Published online Apr 26, 2017. doi: 10.4330/wjc.v9.i4.332
Peer-review started: October 21, 2016
First decision: December 15, 2016
Revised: December 21, 2016
Accepted: January 11, 2017
Article in press: January 14, 2017
Published online: April 26, 2017
Processing time: 196 Days and 10 Hours
To demonstrate the feasibility of blood conservation methods and practice across all ages and risk categories in congenital cardiac surgery.
We retrospectively analyzed a collected database of 356 patients who underwent cardiac surgery using cardiopulmonary bypass (CPB) from 2010-2015. The patients were grouped into blood conservation (n = 138) and non-conservation (n = 218) groups and sub-grouped based on their ages and procedural complexity scores.
There were no statistical differences in gender, weight, pre-operative and pre-CPB hematocrit levels in both groups. Despite equivalent hematocrit levels during and after CPB for both groups, there was significantly less operative homologous blood utilized in blood conservation group across all ages and complexity levels.
Blood conservation surgery can be performed in congenital patients needing cardiac surgery in all age groups and complexity categories. The above findings in addition to attendant risks and side effects of blood transfusion and the rising cost of safer blood products justify blood conservation in congenital cardiac surgery.
Core tip: We evaluated the feasibility of blood conservation pediatric cardiac surgery for all age groups and complexity levels in this retrospective study. We reviewed 356 patients who underwent cardiac surgery from 2010-2015. The patients were grouped into historical non-conservation (NC = 218) and blood conservation (BC = 138) cohorts. The blood conservation was performed by miniaturizing bypass circuit, changing the trigger point for transfusion and adapting protocols and guidelines accepted and implemented by the group. We demonstrated that the blood conservation practice can be performed safely in all ages and complexity levels by reducing cardiopulmonary bypass prime volume and institutional commitment to guidelines and practice of blood conservation cardiac surgery.
- Citation: Karimi M, Sullivan JM, Linthicum C, Mathew A. Blood conservation pediatric cardiac surgery in all ages and complexity levels. World J Cardiol 2017; 9(4): 332-338
- URL: https://www.wjgnet.com/1949-8462/full/v9/i4/332.htm
- DOI: https://dx.doi.org/10.4330/wjc.v9.i4.332
There are accumulating evidences of the association of red blood cell (RBC) transfusion with adverse outcomes in both adult and pediatric patients undergoing cardiac surgery[1-5]. The increasing costs associated with blood transfusion and the need for preservation of limited blood supplies have mandated that RBC transfusion to be included as a quality indicator in cardiac surgery[6].
There are many blood conservation strategies available for children undergoing cardiac surgery depending on age and type of surgery. The main goal of blood conservation is to minimize exposure to allogeneic transfusion while maximizing the use of autologous red cells. Although, the effects and costs of all these methods have not yet been completely assessed, many of these strategies have been implemented in clinical practice collectively with great efficacy.
The purpose of this single-center study is to demonstrate the feasibility of blood conservation cardiac surgery practice across different age groups and complexity scores in congenital cardiac surgery.
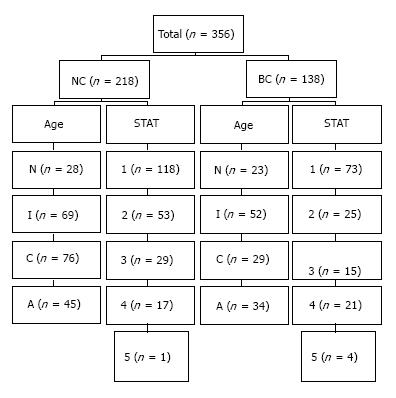
Retrospective analysis of 356 patients who underwent open heart surgery from 2010 to 2015 was investigated. The patients were categorized into blood conservation (BC) and non-conservation (NC) groups and subcategorized by their different age categories and the Society of Thoracic Surgeons-European Association for Cardio-Thoracic Surgery Congenital Heart Surgery Mortality scores (STAT Complexity Scores) (Figure 1). The NC group (n = 218) underwent surgical procedures between 2010 and 2014 using conventional cardiopulmonary bypass (CPB) without utilizing intra-operative blood conservation methods or protocols. The BC group (n = 138) underwent surgical procedures between 2014 and 2015 by incorporating blood conservation equipment, techniques, and intra-operative guidelines for homologous RBC transfusion.
