteria for anemia diagnosis (hemoglobin < 12 g/dL for women and < 13 g/dL for men). Similarly, there were no differences in total iron content, transferrin saturation or concentrations of ferritin, transferrin and vitamin B12. Only folic acid concentration was found to be significantly lower in patients (Table 2). Employing the reference values established by the World Health Organization[16,17], the prevalence of folic acid deficiency resulted to be 10% (< 4 ng/dL), of iron deficiency 15% (ferritin < 15 ng/dL) and of low vitamin B12 7.5% (< 203 pg/mL).
| Patients with celiac disease (n = 20) | Control subjects (n = 20) | P | |
| Erythrocytes (106/mL) | 4.40 ± 0.48 | 4.58 ± 0.27 | ns |
| Hematocrite (%) | 38.5 ± 4.0 | 40.1 ± 2.6 | ns |
| Hemoglobin (g/dL) | 13.0 ± 1.4 | 13.3 ± 0.9 | ns |
| Serum iron (µg/dL) | 73 ± 35 | 105 ± 61 | ns |
| Ferritin (ng/mL) | 33 (13-110) | 92 (43-117) | ns |
| Transferrin (mg/dL) | 261 ± 62 | 293 ± 59 | ns |
| Transferrin Sat. (%) | 25 ± 14 | 29 ± 14 | ns |
| Vitamin B12 (pg/mL) | 337 (251-482) | 315 (265-393) | ns |
| Folic acid (ng/mL) | 5.4 (4.4-7.9) | 12.2 (8.0-14.2) | < 0.01 |
Regarding the lipid and lipoprotein profile, no differences were detected in TG, TC, LDL-C and apo B levels. However, statistically significant decreases in HDL-C and apo A-I concentrations were observed (Table 3). Furthermore, both parameters showed a strong positive correlation between them (r = 0.78; P < 0.0001). TC/HDL-C and apo B/apo A-I ratios, both of which possess high predictive value for CVD, were significantly higher in patients, whilst TG/HDL-C showed no difference between groups. On the other hand, CETP activity was similar in patients and controls (145% ± 32%/mL.h vs 132% ± 33%/mL.h, P > 0.05) and exhibited direct correlations with TG levels (r = 0.52; P < 0.005) and apo B/apo A-I ratio (r = 0.48; P < 0.005), and negative ones with HDL-C (r = -0.58; P < 0.0001) and apo A-I (r = -0.40; P < 0.005).
| Patients with celiac disease (n = 20) | Control subjects (n = 20) | P | |
| TG (mg/dL) | 81 (65-119) | 78 (60-114) | ns |
| TC (mg/dL) | 185 ± 37 | 194 ± 39 | ns |
| VLDL-C (mg/dL) | 18 ± 8 | 17 ± 7 | ns |
| LDL-C (mg/dL) | 139 (89-149) | 107 (95-147) | ns |
| HDL-C (mg/dL) | 45 ±15 | 57 ±17 | < 0.05 |
| Non-HDL-C (mg/dL) | 153 (105-167) | 137 (112-167) | ns |
| Apo A-I (mg/dL) | 130 ± 31 | 155 ± 29 | < 0.05 |
| Apo B (mg/dL) | 93.6 ± 23.8 | 83.5 ± 20.7 | ns |
| TG/HDL-C | 2.09 (1.13-2.98) | 1.29 (1.06-1.93) | ns |
| TC/HDL-C | 4.19 (3.11-5.00) | 3.52 (2.84-4.08) | < 0.05 |
| ApoB/apo A-I | 0.75 ± 0.25 | 0.55 ± 0.16 | < 0.01 |
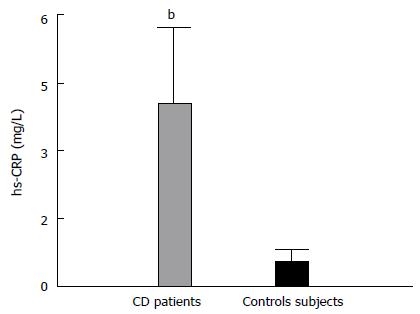
Evaluation of inflammation markers showed an increase in hsCRP levels in CD patients (Figure 1), which also correlated with apo B/apo A-I ratio (r = 0.42; P < 0.01). Even though white blood cell count (WBC) showed no differences between the two groups (6.11 ± 1.31 103/mL vs 6.17 ± 1.15 103/mL), it was directly associated with several parameters of the lipid profile (r/p; TG, 0.33/< 0.05; HDL-C, -0.34/< 0.05; apo B, 0.42/< 0.05; TG/HDL-C, 0.37/< 0.05; TC/HDL-C, 0.44/< 0.01; and apo B/apo A-I, 0.51; < 0.005). Lastly, Lp-PLA2 activity was similar between patients and controls (7.20 ± 1.28 μmol/mL.h vs 7.91 ± 2.02 μmol/mL.h) and was positively associated with LDL-C, main carrier of the enzyme in circulation (r = 0.50; P < 0.005).
