Published online Apr 26, 2017. doi: 10.4330/wjc.v9.i4.363
Peer-review started: September 27, 2016
First decision: October 20, 2016
Revised: December 30, 2016
Accepted: January 14, 2017
Article in press: January 14, 2017
Published online: April 26, 2017
Processing time: 214 Days and 2.7 Hours
To explore regional systolic strain of midwall and endocardial segments using speckle tracking echocardiography in patients with apical hypertrophic cardiomyopathy (HCM).
We prospectively assessed 20 patients (mean age 53 ± 16 years, range: 18-81 years, 10 were male), with apical HCM. We measured global longitudinal peak systolic strain (GLPSS) in the midwall and endocardium of the left ventricle.
The diastolic thickness of the 4 apical segments was 16.25 ± 2.75 mm. All patients had a normal global systolic function with a fractional shortening of 50% ± 8%. In spite of supernormal left ventricular (LV) systolic function, midwall GLPSS was decreased in all patients, more in the apical (-7.3% ± -8.8%) than in basal segments (-15.5% ± -6.93%), while endocardial GLPPS was significantly greater and reached normal values (apical: -22.8% ± -7.8%, basal: -17.9% ± -7.5%).
This study shows that two-dimensional strain was decreased mainly confined to the mesocardium, while endocardium myocardial deformation was preserved in HCM and allowed to identify subclinical LV dysfunction. This transmural heterogeneity in systolic strain had not been previously described in HCM and could be explained by the distribution of myofibrillar disarray in deep myocardial areas. The clinical application of this novel finding may help further understanding of the pathophysiology of HCM.
Core tip: In this study we prospectively assessed 20 patients with apical hypertrophic cardiomyopathy (HCM) in which we used speckle tracking echocardiography for measuring global longitudinal peak systolic strain in the midwall and endocardium of the left ventricle. We showed that two-dimensional strain was decreased mainly confined to the mesocardium, while endocardial deformation was preserved. This finding allowed to identify subclinical left ventricular systolic dysfunction. This transmural heterogeneity in systolic strain had not been previously described in HCM and could be explained by the distribution of myofibrillar disarray in deep myocardial areas. The clinical application of this novel finding may help further understanding of the pathophysiology of HCM.
- Citation: Saccheri MC, Cianciulli TF, Morita LA, Méndez RJ, Beck MA, Guerra JE, Cozzarin A, Puente LJ, Balletti LR, Lax JA. Speckle tracking echocardiography to assess regional ventricular function in patients with apical hypertrophic cardiomyopathy. World J Cardiol 2017; 9(4): 363-370
- URL: https://www.wjgnet.com/1949-8462/full/v9/i4/363.htm
- DOI: https://dx.doi.org/10.4330/wjc.v9.i4.363
Hypertrophic cardiomyopathy (HCM) is a genetic disease transmitted with an autosomal dominant pattern, whereby the direct relatives of affected subjects carry 50% chances of having the disease[1]. Its prevalence in the general population is 0.2% and it is frequent cause of sudden cardiac death in patients younger than age 30, including athletes. The annual mortality rate is 1%, but may be as high as 6% during childhood and adolescence; of note, sudden death may be the first symptom of disease[2]. It is a heterogeneous disease in its clinical as well as genetic aspects, characterized by the presence of primary left ventricular hypertrophy (LVH), with variable clinical expression and outcome[3], and caused by genetic mutations that lead to abnormal sarcomeric proteins[4].
In patients with complete phenotypic expression, characteristic findings are: Hypertrophy, myofibrillar disarray, interstitial fibrosis and microvascular dysfunction, all of which contribute to the progression to heart failure, ventricular arrhythmias and sudden death. Recent studies with MRI have shown that many patients with HCM have multiple areas of myocardial fibrosis, even with normal LV ejection fraction[5].
Epicardial coronary arteries in patients with HCM are usually normal, but coronary flow reserve is diminished due to narrowing of the small intramyocardial arteries[6]. This microvascular ischemia is one of the factors resulting in LV diastolic dysfunction, which in turn is the main functional consequence of this disease.
Although patients with HCM have a normal ejection fraction, studies with Doppler tissue imaging have documented a regional systolic dysfunction in the longitudinal fibers of the LV[7-10] .
Regional LV function may be assessed non-invasively by measuring strain or systolic deformation. Initially, strain calculated with colour tissue Doppler proved to be a useful and sensitive tool to detect early systolic function abnormalities in patients with HCM[11]. However, its clinical application proved to be hindered by the complexity of data collection and limited reproducibility.