The patients were analyzed for the amount of intraoperative RBC usage based on by their age categories and STAT complexity scores. There were no changes in clinical personnel as far as anesthesia, perfusion, intensive care, or cardiology care givers for both groups. A comprehensive database including demographics and intra-operative data was created for all the patients in the cohort using electronic and paper medical records. All the data collection was complete for the primary outcome of total intraoperative RBC usage in eligible patients undergoing cardiac surgery. The subjects requiring extracorporeal membrane oxygenator before or after surgery were excluded from the study groups. The institutional review board has exempted patients’ consent and approved the study.
Intraoperative data included CPB and aortic cross-clamp (CC) times, hematocrit levels, and amount of homologous RBC transfusion. Preoperative hematocrit was measured by the preoperative work up in the core laboratory. Pre-bypass hematocrit was defined as the patient hematocrit measure by the blood gas analyzer prior to CPB. On bypass hematocrit was defined as hematocrit immediately after initiation of CPB. Post-bypass hematocrit was defined as the hematocrit prior to leaving the operating room suite. Intraoperative RBC transfusion was defined as the total amount of homologous RBC that the patient received from the time of arrival to the operating room until leaving the operating room, including prime volume for the CPB circuit. All the patients received irradiated and leukocyte depleted RBC based on the institutional blood bank protocol.
A strategic protocol by the surgeons, anesthesiology, and perfusion staff was formulated and agreed upon to achieve a reduction in hemodilution and trigger points for RBC transfusion. The formulation of the plan was divided into equipment and technique.
The Terumo System One Heart Lung Machine (Terumo Cardiovascular, Ann Arbor, MI) was modified and positioned close to the operating table to reduce tubing lengths. Four different arterio-venous loops were customized specific to the weight of the patient. The Terumo FX05 (weight < 12 kg), FX15 (weight 12-75 kg), and FX25 (weight > 75 kg) oxygenators with integrated arterial filter were utilized for the CPB runs. The Terumo Capiox CP50 was configured for the administration of cold cardioplegia and modified ultrafiltration (MUF). The Hemocor HPH 400TS (Minntech Corporation, Minneapolis, MN) was used to remove excess fluid from the circuit. The Haemonetics Cell Saver 5 (Haemonetics Corporation, Braintree, MA) has allowed for successful return of shed blood during and after surgery. Continuous arterial and venous blood gas monitoring (CDI 500 Terumo Cardiovascular, Ann Arbor, MI), and cerebral saturation monitoring (Somanetics INVOS 5100 C system, Somanetics Corporation, Troy, MI) provided additional hemodynamic information regarding adequacy of patient oxygenation and perfusion in order to tailor the need for blood transfusion. Utilization of point of care testing with i-STAT® (Abbott Point of Care, Princeton, NJ) and the Hemochron Signature Elite (ITC, Edison, NJ) allowed us for micro sampling of 0.5 mL of patient blood throughout the operative management. The differences in perfusion equipment for the two eras are depicted in Table 1.
The anesthesiology staff made every effort to minimize the amount of intravenous crystalloid infusion at the induction and throughout the operation to minimize hemodilution. Our current practice allows the primary perfusion staff to customize the patient circuitry with four available tubing packs and three oxygenators. These selections provided optimal circuit configuration based on patient size in order to decrease hemodilution while working safely within Food and Drug Administration (FDA) product specifications. The differences in priming volume between the two groups are demonstrated in Table 2. The circuit is positioned closed to the operating bed while avoiding crowdedness around the surgeon and assistant. Incorporating vacuum assisted drainage has made it possible to increase the height of the oxygenator to the level of the patient decreasing the arterial and venous tubing length and significantly reducing hemodilution. Retrograde arterial and venous priming have been instrumental in displacing the crystalloid priming volume of the circuit with the patients’ own blood reducing hemodilution effect at the initial stage of CPB.