Moreover, folic acid level was significantly associated with several parameters of the lipid profile (r/p; HDL-C, 0.52/< 0.05; apo A-I, 0.45/< 0.01; TG/HDL-C, -0.36/< 0.05; and apo B/apo A-I, -0.34/< 0.05) and with hsCRP concentration (r = -0.42; P < 0.05).
When comparing patients according to the clinical features, no differences were detected between patients with typical and atypical presentation of the disease in any of the parameters analyzed (data not shown).
Patients with CD showed a slight alteration in carbohydrate metabolism, decreased folic acid levels, a more atherogenic lipoprotein profile and an increase in the inflammatory marker hsCRP, with no difference evidenced between typical and atypical presentation of the disease. Likely, in both groups, the severity of duodenal lession would not be a determining factor in the metabolic alterations nor in the increase of hsCRP observed in this study.
Unlike other pathologies characterized by the presence of systemic inflammation, such as lupus erythematosus and rheumatoid arthritis, in which patients show higher CVD morbidity and mortality[18,19], available evidence for CD appears less solid and more controversial. Even though some studies have described an increase in CVD risk compared with the general population[20-22], this has not been the case in other reports[4,23]. A group of CD patients, retrospectively studied in comparison with data from general population[24], showed less CVD risk employing the Framingham score. Nevertheless, assessment of cardiac functionality, specifically of the left ventricle[25], and the study of the presence of subclinical atherosclerosis, analyzed through aortic stiffness, aortic strain, and aortic distensibility[26,27], evidenced a clear association between CD and CVD. Moreover, a previous study showed higher carotid intima media thickness (an established marker of generalized atherosclerosis that correlates with the extent of coronary artery disease and predicts future cardiovascular events) in CD patients compared to healthy controls and similar to that of patients with type 1 diabetes[28]. Lastly, it is worth noting that a recent meta-analysis[29], based on ten studies performed in CD patients, showed a slight increase in the risk of stroke, acute myocardial infarction, and cardiovascular death, though only in the first case this increase reached statistical significance. As evidenced by the bibliography, the subject remains highly controversial.
In CD patients, a systemic pro-inflammatory status was evidenced through an increase in plasma hsCRP levels. According to the guides of the American Heart Association[30], values above 3 mg/dL, such as those observed in the group of the CD patients studied, would be indicative of high CVD risk. Even though Lp-PLA2 activity, considered a specific marker of vascular inflammation[31], showed no differences between patients and controls, there is solid evidence about the increase of other inflammation markers in CD. In this regard, a previous study reported an increase in tumor necrosis factor (TNF)-α-producing innate lymphoid cells in the intestinal mucosa of untreated CD patients in comparison with treated patients and healthy controls[32]. Moreover, an increase in TNF-α and interleukin (IL)-6 levels has been reported in the epithelium and the lamina propia of the intestinal mucosa of untreated CD patients[33,34]. Additionally, higher levels of IL-6 have been observed in the plasma of untreated CD patients compared to treated ones and healthy controls[35,36].