Recently, a method derived from the two-dimensional (2-D) echocardiogram, called “speckle tracking” of 2-D strain, has been developed to measure systolic strain[12]. The goal of this study was to assess the abnormalities of global and regional systolic LV function using 2-D strain in patients with apical HCM.
The study has a cross-sectional design and included 20 patients with apical HCM who were being followed at our tertiary referral center. Using a retrospective methodology, 2-D strain was measured in 340 myocardial segments.
The diagnostic criteria for apical HCM included demonstration of asymmetric left ventricular hypertrophy (LVH), confined predominantly to the LV apex with an apical wall thickness > 15 mm, a ratio of maximal apical to posterior wall thickness >1.5[13], and a “spade shape” deformity of the left ventricle with apical cavity obliteration in end-systole based on 2-D echocardiography.
Inclusion criteria were: HCM with apical involvement, non-dilated LV, normal global and regional systolic LV function, normal blood pressure, sinus rhythm, absence of comorbidities and without history of hypertension. Noninvasive evaluation of global and regional systolic LV function was done by calculation of LV ejection fraction and visual judgement of segmental function from 2-D echocardiographic images.
Exclusion criteria were obesity, poor echocardiographic window, concomitant diseases that could cause ventricular hypertrophy or abnormal systolic or diastolic function (hypertension, diabetes, coronary heart disease, valve disease, cardiomyopathy, pericardial disease, congenital heart disease or systemic disease).
The study was approved by the Education and Research Committee and the Ethics Committee of the “Dr. Cosme Argerich” Hospital. All patients signed the informed consent form, including the authorization to use the data collected for future studies.
Standard echocardiographic examinations were performed in all patients using a Vivid Seven digital ultrasound system (GE Medical System, Hotern, Norway). Cardiac cycles were stored in digital, cineloop format for off-line analysis performed by two independent observers (TFC and JAL) with a dedicated software package (EchoPAC PC, version 108.1.5).
Both parasternal long- and short-axis views were analyzed. The M-mode echo was derived from the parasternal short-axis at the papillary muscle level, and the following measurements were obtained according to the American and European Societies of Echocardiography[14]: LV end-diastolic diameter (EDD), LV end-systolic diameter (ESD), interventricular septum and posterior LV wall thickness, and end-systolic left atrial diameter. Ejection fraction was measured by Simpson method. Continuous Doppler from the apical 5-chamber view was used to rule out the presence of a dynamic subaortic gradient.
2-D strain is a novel non-Doppler-based method to evaluate strain from standard 2-D acquisitions[15]. By tracing the endocardial contour on an end-systolic frame, the software will automatically track the contour on subsequent frames. Adequate tracking can be verified in real-time and corrected by adjusting the region of interest (ROI) or manually correcting the contour to ensure optimal tracking. A minimum frame rate of 30 Hz was required for reliable operation of this program and frame rates of 30 to 80 Hz were used for routine gray scale imaging. 2-D longitudinal strains were assessed in 2 orthogonal apical views (4- 3 and 2-chambers, 17 segments) starting from the septal, posterior and the inferior atrioventricular wall junction, respectively. The 2-D strain software adequately tracked > 85% of the attempted segments.
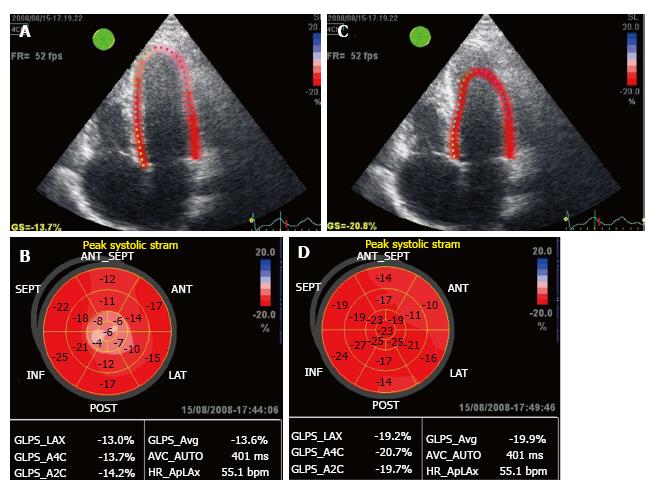
The ROI was reduced and shifted to the mesocardium to obtain the parametric image, which allowed quantifying strain in each of the 17 segments of the LV as a “bull’s eye” (Figure 1A and B), the mean value of peak global systolic strain and strain in the 3 apical views. Later, the ROI was shifted to the endocardium to obtain the endocardial strain of the 17 segments represented as a bull’s eye (Figure 1C and D).
