Published online Mar 26, 2017. doi: 10.4330/wjc.v9.i3.212
Peer-review started: October 31, 2016
First decision: December 1, 2016
Revised: December 15, 2016
Accepted: January 11, 2017
Article in press: January 14, 2017
Published online: March 26, 2017
Processing time: 150 Days and 3.3 Hours
Transcatheter aortic valve replacement (TAVR) has been validated as a new therapy for patients affected by severe symptomatic aortic stenosis who are not eligible for surgical intervention because of major contraindication or high operative risk. Patient selection for TAVR should be based not only on accurate assessment of aortic stenosis morphology, but also on several clinical and functional data. Multi-Imaging modalities should be preferred for assessing the anatomy and the dimensions of the aortic valve and annulus before TAVR. Ultrasounds represent the first line tool in evaluation of this patients giving detailed anatomic description of aortic valve complex and allowing estimating with enough reliability the hemodynamic entity of valvular stenosis. Angiography should be used to assess coronary involvement and plan a revascularization strategy before the implant. Multislice computed tomography play a central role as it can give anatomical details in order to choice the best fitting prosthesis, evaluate the morphology of the access path and detect other relevant comorbidities. Cardiovascular magnetic resonance and positron emission tomography are emergent modality helpful in aortic stenosis evaluation. The aim of this review is to give an overview on TAVR clinical and technical aspects essential for adequate selection.
Core tip: Transcatheter aortic valve replacement (TAVR) has been validated as a new therapy for patients affected by severe symptomatic aortic stenosis who are not eligible for surgical intervention because of major contraindication or high operative risk. Patient selection for TAVR should be based not only on accurate assessment of aortic stenosis morphology, but also on several clinical and functional data. Multi-Imaging modalities are preferred for assessing the anatomy and the dimensions of the aortic valve and annulus before TAVR. The aim of this review is to give an overview on TAVR clinical and technical aspects essential for adequate selection.
- Citation: Cocchia R, D’Andrea A, Conte M, Cavallaro M, Riegler L, Citro R, Sirignano C, Imbriaco M, Cappelli M, Gregorio G, Calabrò R, Bossone E. Patient selection for transcatheter aortic valve replacement: A combined clinical and multimodality imaging approach. World J Cardiol 2017; 9(3): 212-229
- URL: https://www.wjgnet.com/1949-8462/full/v9/i3/212.htm
- DOI: https://dx.doi.org/10.4330/wjc.v9.i3.212
Transcatheter aortic valve replacement (TAVR) has been validated as a new therapy for patients affected by severe symptomatic aortic stenosis who are not eligible for surgical intervention because of major contraindication or high operative risk[1,2]. Recently this option, performed in experienced centers, using next generation devices has demonstrated to be not inferior to standard surgery also in intermediate-risk patients[3].
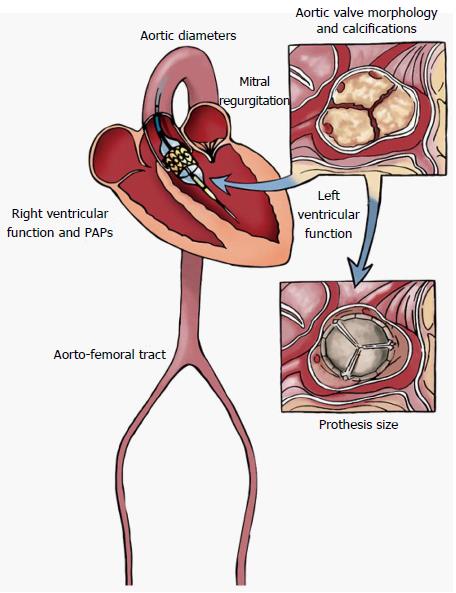
The safety and efficacy of prosthesis implantation depends on a proper patient selection and procedural guidance, based on a multimodality imaging approach[4,5]. A precise measurements of annulus and aortic root allow to make a correct “sizing”, that means to choose the best fitting prosthesis in native aortic seat, representing one of the most important predictor of a successful procedure[6,7].
Patient selection requires a multidisciplinary team approach including interventional cardiologists, surgeons, anesthesiologists and imaging specialists in order to delineate risk profile, study the anatomy of aortic valve, aorta and peripheral vascular structures.
First line risk evaluation is usually performed using the Logistic European System for Cardiac Operative Risk Evaluation (EuroSCORE) and/or the STS Predicted Risk of Mortality Score, defining a high risk in case of logistic EuroSCORE ≥ 15%-20% or a STS score ≥ 10%. These scores present clear limitations mostly in elderly population and have not been created for TAVR procedures but for surgery so that their suitability in percutaneous valve implantation has been questioned and a risk overestimation suspected in this contest[8].