Regarding markers of carbohydrate metabolism, even though insulin levels and HOMA-IR were increased and QUICKI diminished, the analysis of the individual values did not allow the diagnosis of insulin resistance in any of the patients included in the present study. In fact, the results obtained were below the values reported for patients with metabolic syndrome or type 2 diabetes, though above those reported for the general population[37-40]. Nevertheless, even the presence of a subtle alteration in carbohydrate metabolism in untreated CD patients would possess great clinical impact. Actually, GFD, the only available treatment for CD, contains higher caloric density than similar diets based on gluten containing foods, and its implementation could, as a result, increase the risk of developing obesity, and, consequently, metabolic syndrome and diabetes[41-43]. Therefore, assessment of fasting glucose and insulin in patients diagnosed with CD before and during the introduction of GFD should be performed. However, the subject is still controversial. Kabbani et al[44] reported lower prevalence of metabolic syndrome and type 2 diabetes in patients under GFD treatment, regardless of treatment duration. Moreover, experiments carried out in C57BL/6 mice fed on a hyper fat diet with and without gluten showed that GFD reduced insulin resistance, adiposity and inflammation[45], although it is necessary to consider that these mice did not present CD. In the present study, the finding of significantly higher insulin levels and HOMA-IR and lower QUICKI than in controls suggests the presence of a slightly altered carbohydrate metabolism, which could be related to the pro-inflammatory status described in CD patients and evidenced in our study by higher hsCRP levels. It has been previously proposed that inflammation could be a causative agent for alterations in carbohydrate metabolism through the action of cytokines such as TNF-α, IL-6 and IL-1β, among others[46,47].
Studying lipoprotein profile in CD patients appears interesting, since there is evidence for both a decrease in cholesterol absorption and an increase in its synthesis[48-50]. In the current study, patients showed TC and LDL-C levels similar to controls. These findings are in disagreement with the decrease in both parameters previously reported for CD patients[5,51,52]. Patients evaluated in this study also presented lower HDL-C levels. There is prior evidence showing a 12% prevalence of CD in patients with low HDL-C concentration[53], much higher than that reported for the general population, which implies a causal relationship between the presence of CD and the decrease in HDL-C. Unlike in the case of patients with insulin resistance[54,55], this decrease was not found to be associated with higher CETP activity. Therefore, this alteration could result from a lower synthesis and secretion of apo A-I[56]. Furthermore, longitudinal studies described an increase in HDL-C and apo A-I values after initiation of treatment with GFD[51]. In addition, CD patients presented an increase in TC/HDL-C and apo B/apo A-I ratios[57], which reflect an imbalance between proatherogenic and antiatherogenic lipid factors. Another possibility that could explain HDL-C decrease is that HDL particles from CD patients would possess less capability to promote cholesterol efflux from cells. In fact, apo A-I has not only got a structural role in HDL particles, but it is also involved in multiple antiatherogenic functions including cholesterol efflux promotion[58]. Due to the decrease in intestinal apo A-I synthesis, the number of circulating HDL particles would be diminished and, in turn, each particle would be depleted in this apolipoprotein. As a matter of fact, in other inflammatory pathologies such as rheumatoid arthritis, alterations in HDL functionality have been associated with higher risk of CVD[59]. Study of HDL functions in affected patients could provide important evidence linking CD and CVD risk.
Regarding hematological parameters, only folic acid was decreased in CD patients. This finding is consistent with previous reports that show a decrease in folic acid levels as a consequence of impaired intestinal absorption, resulting from the damage to the intestinal epithelium caused by the inflammatory process[60]. This folic acid deficiency persists, in many cases, even after the initiation of treatment with GFD[61]. It is worth noting that a decrease in folic acid levels may lead to an increase in homocysteine concentration. One of the main homocysteine clearance pathways consists of its re-methylation and recycling to methionine, a process catalyzed by the methionine synthase (MTR) enzyme, which links the folate cycle with homocysteine metabolism[62]. In fact, different studies showed higher homocysteine levels in CD patients[26,63,64]. Importantly, this increment was independently associated with increased risk and severity of coronary artery disease[65]. Moreover, high homocysteine levels were also identified as independent predictors of a suboptimal response to antiplatelet therapy with acetyl salicylic acid, thus favouring thrombotic complications in patients with coronary artery disease[66]. In addition, in a meta-analysis of randomized controlled trials, Liu et al[67] demonstrated that folic acid supplementation could improve the endothelial dysfunction observed in patients with coronary artery disease. Nevertheless, studies on homocysteine-lowering interventions with vitamin B6, folic acid (vitamin B9) or vitamin B12, administrated alone or in combination with the purpose of preventing cardiovascular events, failed to consistently demonstrate their efficacy[68]. Therefore, consideration of increased homocysteine levels as a risk factor for CVD is still a controversial topic[69].