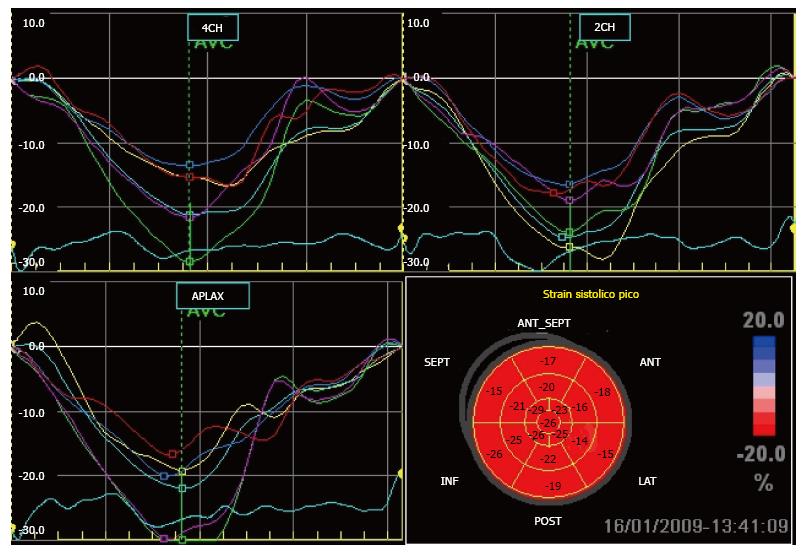
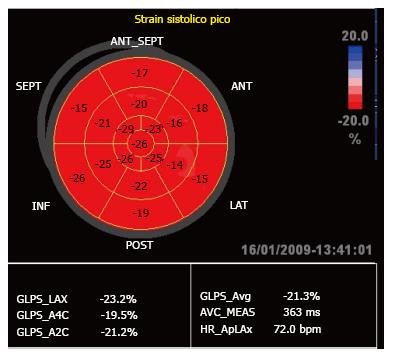
In the present study we only used global longitudinal peak systolic strain (GLPSS), which was plotted as a negative curve with a peak close to the aortic closure (Figure 2). These GLPSS curves represent the maximum myocardial longitudinal shortening during contraction in each of the 17 segments. In a normal subject (Figure 3) GLPSS varies between -15% and -20%[15].
The first 10 studies were analyzed blindly by a second operator who measured longitudinal 2-D strain in 170 myocardial segments. Intraobserver variability was calculated from the mean of the differences obtained in the 170 segments. Interobserver variability was calculated as the absolute difference divided by the mean of the 2 observations for all segments measured[16].
Quantitative data with a normal distribution were expressed as the mean ± SD and data without a Gaussian distribution were expressed as medians (interquartile interval).
For the comparison of quantitative variables with a normal distribution we used the Student’s t test for paired data; for variables without a normal distribution we used the Wilcoxon or Signed Rank Test.
All P values < 0.05 were considered statistically significant. Statistical analyses were performed with Statistix 7.0 software for Windows.
The clinical and echocardiographic characteristics of patients with apical HCM are summarized in Table 1. No patient was receiving medication at the time of inclusion in this study.
| No. of patients | 20 |
| Age (yr) | 53 ± 16 |
| Women, n (%) | 10 (50) |
| RV (mm) | 15 ± 5 |
| LVDD (mm) | 48 ± 5 |
| LVSD (mm) | 24 ± 5 |
| EF (%) | 69 ± 5 |
| LA (mm) | 44 ± 7 |
| Antero-apical (mm) | 16 ± 2 |
| Infero-apical (mm) | 15 ± 3 |
| Lateral-apical (mm) | 17 ± 3 |
| Septal-apical (mm) | 17 ± 3 |
| Apex/LVPW ratio | 2.1 ± 0.4 |
All patients exhibited apical hypertrophy, (the diastolic thickness of the 4 apical segments is described in Table 1). All patients had a normal ejection fraction (69% ± 5%).