In patient with prior cardiac surgery, including degeneration of an implanted aortic bioprosthesis (valve in valve implantation), chest radiation therapy, porcelain aorta, liver cirrhosis, pulmonary hypertension and/or right ventricular dysfunction a TAVR approach should be reasonably preferred.
On the other hand, in elderly population, frailty has been associated with worst prognosis in several pathological conditions and also after TAVR and must be considered in patient evaluation. It can be definite as a syndrome of impaired physiologic reserve with decreased resistance to stressors[9] and can be quantified using a composite of four markers: Serum albumin, dominant hand grip strength, gait speed on a 15 ft (4.57 m) walk and independence in activities in daily living.These components can be summed to derive a frailty score (ranging 0 to 12) able to identify frail patients in case of score ≥ 5.
Moreover, patients with poor life expectancy (less than 1 year) or in which TAVR has not expected to significantly improve quality of life should be excluded from this selection[10].
Relative and absolute contraindications to TAVR are listed in Table 1.
| Absolute contraindications |
| Absence of heart team or surgery on the site |
| Estimated life expectancy < 1 yr |
| Improvement of quality of life by TAVI unlikely because of comorbidities |
| Severe primary associated disease of other valves with major contribution to the patient’s symptoms, that can be treated only by surgery |
| Inadequate annulus size (< 18 mm, > 29 mm) |
| Thrombus in the left ventricle |
| Active endocarditis |
| Elevated risk of coronary ostium obstruction (asymmetric valve calcification, short distance between annulus and coronary ostium, small aortic sinuses) |
| Plaques with mobile thrombi in the ascending aorta, or arch |
| For transfemoral/subclavian approach: inadequate vascular access (vessel size, calcification, tortuosity) |
| Relative contraindications |
| Bicuspid or non-calcified valves |
| Untreated coronary artery disease requiring revascularization |
| Haemodynamic instability |
| LVEF < 20% |
| For transapical approach: severe pulmonary disease, LV apex not accessible |
One of the main advantages of TAVR vs SAVR is the more rapid recovery from TAVR and this benefit is different according to access site and is greater for transfemoral approach. Transapical access for TAVR is an accepted approach for patients in whom vascular anatomy do not permit a transfemoral approach and if on one hand it avoids potential site complications of iliac and femoral vessels, on the other hand has some limitations including an increase in respiratory complications[11].
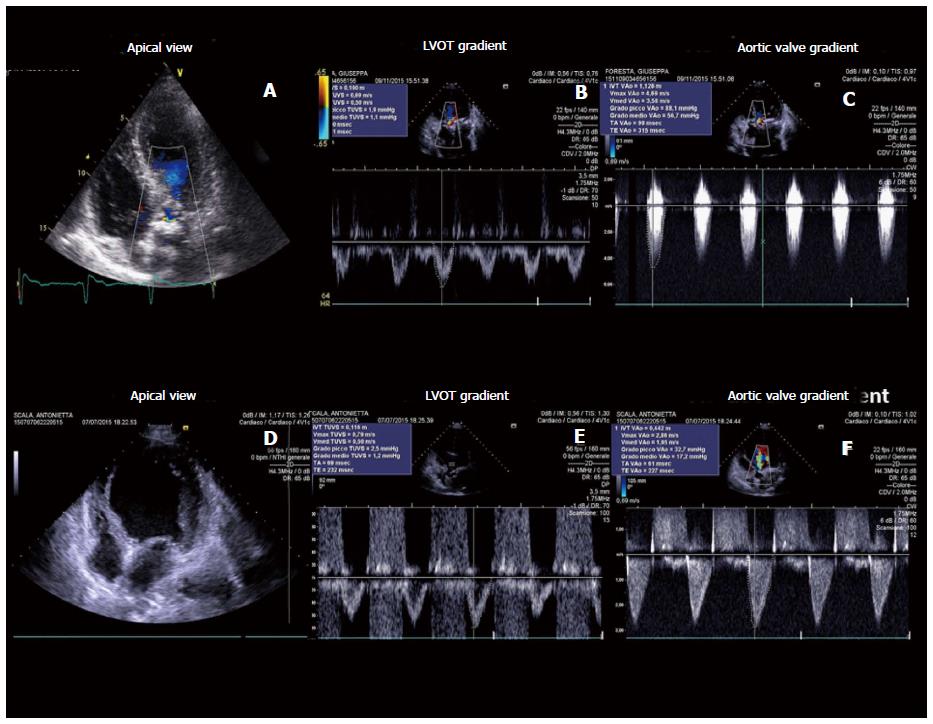
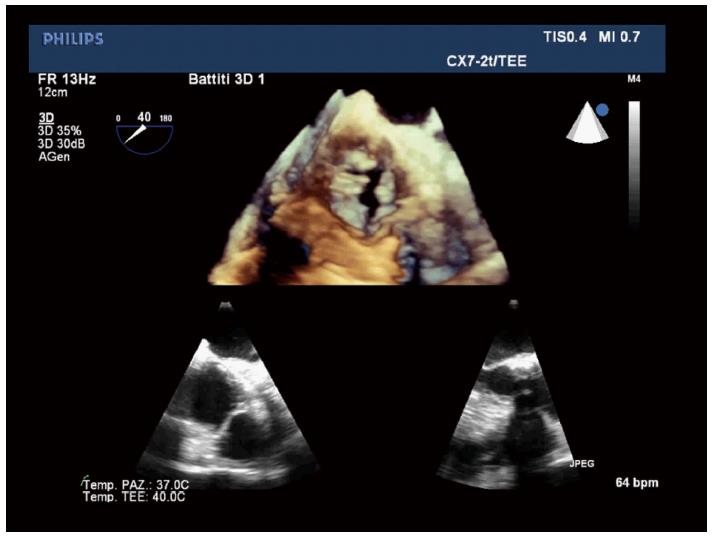
Echocardiography represents the first line tool in the setting of pre- and post-interventional evaluation and planning of Transcatheter Aortic Valve Replacement procedures (Figures 1-3).