In the present study, newly diagnosed CD patients, who were not following a GFD, presented higher insulin levels, HOMA-IR index, apo B/apo A-I ratio and hsCRP concentration, as well as lower QUICKI index, HDL-C and apo A-I levels in comparison with sex and aged-matched healthy controls.
The main limitation of the present study is that, due to its cross-sectional design, it only provides a “snapshot” of the outcome and the characteristics associated with it, at a specific point in time. Then, only associations that may exist and are therefore useful in generating hypotheses for future research may be established. Another limitation is the sample size, which may be attributed to the fact that this study only included newly diagnosed CD patients, but that hampered the search for a possible correlation between intestinal inflammation factors and the risk of atherosclerosis.
According to the results reported in the current study, untreated CD patients would present modifications in carbohydrate and lipoprotein metabolism and a pro-inflammatory status. Even though the magnitude of the alterations here described is not major, their presence and interaction through long periods of time in a chronic pathologic condition, as it is the case with CD, would constitute a high risk of developing atherosclerotic CVD.
Celiac disease (CD) is a multisystemic disease which main trait is chronic and diffuse inflammation of the mucosa of the small intestine. The only available therapy for CD consists of the implementation of a gluten free diet (GFD). It is well known that CD patients do not show classical cardiovascular disease (CVD) risk factors suggesting that CD would be associated with novel atherogenic risk factors or even with other non-identified risk factors such as inflammatory markers.
The role of novel atherogenic risk factors or inflammatory markers for CVD in CD patients has been poorly studied. The research hotspot is to assess which factors or markers for cardiovascular disease are found in CD patients in order to be able to prevent of treat them.
It is well known that CD patients do not show classical CVD risk factors. Therefore in these patients, detection of novel atherogenic risk factors would be crucial to reduce the risk of CVD.
The detection of CVD risk factors in CD patients is an important tool for the implementation of an adequate treatment.
CD is a disease which mainly affects the digestive system. Its main trait is chronic and diffuse inflammation of the mucosa of the small intestine and it can present a wide variety of clinical symptoms. It is remarkable that most cases of CD lack typical gastrointestinal symptoms and are, instead, very frequently associated with presentations known as atypical or extra-intestinal. Thus, its diagnosis represents one of the main challenges for health professionals
This is an interesting study showing that patients with CD do have an atherogenic lipoprotein profile that may dispose them to develop CVD.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: Argentina
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Holmes GKT, De Luca G, Pallav K S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Tack GJ, Verbeek WH, Schreurs MW, Mulder CJ. The spectrum of celiac disease: epidemiology, clinical aspects and treatment. Nat Rev Gastroenterol Hepatol. 2010;7:204-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 176] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 2. | Katz KD, Rashtak S, Lahr BD, Melton LJ, Krause PK, Maggi K, Talley NJ, Murray JA. Screening for celiac disease in a North American population: sequential serology and gastrointestinal symptoms. Am J Gastroenterol. 2011;106:1333-1339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 3. | Armstrong MJ, Hegade VS, Robins G. Advances in coeliac disease. Curr Opin Gastroenterol. 