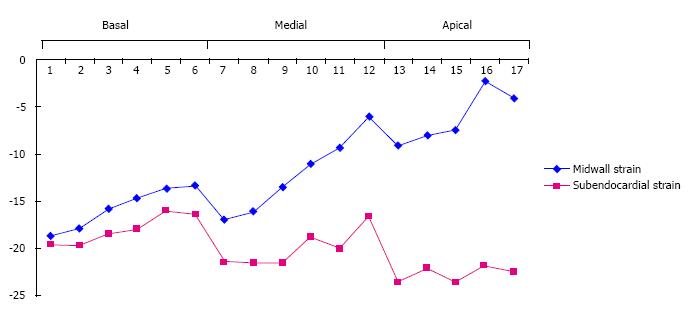
A total of 20 patients with apical HCM were assessed and 340 myocardial segments were analyzed; midwall longitudinal peak systolic strain (LPSS) was measured and compared to endocardial LPSS (Table 2 and Figure 4). We confirmed that, in spite of a supernormal systolic LV function, midwall GLPSS exhibited a diminished percent of strain, which was more marked in the apical than in basal segments. By contrast, endocardial GLPSS was significantly higher and reached normal values.
| Segments | Midwall LPSS(%) | Endocardial LPSS (%) | P value |
| Mean GLPSS | -13 (-14/-8.8) | -19.4 (-23.9/-16.2) | < 0.001 |
| Antero-basal | -14.5 (-18/-8) | -16 (-19.5/-12.3) | NS |
| Latero-basal | -12 (-14/-10) | -15 (-18.7/-12) | NS |
| Postero-basal | -15 (-20/-9) | -17 (-19.7/-14.2) | NS |
| Infero-basal | -19 (-22.7/-13.7) | -21 (-22/-17.2) | NS |
| Postero-basal septum | -16 (-23.5/-14) | -18 (-22.7/-13.2) | NS |
| Antero-basal septum | -17.5 (-21/-8.25) | -18 (-25.2/-14) | NS |
| Antero-medial | -11.5 (-15/-7.2) | -19 (-23.5/-12) | < 0.001 |
| Latero-medial | -7.5 (-8.7/-2.5) | -18 (-20.7/-10.5) | < 0.001 |
| Postero-medial | -10.5 (-13.7/-7.2) | -17.5 (-23/-15) | < 0.001 |
| Infero-medial | -16 (-20.7/-12.5) | -20.5 (-22.7/-18.2) | < 0.001 |
| Postero-medial septum | -18 (-22.5/-11.5) | -20 (-29/-15) | < 0.001 |
| Antero-medial septum | -16.5 (-18.7/-9.2) | -23.5 (-27.7/-16.2) | < 0.001 |
| Antero-apical | -8 (-16/-1.5) | -21.5 (-29.7/-16.2) | < 0.001 |
| Lateral-apical | -2 (-8/-2.5) | -22.5 (-28.7/-15) | < 0.001 |
| Infero-apical | -8 (-18.2/-0.25) | -22.5 (-28.7/-18) | < 0.001 |
| Septal-apical | -9 (-17.2/-5.2) | -23.5 (-31.5/-16.2) | < 0.001 |
| Apex | -8 (-16/-1.5) | -21.5 (-29.7/-10.2) | < 0.001 |
Midwall GLPSS in the basal segments (Table 3) was lower than the endocardial GLPSS, but without significant differences (-15.5% ± -6.93% vs -17.9% ± -7.5%, P = NS). Midwall GLPSS was significantly decreased in the medial segments (-12.4% ± -7.3% vs -19.7% ± -7.6%, P < 0.001), with a 52% increase in endocardium strain. But the largest difference between midwall and endocardial strain was found in the apical segments, with a 168% increase in endocardial strain (-7.3% ± -8.8% vs -22.8% ± -7.8%, P < 0.001). The increase of the GLPSS from basal to apex segments can be seen in the dotted line (Figure 4).
| Segments | Mesocardial GLPSS (%) | Endocardial GLPSS (%) | Media of increment | CI | Increase of GLPSS) | P value |
| Basal | -15.5 ± -6.93 | -17.9 ± -7.5 | -2.4 | -3/-1.3 | 18% | NS |
| Medial | -12.4 ± -7.3 | -19.7 ± -7.6 | -7.2 | -8.4/-6 | 52% | < 0.001 |
| Apical | -7.3 ± -8.8 | - 22.8 ± -7.8 | -15.5 | -17/-13 | 168% | < 0.001 |
Using 2D-based method for myocardial velocity strain (XStrain) that allows analyse the endocardial and epicardial border, this transmural gradient between the midwall and endocardial of global longitudinal peak systolic strain were seen in normal subjects, but without significant differences.