Transthoracic echocardiography (TTE) gives detailed anatomic description of aortic valve complex and allows to estimate with enough reliability the haemodynamic entity of valvular stenosis.
An adequate TTE examination in a patient presenting with aortic valve stenosis should include information about valve anatomy (bicuspid or tricuspid valve) and severity of impairment of cusp motion. Moreover TTE provides an accurate evaluation of alterations in left and right ventricular morphology and function induced by the increase in afterload and allows to structurally and functionally investigate the other cardiac valves[12].
Ultrasounds allow to underlie factors associated with outcome: In a longitudinal study of echo parameters in cohort A of the PARTNER trial authors showed that the TAVR and the SAVR groups had different univariate factors associated with outcome. In fact, in TAVR group, baseline low peak gradients predicted worse outcome expressing a low stroke volume status while in SAVR population the strongest determinant of mortality was mitral regurgitation[13].
An appropriate haemodynamic evaluation of aortic valve stenosis requires the assessment of functional aortic valve area (AVA) or indexed AVA by body surface area, derived using continuity equation, peak transvalvular gradient and velocity (Vmax), mean transvalvular pressure gradient (MPG) and Stroke Volume index (SVi). According to latest recommendations by American College of Cardiology/American Heart Association, aortic valve stenosis is considered severe when Vmax is above 4 m/s, mean pressure exceeds 40 mmHg and estimated or measured AVA is under 1 cm2 (< 0.6 cm2/m2 if indexed for body surface area), assuming a normal left ventricular EF (LVEF)[14].
When performing continuity equation it should be remembered that diameter of left ventricular outflow tract (LVOT) diameter should be taken within 1 to 5 mm from aortic valve annulus in order to obtain maximum diameter[15]. LVOT often is elliptical so in case of measurement of the shortest dimension the continuity equation may still under-estimate the AVA and the stroke volume.
The calculation of the valvuloarterial impedance (Zva) should be part of a routine echocardiographicexaminationbecause this parameter provide an estimate of the global hemodynamic load[16] and can be an useful parameters in the evaluation of paradoxical aortic stenosis.
In clinical practice discordance between these parameters is often encountered so that commonly a severely restricted AVA can be found concomitantly with mean and peak pressure gradients falling into the moderate or mild category. This pattern is typically observed when systolic stroke volume and consequently transvalvular flow are reduced, thus realizing a so called low-flow low-gradient (LF-LG) aortic stenosis. In this condition visual assessment of structure, calcification and mobility of aortic valve is a crucial element as it can allow suspecting the diagnosis of severe aortic stenosis regardless of Doppler values.
Two forms of LF-LG aortic stenosis have been described[17]: (1) classical LF-LG aortic stenosis defined as an AVA < 1 cm2 in presence of LVEF < 50% and MPG < 40 mmHg or Vmax < 4 m/s; (2) paradoxical LF-LG aortic stenosis in presence of an AVA < 1 cm2, LVEF > 50%, a reduced left ventricular stroke volume (< 35 mL/m2), MPG < 40 mmHg or Vmax < 4 m/s. In this case stroke volume is low usually because of a markedly hypertrophied left ventricle with a small cavity that is unable to be filled appropriately and subsequently eject a normal stroke volume, in case of reduced volume load due to diuretic therapy[18,19] or in presence of a high valvulo-arterial impedance (ZVa > 5.5 mmHg/mL/mq)[20]. These patients seem to have a dismal prognosis, which can be improved by aortic valve replacement or TAVI, as demonstrated in a PARTNER study sub-group analysis[21].