2012;28:104-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | West J, Logan RF, Card TR, Smith C, Hubbard R. Risk of vascular disease in adults with diagnosed coeliac disease: a population-based study. Aliment Pharmacol Ther. 2004;20:73-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Lewis NR, Sanders DS, Logan RF, Fleming KM, Hubbard RB, West J. Cholesterol profile in people with newly diagnosed coeliac disease: a comparison with the general population and changes following treatment. Br J Nutr. 2009;102:509-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Ludvigsson JF, de Faire U, Ekbom A, Montgomery SM. Vascular disease in a population-based cohort of individuals hospitalised with coeliac disease. Heart. 2007;93:1111-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Leffler DA, Green PH, Fasano A. Extraintestinal manifestations of coeliac disease. Nat Rev Gastroenterol Hepatol. 2015;12:561-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 187] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 8. | Meroño T, Dauteuille C, Tetzlaff W, Martín M, Botta E, Lhomme M, Saez MS, Sorroche P, Boero L, Arbelbide J. Oxidative stress, HDL functionality and effects of intravenous iron administration in women with iron deficiency anemia. Clin Nutr. 2017;36:552-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Libby P, Ridker PM, Hansson GK. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54:2129-2138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1352] [Cited by in RCA: 1534] [Article Influence: 102.3] [Reference Citation Analysis (0)] |
| 10. | Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, Monauni T, Muggeo M. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23:57-63. [PubMed] |
| 11. | Yokoyama H, Emoto M, Fujiwara S, Motoyama K, Morioka T, Komatsu M, Tahara H, Shoji T, Okuno Y, Nishizawa Y. Quantitative insulin sensitivity check index and the reciprocal index of homeostasis model assessment in normal range weight and moderately obese type 2 diabetic patients. Diabetes Care. 2003;26:2426-2432. [PubMed] |
| 12. | Warnick GR, Benderson J, Albers JJ. Dextran sulfate-Mg2+ precipitation procedure for quantitation of high-density-lipoprotein cholesterol. Clin Chem. 1982;28:1379-1388. [PubMed] |
| 13. | Assmann G, Jabs HU, Kohnert U, Nolte W, Schriewer H. LDL-cholesterol determination in blood serum following precipitation of LDL with polyvinylsulfate. Clin Chim Acta. 1984;140:77-83. [PubMed] |
| 14. | Lagrost L, Gandjini H, Athias A, Guyard-Dangremont V, Lallemant C, Gambert P. Influence of plasma cholesteryl ester transfer activity on the LDL and HDL distribution profiles in normolipidemic subjects. Arterioscler Thromb. 1993;13:815-825. [PubMed] |
| 15. | Blank ML, Hall MN, Cress EA, Snyder F. Inactivation of 1-alkyl-2-acetyl-sn-glycero-3-phosphocholine by a plasma acetylhydrolase: higher activities in hypertensive rats. Biochem Biophys Res Commun. 1983;113:666-671. [PubMed] |
| 16. | World Health Organization. Serum ferritin concentrations for the assessment of iron status and iron deficiency in populations. ([database on the Internet]. 2016. [accessed. 2016;Mar 30]) Available from: http://www.who.int/vmnis/indicators/serum_ferritin.pdf. |
| 17. | World Health Organization. Conclusions of a WHO Technical Consultation on folate and vitamin B12 deficiencies [database on the Internet]. (. 2016;[accessed 2016 Mar 30]) Available from: http://www.who.int/vmnis/indicators/en/. |
| 18. | Aviña-Zubieta JA, Choi HK, Sadatsafavi M, Etminan M, Esdaile JM, Lacaille D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690-1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 926] [Cited by in RCA: 1047] [Article Influence: 65.4] [Reference Citation Analysis (0)] |
| 19. | Hak AE, Karlson EW, Feskanich D, Stampfer MJ, Costenbader KH. Systemic lupus erythematosus and the risk of cardiovascular disease: results from the nurses’ health study. Arthritis Rheum. 2009;61:1396-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 199] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 20. | Jansen S, Bhangu J, de Rooij S, Daams J, Kenny RA, van der Velde N. The Association of Cardiovascular Disorders and Falls: A Systematic Review. J Am Med Dir Assoc. 2016;17:193-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 21. | Ludvigsson JF, James S, Askling J, Stenestrand U, Ingelsson E. Nationwide cohort study of risk of ischemic heart disease in patients with celiac disease. Circulation. 2011;123:483-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 22. | Wei L, Spiers E, Reynolds N, Walsh S, Fahey T, MacDonald TM. The association between coeliac disease and cardiovascular disease. Aliment Pharmacol Ther. 2008;27:514-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Whorwell PJ, Alderson MR, Foster KJ, Wright R. Death from ischaemic heart-disease and malignancy in adult patients with coeliac disease. Lancet. 1976;2:113-114. [PubMed] |