In our laboratory, intraobserver and interobserver variability of 2-D strain was low and varied between 3.6% and 5.3% and 7% and 11.8% respectively.
To our knowledge, this is the first study to show that in a selected population of patients with apical HCM and normal LV ejection fraction, the regional systolic strain is decreased in the mesocardium, with a compensatory effect in the endocardium. The clinical application of this new finding may help to further understanding the pathophysiology of apical HCM.
Mutations of genes that code for contractile proteins of the sarcomere are responsible for the structural and functional changes seen in patients with HCM, and cause ventricular hypertrophy, myofibrillar disarray and interstitial fibrosis. In spite of the hyperdynamic systolic function seen by echo, midwall 2-D strain detected a decrease in myocardial strain in all of our patients.
All patients had hypertrophy of the LV apex with normal apical wall motion, but they exhibited a decreased midwall 2-D strain, predominantly in the apex. One might postulate that this finding expresses myofibrillar disarray with microvascular ischemia, which contributes to increased myocardial fibrosis in those segments with greater hypertrophy.
In patients with HCM, Popović et al[17] have shown that 2-D strain was lower in patients whose MRI showed myocardial fibrosis than in patients without fibrosis, but they did not analyze whether longitudinal strain had transmural heterogeneity, as shown in our study.
The normal LV contracts longitudinally in systole and also radially. The array of myocardial fibers in the ventricular wall is quite unique; endocardial and subepicardial fibers align longitudinally, in a spiral shape, and midwall fibers are aligned circumferentially. This latter group is responsible for the radial contraction in the minor axis of the LV (similar to the movement of the bellows of an accordion), while the former cause longitudinal contraction similar to the movement of a piston. This fiber orientation is so efficient that a 15%-20% reduction in the myocite’s length can result in a 40%-60% radial wall thickening, thus allowing the LV to achieve an ejection fraction of 60%.
In patients with apical HCM, longitudinal midwall strain allowed to identify subclinical global systolic dysfunction, with a lower intra and interobserver variability than for strain derived from colour tissue Doppler[18].
In our study of 20 patients with apical HCM, we analyzed 340 myocardial segments with midwall LPSS and compared it to endocardial LPSS. We confirmed that although systolic ventricular function was supernormal, midwall GLPSS exhibited a decrease in the percent of strain, more evident in apical than in medial segments, whereas endocardial GLPSS was significantly greater, and reached normal values[19]. These findings indicate that in spite of the apical ventricular hypertrophy with excellent ejection fraction parameters, there is subclinical abnormality in midwall strain, while endocardial function is preserved. An explanation for this phenomenon could be that myofiber disarray[5,20] and interstitial fibrosis[21-23] are mostly located in the mid third of the ventricular wall. This particular distribution of histological abnormalities in apical HCM also explains why endomyocardial biopsy is not useful in the diagnosis of HCM, since the biotome does not reach the myocardium with fiber disarray and interstitial fibrosis[24].
One limitation of this study is that we only measured longitudinal strain. It is possible that measurement of radial and circumferential strain will add useful information to the data obtained in this work. 2-D strain is a sensitive method to measure myocardial strain, but it is very much dependent on echo image quality, and in patients with necrotic scars strain may be measured in 80% of segments analyzed[12]. Such limitation is not applicable to our population, since the presence of LVH helped in obtaining a good quality image.
Another limitation is that the ROI of speckle tracking method cannot be diminished more than 10 mm. The most patients did not exhibit hypertrophy of the basal segments; hence, further midwall strain overlapped with endocardial strain, which might explain the smaller difference between them.
In conclusion, this study shows that 2-D strain assessed by “speckle tracking” is a sensitive method to detect subclinical systolic LV dysfunction. When midwall and endocardial strain values were compared, we confirmed that the decrease in strain was confined to the midwall, while endocardial myocardial function was preserved. This transmural heterogeneity of systolic deformity in apical HCM has not been previously described. A possible explanation could be that myofibrillar disarray and interstitial fibrosis are distributed in deeper areas of the myocardium. The clinical application of this new finding may help in the pathophysiological interpretation of HCM.