| Body weight (kg) | NC (mL) | BC (mL) | Reduction (%) |
| Neonate-12 | 400 | 160 | 60 |
| 12-35 | 600 | 445 | 26 |
| 35-55 | 800 | 520 | 35 |
| 55-75 | 1000 | 765 | 24 |
| > 75 | 1000 | 880 | 12 |
We also aggressively ultrafiltrated the added crystalloid volume to the circuit to maintain an even fluid balance throughout the case. In addition, we performed arteriovenous MUF on all patients at the conclusion of the CPB. We routinely ultrafiltrated the remainder of the volume in the circuit and checked the hematocrit to ensure it was greater than or equal to the patient’s most recent hematocrit before reinfusion. This whole blood containing clotting factors and the cell saver processed blood are given to the patient prior to leaving the operating room or taken to the intensive care unit for postoperative transfusion as needed.
Standard descriptive statistics were used for patient demographic information. Values were calculated as mean ± SD. Continuous variables were compared between blood conservation and non-conservation groups using independent sample t-tests, for each of the 4 age groups (neonate, infant, child, and adolescent) and 5 STAT categories. P value < 0.05 was considered to be statistically significant. SPSS software (IBM, Armonk, New York) was used for statistical analysis.
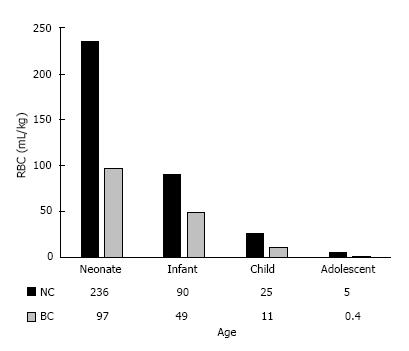
There were a total of 356 patients with 218 patients in NC and 138 in BC arms. The breakdown of the patients in the ages and STAT categories for both groups are depicted in Figure 1. There were in general no statistically discernible differences in gender, weight, preoperative hematocrit, or pre-bypass hematocrit levels between the two groups across all ages and STAT categories. There was a trend toward longer bypass and cross clamp times in neonates, infants, and patients with STAT scores of 3 and 4 in NC cohort than BC counterpart, but did not consistently reach statistical significance (Tables 3 and 4). The neonates in NC group had higher post-bypass hematocrit (P = 0.001) despite comparable on-bypass hematocrit due to usage of larger volume of RBC (P > 0.001). The infants in BC group were younger (P = 0.001) and had shorter CBP and CC times and much less RBC transfusion (P < 0.001) despite comparable on-bypass hematocrits. The children in BC group had shorter bypass time (P = 0.04) but comparable CC time, on-bypass, and post-bypass hematocrit, but statistically less RBC usage (P = 0.002). Interestingly, the adolescence patients had higher on-bypass and post-bypass hematocrit (P < 0.001) despite less RBC usage (P = 0.02) signifying the efficacy of our circuit and protocol to conserve blood (Table 3). Overall, there was significantly less homologous RBC utilization across all age groups in BC than NC cohorts (Figure 2).