| 24. | Stein AC, Liao C, Paski S, Polonsky T, Semrad CE, Kupfer SS. Obesity and Cardiovascular Risk in Adults With Celiac Disease. J Clin Gastroenterol. 2016;50:545-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Akin FE, Sari C, Ozer-Sari S, Demirezer-Bolat A, Durmaz T, Keles T, Ersoy O, Bozkurt E. The evaluation of left ventricular functions with tissue doppler echocardiography in adults with celiac disease. Saudi J Gastroenterol. 2015;22:116-121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Korkmaz H, Sozen M, Kebapcilar L. Increased arterial stiffness and its relationship with inflammation, insulin, and insulin resistance in celiac disease. Eur J Gastroenterol Hepatol. 2015;27:1193-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Sari S, Dogu F, Hwa V, Haskologlu S, Dauber A, Rosenfeld R, Polat M, Kuloglu Z, Kansu A, Dalgic B. A Successful HSCT in a Girl with Novel LRBA Mutation with Refractory Celiac Disease. J Clin Immunol. 2016;36:8-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Pitocco D, Giubilato S, Martini F, Zaccardi F, Pazzano V, Manto A, Cammarota G, Di Stasio E, Pedicino D, Liuzzo G. Combined atherogenic effects of celiac disease and type 1 diabetes mellitus. Atherosclerosis. 2011;217:531-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 29. | Emilsson L, Lebwohl B, Sundström J, Ludvigsson JF. Cardiovascular disease in patients with coeliac disease: A systematic review and meta-analysis. Dig Liver Dis. 2015;47:847-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 30. | Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499-511. [PubMed] |
| 31. | Zalewski A, Macphee C. Role of lipoprotein-associated phospholipase A2 in atherosclerosis: biology, epidemiology, and possible therapeutic target. Arterioscler Thromb Vasc Biol. 2005;25:923-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 363] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 32. | Marafini I, Monteleone I, Di Fusco D, Cupi ML, Paoluzi OA, Colantoni A, Ortenzi A, Izzo R, Vita S, De Luca E. TNF-α Producing Innate Lymphoid Cells (ILCs) Are Increased in Active Celiac Disease and Contribute to Promote Intestinal Atrophy in Mice. PLoS One. 2015;10:e0126291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 33. | Iacomino G, Marano A, Stillitano I, Aufiero VR, Iaquinto G, Schettino M, Masucci A, Troncone R, Auricchio S, Mazzarella G. Celiac disease: role of intestinal compartments in the mucosal immune response. Mol Cell Biochem. 2016;411:341-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Przemioslo RT, Kontakou M, Nobili V, Ciclitira PJ. Raised pro-inflammatory cytokines interleukin 6 and tumour necrosis factor alpha in coeliac disease mucosa detected by immunohistochemistry. Gut. 1994;35:1398-1403. [PubMed] |
| 35. | Kapoor A, Patwari AK, Kumar P, Jain A, Narayan S. Serum soluble interleukin-2 receptor, interleukin-6 and tumor necrosis factor alpha as markers of celiac disease activity. Indian J Pediatr. 2013;80:108-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 36. | Romaldini CC, Barbieri D, Okay TS, Raiz R, Cançado EL. Serum soluble interleukin-2 receptor, interleukin-6, and tumor necrosis factor-alpha levels in children with celiac disease: response to treatment. J Pediatr Gastroenterol Nutr. 2002;35:513-517. [PubMed] |
| 37. | Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412-419. [PubMed] |