Future studies, with more subjects, will allow assessing whether patients with greater change in midwall strain may be at higher risk for ventricular arrhythmias, sudden death or progression to heart failure due to systolic dysfunction. Additionally, the method could help in evaluating the benefit of conventional treatment and new therapeutic strategies.
We would like to thank Ing. Ariel Desseno (General Electric) for his technical assistance.
Hypertrophic cardiomyopathy (HCM) is associated with normal left ventricular (LV) ejection fraction and impaired LV strain, but there are no studies so far comparing midwall and endocardial strain.
The diagnostic criteria for apical HCM included demonstration of asymmetric left ventricular hypertrophy (LVH), confined predominantly to the LV apex with an apical wall thickness > 15 mm, a ratio of maximal apical to posterior wall thickness > 1.5, and a “spade shape” deformity of the left ventricle with apical cavity obliteration in end-systole based on 2-D echocardiography.
This study shows that two-dimensional strain assessed by “speckle tracking” is a sensitive method to detect subclinical systolic LV dysfunction. When midwall and endocardial strain values were compared, the authors confirmed that the decrease in strain was confined to the midwall, while endocardial myocardial function was preserved. This transmural heterogeneity of systolic deformity in apical HCM has not been previously described. A possible explanation could be that myofibrillar disarray and interstitial fibrosis are distributed in deeper areas of the myocardium. The clinical application of this new finding may help in the pathophysiological interpretation of HCM.
Two-dimensional strain is a novel non-Doppler-based method to evaluate strain from standard two-dimensional acquisitions. By tracing the endocardial contour on an end-systolic frame, the software will automatically track the contour on subsequent frames. Two-dimensional longitudinal strains were assessed in 2 orthogonal apical views (4- 3 and 2-chambers, 17 segments). The region of interest (ROI) was reduced and shifted to the mesocardium to obtain the parametric image, which allowed quantifying strain in each of the 17 segments of the LV. Later, the ROI was shifted to the endocardium to obtain the endocardial strain of the 17 segments.
The study by Saccheri et al reports the data obtained by speckle tracking echocardiography in patients with apical hypertrophic cardiomyopathy. The authors show that two-dimensional strain is able to identify subclinical systolic left ventricular dysfunction in this patient population. The manuscript is interesting and well written.
Manuscript source: Unsolicited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: Argentina
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: De Ponti R, Kettering K, Vermeersch P S- Editor: Song XX L- Editor: A E- Editor: Lu YJ
| 1. | Navarro-López F. [Hypertrophic cardiomyopathy. Genetic basis and clinical implications]. Rev Esp Cardiol. 2004;57 Suppl 1:22-32. [PubMed] |
| 2. | García-Castro M, Reguero JR, Batalla A, Catalán F, Mayordomo J, Coto E. [Direct detection of malignant mutations in patients with hypertrophic cardiomyopathy]. Rev Esp Cardiol. 2003;56:1022-1025. [PubMed] |
| 3. | Maron BJ. Hypertrophic cardiomyopathy. Lancet. 1997;350:127-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 279] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 4. | McKenna WJ, Coccolo F, Elliott PM. Genes and disease expression in hypertrophic cardiomyopathy. Lancet. 1998;352:1162-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Choudhury L, Mahrholdt H, Wagner A, Choi KM, Elliott MD, Klocke FJ, Bonow RO, Judd RM, Kim RJ. Myocardial scarring in asymptomatic or mildly symptomatic patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2002;40:2156-2164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 456] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 6. | Lim DS, Roberts R, Marian AJ. Expression profiling of cardiac genes in human hypertrophic cardiomyopathy: insight into the pathogenesis of phenotypes. J Am Coll Cardiol. 2001;38:1175-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 115] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 7. | Tabata T, Oki T, Yamada H, Abe M, Onose Y, Thomas JD. Subendocardial motion in hypertrophic cardiomyopathy: assessment from long- and short-axis views by pulsed tissue Doppler imaging. J Am Soc Echocardiogr. 2000;13:108-115. [PubMed] |