| Operative variables | NC neonate n = 28 (12%) | BC neonate n = 23 (16%) | P | NC infant n = 69 (32%) | BC infant n = 52 (38%) | P | NC child n = 76 (35%) | BC child n = 29 (21%) | P | NC adolescent n = 45 (21%) | BC adolescent n = 34 (25%) | P |
| Average age (d) | 10 ± 9 | 12 ± 10 | 0.08 | 187 ± 109 | 139 ± 86 | 0.001 | 1638 ± 806 | 1730 ± 1012 | 0.44 | 6280 ± 1977 | 6671 ± 3103 | 0.78 |
| Weight (kg) | 3 ± 0.7 | 3 ± 1 | 0.94 | 6 ± 2 | 6 ± 2 | 0.3 | 19 ± 9 | 20 ± 9 | 0.49 | 60 ± 20 | 57 ± 17 | 0.19 |
| % Male | 60% | 52% | 0.35 | 45% | 35% | 0.59 | 45% | 52% | 0.83 | 69% | 47% | 0.07 |
| Bypass (min) | 161 ± 74 | 136 ± 53 | 0.15 | 124 ± 66 | 85 ± 39 | < 0.001 | 83 ± 48 | 71 ± 45 | 0.04 | 116 ± 63 | 106 ± 59 | 0.54 |
| Cross clamp (min) | 75 ± 47 | 75 ± 39 | 0.99 | 74 ± 45 | 45 ± 30 | < 0.001 | 37 ± 38 | 30 ± 32 | 0.16 | 46 ± 53 | 57 ± 46 | 0.3 |
| Pre-operative Hct (%) | 32 ± 2 | 37 ± 5 | 0.11 | 30 ± 5 | 32 ± 7 | 0.19 | 31 ± 4 | 31 ± 5 | 0.63 | 32 ± 7 | 34 ± 4 | 0.26 |
| Pre-bypass Hct (%) | 36 ± 7 | 35 ± 6 | 0.19 | 30 ± 6 | 31 ± 7 | 0.89 | 33 ± 5 | 31 ± 5 | 0.1 | 34 ± 5 | 33 ± 3 | 0.54 |
| On-bypass Hct (%) | 25 ± 3 | 23 ± 3 | 0.07 | 25 ± 3 | 23 ± 3 | 0.09 | 24 ± 3 | 23 ± 3 | 0.1 | 25 ± 3 | 28 ± 3 | < 0.001 |
| Post-bypass Hct (%) | 47 ± 4 | 34 ± 5 | 0.001 | 35 ± 5 | 34 ± 4 | 0.3 | 31 ± 5 | 32 ± 4 | 0.75 | 28 ± 3 | 34 ± 4 | < 0.001 |
| Operative RBC (mL/kg) | 236 ± 220 | 97 ± 34 | 0.01 | 90 ± 58 | 49 ± 24 | < 0.001 | 25 ± 26 | 11 ± 10 | 0.01 | 5 ± 13 | 0.4 ± 2 | 0.02 |
| RBC exposure (unit) | 2 ± 0.5 | 1.0 ± 0.2 | < 0.001 | 1.6 ± 0.7 | 0.8 ± 0.3 | < 0.001 | 1.2 ± 0.9 | 0.6 ± 0.6 | 0.002 | 0.9 ± 2 | 0.04 ± 0.2 | 0.02 |
| Operative variables | NC STAT 1 n = 118 (54%) | BC STAT 1 n = 73 (53%) | P | NC STAT 2 n = 53 (24%) | BC STAT 2 n = 25 (18%) | P | NC STAT 3 n = 29 (13%) | BC STAT 3 n = 15 (11%) | P | NC TAT 4 n = 17 (8%) | BC STAT 4 n = 21 (15%) | P |
| Average age (d) | 2419 ± 2667 | 3088 ± 3498 | 0.12 | 1500 ± 1947 | 1012 ± 2176 | 0.001 | 899 ± 1762 | 619 ± 1659 | 0.62 | 1724 ± 3559 | 1447 ± 2817 | 0.81 |
| Weight (kg) | 27 ± 26 | 29 ± 24 | 0.53 | 17 ± 18 | 15 ± 23 | 0.58 | 11 ± 13 | 9 ± 12 | 0.65 | 16 ± 25 | 14 ± 21 | 0.81 |
| % Male | 58% | 42% | 0.07 | 47% | 56% | 0.63 | 45% | 40% | 0.76 | 47% | 38% | 0.74 |
| Bypass (min) | 82 ± 37 | 74 ± 39 | 0.2 | 127 ± 68 | 94 ± 47 | 0.03 | 185 ± 73 | 131 ± 73 | 0.02 | 155 ± 66 | 132 ± 49 | 0.22 |
| Cross clamp (min) | 42 ± 29 | 38 ± 31 | 0.35 | 59 ± 55 | 43 ± 43 | 0.21 | 87 ± 64 | 81 ± 45 | 0.77 | 81 ± 58 | 71 ± 37 | 0.52 |