| 38. | McLaughlin T, Reaven G, Abbasi F, Lamendola C, Saad M, Waters D, Simon J, Krauss RM. Is there a simple way to identify insulin-resistant individuals at increased risk of cardiovascular disease? Am J Cardiol. 2005;96:399-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 443] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 39. | Radziuk J. Insulin sensitivity and its measurement: structural commonalities among the methods. J Clin Endocrinol Metab. 2000;85:4426-4433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 40. | Skrha J, Haas T, Sindelka G, Prázný M, Widimský J, Cibula D, Svacina S. Comparison of the insulin action parameters from hyperinsulinemic clamps with homeostasis model assessment and QUICKI indexes in subjects with different endocrine disorders. J Clin Endocrinol Metab. 2004;89:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 66] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 41. | Kaukinen K, Lindfors K, Collin P, Koskinen O, Mäki M. Coeliac disease--a diagnostic and therapeutic challenge. Clin Chem Lab Med. 2010;48:1205-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 42. | Norsa L, Shamir R, Zevit N, Verduci E, Hartman C, Ghisleni D, Riva E, Giovannini M. Cardiovascular disease risk factor profiles in children with celiac disease on gluten-free diets. World J Gastroenterol. 2013;19:5658-5664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 61] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 43. | Tortora R, Capone P, De Stefano G, Imperatore N, Gerbino N, Donetto S, Monaco V, Caporaso N, Rispo A. Metabolic syndrome in patients with coeliac disease on a gluten-free diet. Aliment Pharmacol Ther. 2015;41:352-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 123] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 44. | Kabbani TA, Kelly CP, Betensky RA, Hansen J, Pallav K, Villafuerte-Gálvez JA, Vanga R, Mukherjee R, Novero A, Dennis M. Patients with celiac disease have a lower prevalence of non-insulin-dependent diabetes mellitus and metabolic syndrome. Gastroenterology. 2013;144:912-917.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 45. | Soares FL, de Oliveira Matoso R, Teixeira LG, Menezes Z, Pereira SS, Alves AC, Batista NV, de Faria AM, Cara DC, Ferreira AV. Gluten-free diet reduces adiposity, inflammation and insulin resistance associated with the induction of PPAR-alpha and PPAR-gamma expression. J Nutr Biochem. 2013;24:1105-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 46. | Shoelson SE. Point-counterpoint: Interleukin-6 does/does not have a beneficial role in insulin sensitivity and glucose homeostasis. J Appl Physiol (1985). 2007;102:820; author reply 825. [PubMed] |
| 47. | Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793-1801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2726] [Cited by in RCA: 3078] [Article Influence: 162.0] [Reference Citation Analysis (0)] |
| 48. | Vuoristo M, Kesäniemi YA, Gylling H, Miettinen TA. Metabolism of cholesterol and apolipoprotein B in celiac disease. Metabolism. 1993;42:1386-1391. [PubMed] |
| 49. | Vuoristo M, Miettinen TA. Cholesterol absorption, elimination and synthesis in coeliac disease. Eur J Clin Invest. 1982;12:285-291. [PubMed] |
| 50. | Vuoristo M, Miettinen TA. Enhanced synthesis of cholesterol and its precursors in jejunal mucosa in coeliac disease. Gut. 1986;27:399-404. [PubMed] |
| 51. | Capristo E, Malandrino N, Farnetti S, Mingrone G, Leggio L, Addolorato G, Gasbarrini G. Increased serum high-density lipoprotein-cholesterol concentration in celiac disease after gluten-free diet treatment correlates with body fat stores. J Clin Gastroenterol. 2009;43:946-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 52. | Médiène S, Hakem S, Bard JM, Medjaoui I, Benhamamouch S, Lebel P, Fruchart JC, Clavey V. Serum lipoprotein profile in Algerian patients with celiac disease. Clin Chim Acta. 1995;235:189-196. [PubMed] |
| 53. | Capristo E, Addolorato G, Mingrone G, Scarfone A, Greco AV, Gasbarrini G. Low-serum high-density lipoprotein-cholesterol concentration as a sign of celiac disease. Am J Gastroenterol. 2000;95:3331-3332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 54. | Borggreve SE, De Vries R, Dullaart RP. Alterations in high-density lipoprotein metabolism and reverse cholesterol transport in insulin resistance and type 2 diabetes mellitus: role of lipolytic enzymes, lecithin: cholesterol acyltransferase and lipid transfer proteins. Eur J Clin Invest. 2003;33:1051-1069. [PubMed] |