| 8. | Vinereanu D, Florescu N, Sculthorpe N, Tweddel AC, Stephens MR, Fraser AG. Differentiation between pathologic and physiologic left ventricular hypertrophy by tissue Doppler assessment of long-axis function in patients with hypertrophic cardiomyopathy or systemic hypertension and in athletes. Am J Cardiol. 2001;88:53-58. [PubMed] |
| 9. | Saccheri MC, Cianciulli TF, Konopka IV, Vilkas Ansorena J, Serans DF, Acunzo RS, Redruello HJ, Prezioso HA. Evaluación de la función miocárdica regional con Doppler tisular en los 3 tipos más frecuentes de miocardiopatia hipertrófica. Rev Argent Cardiol. 2005;73:127. |
| 10. | Saccheri MC, Cianciulli TF, Lax JA, Guerra JE, Redruello HJ, Weich Glogier FL, Gagliardi JA, Dorelle AN, Prezioso HA, Vidal LA. Impaired myocardial function in hypertrophic cardiomyopathy. Echocardiography. 2009;26:657-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Yang H, Sun JP, Lever HM, Popovic ZB, Drinko JK, Greenberg NL, Shiota T, Thomas JD, Garcia MJ. Use of strain imaging in detecting segmental dysfunction in patients with hypertrophic cardiomyopathy. J Am Soc Echocardiogr. 2003;16:233-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 87] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Leitman M, Lysyansky P, Sidenko S, Shir V, Peleg E, Binenbaum M, Kaluski E, Krakover R, Vered Z. Two-dimensional strain-a novel software for real-time quantitative echocardiographic assessment of myocardial function. J Am Soc Echocardiogr. 2004;17:1021-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 852] [Cited by in RCA: 873] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 13. | Webb JG, Sasson Z, Rakowski H, Liu P, Wigle ED. Apical hypertrophic cardiomyopathy: clinical follow-up and diagnostic correlates. J Am Coll Cardiol. 1990;15:83-90. [PubMed] |
| 14. | Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise J. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2471] [Cited by in RCA: 2606] [Article Influence: 137.2] [Reference Citation Analysis (0)] |
| 15. | Marwick TH, Leano RL, Brown J, Sun JP, Hoffmann R, Lysyansky P, Becker M, Thomas JD. Myocardial strain measurement with 2-dimensional speckle-tracking echocardiography: definition of normal range. JACC Cardiovasc Imaging. 2009;2:80-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 392] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 16. | Prieto L, Lamarca R, Casado A. [Assessment of the reliability of clinical findings: the intraclass correlation coefficient]. Med Clin (Barc). 1998;110:142-145. [PubMed] |
| 17. | Popović ZB, Kwon DH, Mishra M, Buakhamsri A, Greenberg NL, Thamilarasan M, Flamm SD, Thomas JD, Lever HM, Desai MY. Association between regional ventricular function and myocardial fibrosis in hypertrophic cardiomyopathy assessed by speckle tracking echocardiography and delayed hyperenhancement magnetic resonance imaging. J Am Soc Echocardiogr. 2008;21:1299-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 192] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 18. | Afonso LC, Bernal J, Bax JJ, Abraham TP. Echocardiography in hypertrophic cardiomyopathy: the role of conventional and emerging technologies. JACC Cardiovasc Imaging. 2008;1:787-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | Saccheri MC, Cianciulli TF, Beck MA, Lax JA, Dorelle AN, Chazarreta EL, Prezioso HA, Vidal LA. Strain bidimensional en las miocardiopatías hipertróficas. Rev Argent Cardiol. 2009;77:121. |
| 20. | The Endomyocardial biopsy: Techniques and role in diagnosis of heart diseases. In: Cardiovascular Pathology. Virmany R, Burke A, Farb A, Atkinson JB. Second Edition. Volume 40 in the serie: Major problems in pathology. WB: Saunders Company 2001; 301-302. |
| 21. | Phadke RS, Vaideeswar P, Mittal B, Deshpande J. Hypertrophic cardiomyopathy: an autopsy analysis of 14 cases. J Postgrad Med. 2001;47:165-170. [PubMed] |
| 22. | Tazelaar HD, Billingham ME. The surgical pathology of hypertrophic cardiomyopathy. Arch Pathol Lab Med. 1987;111:257-260. [PubMed] |
| 23. | Shirani J, Pick R, Roberts WC, Maron BJ. Morphology and significance of the left ventricular collagen network in young patients with hypertrophic cardiomyopathy and sudden cardiac death. J Am Coll Cardiol. 2000;35:36-44. [PubMed] |
| 24. | Hauck AD, Edwards WD, Histopathologic examination of tissue obtained by endomyocardial biopsy. In: Fowles RE. Cardiac biopsy. Mount Kisco: Futura Publishing CO 1992; 95-153. |