| Pre-operative Hct (%) | 31 ± 4 | 31 ± 4 | 0.77 | 29 ± 5 | 36 ± 8 | 0.09 | 34 ± 11 | 34 ± 6 | 0.95 | 37 ± 8 | 37 ± 7 | 0.7 |
| Pre-bypass Hct (%) | 31 ± 4 | 30 ± 4 | 0.62 | 35 ± 8 | 33 ± 7 | 0.39 | 35 ± 7 | 32 ± 6 | 0.14 | 35 ± 7 | 35 ± 6 | 0.7 |
| On-bypass Hct (%) | 24 ± 3 | 25 ± 4 | 0.07 | 25 ± 4 | 25 ± 4 | 0.45 | 25 ± 4 | 23 ± 3 | 0.04 | 25 ± 3 | 24 ± 4 | 0.37 |
| Post-bypass Hct (%) | 31 ± 4 | 33 ± 3 | 0.02 | 35 ± 5 | 36 ± 4 | 0.63 | 38 ± 5 | 32 ± 3 | 0.02 | 39 ± 12 | 34 ± 5 | 0.39 |
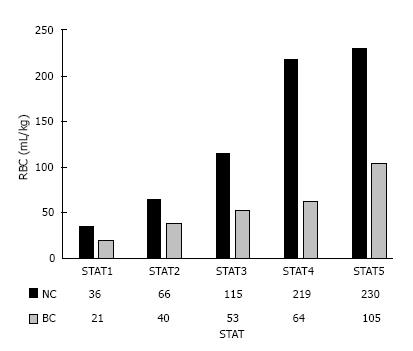
| Operative RBC (mL/kg) | 36 ± 50 | 21 ± 30 | 0.02 | 66 ± 65 | 40 ± 39 | 0.03 | 115 ± 78 | 53 ± 31 | 0.03 | 219 ± 301 | 64 ± 47 | 0.05 |
| RBC exposure (unit) | 0.9 ± 0.7 | 0.5 ± 0.6 | < 0.001 | 1.7 ± 1 | 0.7 ± 0.4 | < 0.001 | 2.2 ± 2.2 | 0.8 ± 0.5 | < 0.001 | 2.0 ± 0.9 | 0.7 ± 0.4 | < 0.001 |
The data was also further analyzed looking at the differences in complexity of the procedures based on STAT mortality scores (1, least complex - 5, more complex) (Table 4). Patients in BC STAT 1 complexity level had higher post-bypass hematocrit (P = 0.02) with comparable on-bypass hematocrit despite less RBC usage (P < 0.001). Patients in BC STAT 2 group were younger (P = 0.001) and had shorter bypass time (P = 0.03), which was also evident in BC STAT 3 category (P = 0.02). The BC STAT 1-4 categories in general had equivalent on-bypass hematocrit with less intraoperative RBC transfusion, which were all statistically significant. The STAT 5 groups could not be compared due to lack of sufficient subjects and power in NC group. Overall, there was significantly less homologous RBC utilization in BC group than NC group across all STAT complexity scores (Figure 3).
Blood conservation in pediatric cardiac surgery has been a panacea and quest of cardiac surgeon due to societal and institutional push for quality care. Despite its challenges, blood conservation cardiac surgery has been practiced in all stages of cardiac surgery in adult and pediatric in certain circumstances with a great success[7,8]. There is a great variability in practice of blood transfusion for a given diagnostic code and complexity and pediatric population is no exception to the rule[9]. By far, pediatric patients undergoing cardiac surgery are exposed to more blood transfusion intra and post-operatively with no consensus or scientific evidence to what would be the optimal hematocrit level across different diagnosis and physiologic status[10-15].