| 55. | Florén CH, Alm P. Defective synthesis of apolipoprotein A-I in jejunal mucosa in coeliac disease. Scand J Gastroenterol. 1988;23:856-860. [PubMed] |
| 56. | Rao SN, Magill PJ, Miller NE, Lewis B. Plasma high-density lipoprotein metabolism in subjects with primary hypertriglyceridaemia: altered metabolism of apoproteins AI and AII. Clin Sci (Lond). 1980;59:359-367. [PubMed] |
| 57. | McQueen MJ, Hawken S, Wang X, Ounpuu S, Sniderman A, Probstfield J, Steyn K, Sanderson JE, Hasani M, Volkova E. Lipids, lipoproteins, and apolipoproteins as risk markers of myocardial infarction in 52 countries (the INTERHEART study): a case-control study. Lancet. 2008;372:224-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 569] [Cited by in RCA: 643] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 58. | Vedhachalam C, Liu L, Nickel M, Dhanasekaran P, Anantharamaiah GM, Lund-Katz S, Rothblat GH, Phillips MC. Influence of ApoA-I structure on the ABCA1-mediated efflux of cellular lipids. J Biol Chem. 2004;279:49931-49939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 59. | Charles-Schoeman C, Lee YY, Grijalva V, Amjadi S, FitzGerald J, Ranganath VK, Taylor M, McMahon M, Paulus HE, Reddy ST. Cholesterol efflux by high density lipoproteins is impaired in patients with active rheumatoid arthritis. Ann Rheum Dis. 2012;71:1157-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 166] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 60. | García-Manzanares A, Lucendo AJ. Nutritional and dietary aspects of celiac disease. Nutr Clin Pract. 2011;26:163-173. [PubMed] [DOI] [Full Text] |
| 61. | Caruso R, Pallone F, Stasi E, Romeo S, Monteleone G. Appropriate nutrient supplementation in celiac disease. Ann Med. 2013;45:522-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 62. | Blom HJ, Smulders Y. Overview of homocysteine and folate metabolism. With special references to cardiovascular disease and neural tube defects. J Inherit Metab Dis. 2011;34:75-81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 292] [Cited by in RCA: 298] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 63. | Dickey W, Ward M, Whittle CR, Kelly MT, Pentieva K, Horigan G, Patton S, McNulty H. Homocysteine and related B-vitamin status in coeliac disease: Effects of gluten exclusion and histological recovery. Scand J Gastroenterol. 2008;43:682-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 64. | Saibeni S, Lecchi A, Meucci G, Cattaneo M, Tagliabue L, Rondonotti E, Formenti S, De Franchis R, Vecchi M. Prevalence of hyperhomocysteinemia in adult gluten-sensitive enteropathy at diagnosis: role of B12, folate, and genetics. Clin Gastroenterol Hepatol. 2005;3:574-580. [PubMed] |
| 65. | Schaffer A, Verdoia M, Cassetti E, Marino P, Suryapranata H, De Luca G. Relationship between homocysteine and coronary artery disease. Results from a large prospective cohort study. Thromb Res. 2014;134:288-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 66. | Verdoia M, Schaffer A, Pergolini P, Rolla R, Barbieri L, Bellomo G, Sinigaglia F, Marino P, Suryapranata H, De Luca G. Homocysteine Levels Influence Platelet Reactivity in Coronary Artery Disease Patients Treated With Acetylsalicylic Acid. J Cardiovasc Pharmacol. 2015;66:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 67. | Liu Y, Tian T, Zhang H, Gao L, Zhou X. The effect of homocysteine-lowering therapy with folic acid on flow-mediated vasodilation in patients with coronary artery disease: a meta-analysis of randomized controlled trials. Atherosclerosis. 2014;235:31-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 68. | Martí-Carvajal AJ, Solà I, Lathyris D. Homocysteine-lowering interventions for preventing cardiovascular events. Cochrane Database Syst Rev. 2015;1:CD006612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 69. | Ganguly P, Alam SF. Role of homocysteine in the development of cardiovascular disease. Nutr J. 2015;14:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 490] [Cited by in RCA: 671] [Article Influence: 67.1] [Reference Citation Analysis (0)] |