In this retrospective study we have looked at the effectiveness of intra-operative blood conservation practice as compared to the historical non-conservation cohort. We have adapted novel techniques in CPB by miniaturizing and customizing the circuit to the patient’s weight and using parameters such as mixed venous saturation, regional cerebral saturations, and serum lactic acid levels to tailor our decision about RBC transfusion. The bypass circuit was primed with the patients’ own blood by performing retrograde arterial and venous priming once the aortic and venous cannulas were in place for majority of the operative procedures. We also performed aggressive hemofiltration during the bypass run and performed arteriovenous MUF after termination of CPB to remove excessive intravascular volume. Pediatric cell saver also was used throughout the operation and the salvaged blood was infused before leaving the operating room or immediately after arrival to the PICU. The efficacies of these conservation measures and practices have been reported by others as the result of greater emphasis that has recently been place in performing blood conservation cardiac surgery in pediatric population[16-19].
Our general trigger point for RBC transfusion was hematocrit of less than 21% for older age and low complexity category patients with biventricular physiology, and less than 25% for neonates, high complexity, and cyanotic univentricular patients. This protocol was followed as long other critical parameters of adequate systemic and cerebral perfusion remained within acceptable range. There were no patients in the blood conservation group whom experienced adverse neurologic events or other complications as the result of these changes in philosophy of RBC transfusion trigger points. We consistently maintained mixed venous saturation at greater than 60% and regional cerebral saturation at greater than the baseline level by increasing flow and cerebral vasodilation using pH-stat during cooling. The trend in serum lactate level after CPB was also used to determine the need for blood transfusion prior to leaving the operating room. Implementation of intraoperative transfusion algorithms in pediatric cardiac operations has also been shown to significantly reduce perioperative blood product use and morbidity[20]. Similarly, a comprehensive intraoperative blood-sparing approach that resulted in no transfusion in 25% of children undergoing cardiac operations compared with children who received a transfusion during the surgery demonstrated a shorter length of postoperative mechanical ventilation and a shorter PICU stay[21]. This has also been demonstrated that blood conservation in pediatric cardiac surgery was associated with a decrease in post-operative inotropic needs, days on ventilator, and length of stay in patients with biventricular physiology[22].
Historically, hemodilution during CPB was introduced to decrease homologous blood use and has been thought to improve microcirculatory flow[23,24]. Hemodilution also potentially could reduce perfusion pressure, which increases the risk of an adverse neurologic outcome after CPB, increases cerebral blood flow and thereby increases the microembolic load to the brain, and reduces the oxygen carrying capacity of blood which might critically limit oxygen delivery to neurons and other cells[25]. Intraoperative implementation of hemodilution restriction prior to CPB to maintain the hematocrit close to preoperative hematocrit is paramount to a successful blood conservation cardiac surgery practice. By limiting crystalloid infusion during and after anesthesia induction, we significantly reduced the additional hemodilution that the patient will invariably face upon initiation of CPB. Having higher hematocrit prior to CPB will allow for retrograde priming of the bypass circuit and higher hematocrit during and after bypass. This was consistently achieved with our protocol and circuit modification throughout the operation despite lower RBC utilization signifying the efficacy of our conservation strategies across all ages and complexity levels.
This study carries some of the known limitations of a retrospective study design. It precludes accurate assessment of practice pattern and trigger points for RBC transfusion in the non-conservation group. There were also differences in surgeons as well as perfusion techniques and equipment that collectively could affect the variability in RBC transfusion trigger point and practices. Also, because of the lack of electronic charting and the absence of specific intraoperative measurements (i.e., cerebral and somatic saturation, serum lactic acid level) for non-conservation cohorts, we could not perform any statistical comparison for some variables between the two groups.
This study has shown that blood conservation in pediatric cardiac surgery is reproducible across different ages and complexity categories. Miniaturization of the CPB circuit, contemporary techniques and equipment, and institutional commitments and protocols were paramount in establishing a successful blood conservation program. Future improvements in perfusion technology and blood conservation protocols in association with additional prospective randomized trials will further capitalize our understanding of the benefit of blood conservation in pediatric cardiac surgery.
Transfusion of homologous red blood cell has been associated with increase in morbidity in pediatric patients undergoing cardiac surgery. Pediatric cardiac surgical patients are at high risk of receiving blood transfusion as the result of cardiopulmonary bypass. Blood conservation surgery practice has been encouraged to reduced or eliminate related risks.
The principle author has demonstrated previously that blood conservation in pediatric cardiac operations is associated with fewer ventilator days, lower inotropic scores, and shorter lengths of stay in patients with biventricular physiology. This study has expanded the safety and applicability of blood conservation cardiac surgery practice to all ages and complexity levels in patients with biventricular and univentricular hearts.
Blood conservation cardiac surgery has been a quest of surgeons for a long time due to associated risks inherent to homologous blood transfusion. There are sporadic successful reports of blood conservation surgery in pediatric population with no concrete methodology or guidelines accepted by most practices to adapt and implement it in their practices. In most part, there are no accepted guidelines in what would be a safe hematocrit range during circulatory support in order to avoid cerebral and end organ ischemic injuries. This single-intuition retrospective study is the only study that has demonstrated blood conservation pediatric cardiac surgery can safely be performed in all ages and complexity categories in a wide spectrum of structural congenital cardiac defects.
The authors present their experience with implementing a blood conservation strategy for pediatric cardiac surgery at their institution. Overall, the manuscript is best categorized as a quality improvement evaluation.
Manuscript source: Unsolicited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Chello M, Lin JA, Said SAM S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Iyengar A, Scipione CN, Sheth P, Ohye RG, Riegger L, Bove EL, Devaney EJ, Hirsch-Romano JC. Association of complications with blood transfusions in pediatric cardiac surgery patients. Ann Thorac Surg. 2013;96:910-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 2. | Salvin JW, Scheurer MA, Laussen PC, Wypij D, Polito A, Bacha EA, Pigula FA, McGowan FX, Costello JM, Thiagarajan RR. Blood transfusion after pediatric cardiac surgery is associated with prolonged hospital stay. Ann Thorac Surg. 2011;91:204-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 123] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 3. | Székely A, Cserép Z, Sápi E, Breuer T, Nagy CA, Vargha P, Hartyánszky I, Szatmári A, Treszl A. Risks and predictors of blood transfusion in pediatric patients undergoing open heart operations. Ann Thorac Surg. 2009;87:187-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 118] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 4. | Kilic A, Whitman GJ. Blood transfusions in cardiac surgery: indications, risks, and conservation strategies. Ann Thorac Surg. 2014;97:726-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 5. | Horvath KA, Acker MA, Chang H, Bagiella E, Smith PK, Iribarne A, Kron IL, Lackner P, Argenziano M, Ascheim DD. Blood transfusion and infection after cardiac surgery. Ann Thorac Surg. 2013;95:2194-2201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 232] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 6. | Shander AS, Goodnough LT. Blood transfusion as a quality indicator in cardiac surgery. JAMA. 2010;304:1610-1611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Ovrum E, Holen EA, Lindstein Ringdal MA. Elective coronary artery bypass surgery without homologous blood transfusion. Early results with an inexpensive blood conservation program. Scand J Thorac Cardiovasc Surg. 1991;25:13-18. [PubMed] |
| 8. | Gombotz H, Rigler B, Matzer C, Metzler H, Winkler G, Tscheliessnigg KH. [10 years’ experience with heart surgery in Jehovah’s witnesses]. Anaesthesist. 1989;38:385-390. [PubMed] |
| 9. | McQuilten ZK, Andrianopoulos N, Wood EM, Cole-Sinclair MF, McNeil JJ, Cameron PA, Reid CM, Newcomb AE, Smith JA, Phillips LE. Transfusion practice varies widely in cardiac surgery: Results from a national registry. J Thorac Cardiovasc Surg. 2014;147:1684-1690.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Mazine A, Rached-D’Astous S, Ducruet T, Lacroix J, Poirier N. Blood Transfusions After Pediatric Cardiac Operations: A North American Multicenter Prospective Study. Ann Thorac Surg. 2015;100:671-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 11. | Bateman ST, Lacroix J, Boven K, Forbes P, Barton R, Thomas NJ, Jacobs B, Markovitz B, Goldstein B, Hanson JH. Anemia, blood loss, and blood transfusions in North American children in the intensive care unit. Am J Respir Crit Care Med. 2008;178:26-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 202] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 12. | Demaret P, Tucci M, Ducruet T, Trottier H, Lacroix J. Red blood cell transfusion in critically ill children (CME). Transfusion. 2014;54:365-375; quiz 364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Cholette JM, Rubenstein JS, Alfieris GM, Powers KS, Eaton M, Lerner NB. Children with single-ventricle physiology do not benefit from higher hemoglobin levels post cavopulmonary connection: results of a prospective, randomized, controlled trial of a restrictive versus liberal red-cell transfusion strategy. Pediatr Crit Care Med. 2011;12:39-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 14. | Meliones JN, Nichols DG, Wetzel RC. Perioperative management of patients with congenital heart disease: a multidisciplinary approach. Critical heart disease in infants and children. St. Louis, MO: Mosby 1995; 553-579. |
| 15. | Paridon SM. Consequence of chronic hypoxemia and pulmonary vascular disease. The science and practice of pediatric cardiology. Baltimore, MD: Williams & Wilkins 1998; 2261-2272. |
| 16. | Redlin M, Habazettl H, Boettcher W, Kukucka M, Schoenfeld H, Hetzer R, Huebler M. Effects of a comprehensive blood-sparing approach using body weight-adjusted miniaturized cardiopulmonary bypass circuits on transfusion requirements in pediatric cardiac surgery. J Thorac Cardiovasc Surg. 2012;144:493-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Richmond ME, Charette K, Chen JM, Quaegebeur JM, Bacha E. The effect of cardiopulmonary bypass prime volume on the need for blood transfusion after pediatric cardiac surgery. J Thorac Cardiovasc Surg. 2013;145:1058-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Ye L, Lin R, Fan Y, Yang L, Hu J, Shu Q. Effects of circuit residual volume salvage reinfusion on the postoperative clinical outcome for pediatric patients undergoing cardiac surgery. Pediatr Cardiol. 2013;34:1088-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Cholette JM, Powers KS, Alfieris GM, Angona R, Henrichs KF, Masel D, Swartz MF, Daugherty LE, Belmont K, Blumberg N. Transfusion of cell saver salvaged blood in neonates and infants undergoing open heart surgery significantly reduces RBC and coagulant product transfusions and donor exposures: results of a prospective, randomized, clinical trial. Pediatr Crit Care Med. 2013;14:137-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 20. | Whitney G, Daves S, Hughes A, Watkins S, Woods M, Kreger M, Marincola P, Chocron I, Donahue B. Implementation of a transfusion algorithm to reduce blood product utilization in pediatric cardiac surgery. Paediatr Anaesth. 2013;23:639-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Redlin M, Kukucka M, Boettcher W, Schoenfeld H, Huebler M, Kuppe H, Habazettl H. Blood transfusion determines postoperative morbidity in pediatric cardiac surgery applying a comprehensive blood-sparing approach. J Thorac Cardiovasc Surg. 2013;146:537-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 22. | Karimi M, Florentino-Pineda I, Weatherred T, Qadeer A, Rosenberg CA, Hudacko A, Ryu D. Blood conservation operations in pediatric cardiac patients: a paradigm shift of blood use. Ann Thorac Surg. 2013;95:962-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Neptune WB, Bougas JA, Panico FG. Open-heart surgery without the need for donor-blood priming in the pump oxygenator. N Engl J Med. 1960;263:111-115. [PubMed] [DOI] [Full Text] |
| 24. | Cooper JR, Slogoff S. Hemodilution and priming solutions for cardiopulmonary bypass. Cardiopulmonary bypass principles and practice. Baltimore, MD: Williams and Wilkins 1993; 124-137. |
| 25. | Sungurtekin H, Cook DJ, Orszulak TA, Daly RC, Mullany CJ. Cerebral response to hemodilution during hypothermic cardiopulmonary bypass in adults. Anesth Analg. 1999;89:1078-1083. [PubMed] |