On the other hand, a severely reduced functional AVA associated with low transvalvular gradient may be consequent to a reduced transvalvularflowdue to left ventricular dysfunction that cannot allow cusps opening, defined as “pseudo-severe” aortic stenosis. It is important to distinguish these two conditions since in this last case aortic valve intervention may not improve prognosis. In patients with reduced EF low-dose dobutamine stress echocardiography (≤ 20 μg/kg per minute) can be used to discriminate LF-LG severe aortic stenosis from pseudosevere aortic stenosis as,in the case of a severely stenotic valve, estimated AVA remains < 1 cm2 and contemporarily transvalvular gradient increase[14,10], but this variation can only be achieved in presence of a significant flow reserve (stroke volume increase > 20%).
In patients with asymptomatic severe aortic stenosis echo stress can be used, with caution and in expert centre, for unmask exercise-limiting symptoms, a drop in systolic blood pressure by > 20 mmHg, exercise increase in mean gradient ≥ 18 to 20 mmHg, the absence of contractile reserve (no or < 5% exercise increase in LVEF) or the presence of exercise pulmonary hypertension (> 60 mmHg) that are all strong predictors of cardiac events[22-25].
When performing TTE evaluation of a stenotic aortic valve multiple windows should be investigated, including apical three or five chambers views and right parasternal approach, in order to obtain the best alignment of Doppler beam to transvalvular flow, thus avoiding inconsistency between estimated functional AVA and pressure gradient[26-28]. Recently in a study including 100 patients it has been shown that right parasternal window is more accurate than apical approach; in fact, when only apical approach is used a quarter of patients was incorrectly classified, underestimating severity in two thirds of patients deemed as moderate and misjudging a third as paradoxical LF-LG[29].
Systemic blood pressure and calibre of ascending aorta can influence severity estimation, increased left ventricular global afterload due to hypertension may cause a reduction in transvalvular flow, thus leading to stenosis underestimation[30].
Whereas if ascending aorta diameter is smaller than 30 mm, transvalvular pressure gradient may be overestimated because of a pressure recovery phenomenon distally to the aortic valve[31].
Conventional 2D-TTE allows in majority of patients to determine the number and disposition of aortic valve cusps. Bicuspid aortic valve with its asymmetrical closure line tends to develop degenerative alterations earlier than normal tricuspid valves and has a markedly elliptical annulus with eccentrically disposed calcium deposition[32].
In presence of a bicuspid aortic valve percutaneous implanted prosthesis may fail to expand completely with consequent periprosthetic regurgitation (up to 28% of cases) and the risk of valve misplacement[33,34]. Dilation of ascending aorta, which can be a contro-indication to TAVI, is also common in bicuspid aortic valve disease, moreover TAVR could increase the risk of aortic dissection in these subjects[35] Because of these technical conundrums PARTNER trial did not include subjects with bicuspid aortic valvular stenosis[1]. Anyway TAVR is still possible in these patients and several cases have been reported up today[36]. Phan et al[37] have published a meta-analysis and systematic review of literature collecting 149 patients undergone TAVR procedure there was no significant difference for patients with bicuspid aortic valves in 30-d mortality, post-procedural prosthetic haemodynamics and presence of moderate to severe perivalvular aortic regurgitation or rate of bleeding or vascular complications, indicating that TAVR can be an effective treatment also in this setting. No difference in 30-d and one year mortality between bicuspid and tricuspid stenotic valves undergoing TAVR was also found in the Poland National Registry[38]. In light of this evidence more and more centres are proposing TAVR as a valuable option for treatment patients carrying a bicuspid aortic valve, considering this condition no more an absolute but a relative contraindication to the procedure.
The presence of a left ventricle systolic dysfunction, defined as aEF < 50%, constitutes a negative prognostic marker both in symptomatic and asymptomatic severe aortic stenosis. In patients considered unsuitable for surgical aortic valve replacement enrolled in PARTNER B cohort 30-d and 1-year prognosis was not different for patients with a LVEF over 50% confronted with those with reduced LVEF[39] Moreover in this arm of PARTNER study an increase in LVEF > 10% subsequently TAVR was found in 50% of patients considered unfit for surgery, especially for those with smaller LV chamber diameters and lower grade of mitral regurgitation before TAVR. Although LVEF improvement was not associated with improvement in survival, in those with no post-procedural increase in LVEF there was a worse prognosis at one year of follow-up.
In light of these evidences TAVR represents a valuable option in severe aortic stenosis and markedly reduced left ventricular systolic function and should be taken in consideration by the Heart Team, because in these very high risk patients for surgery TAVR may show a better outcome.
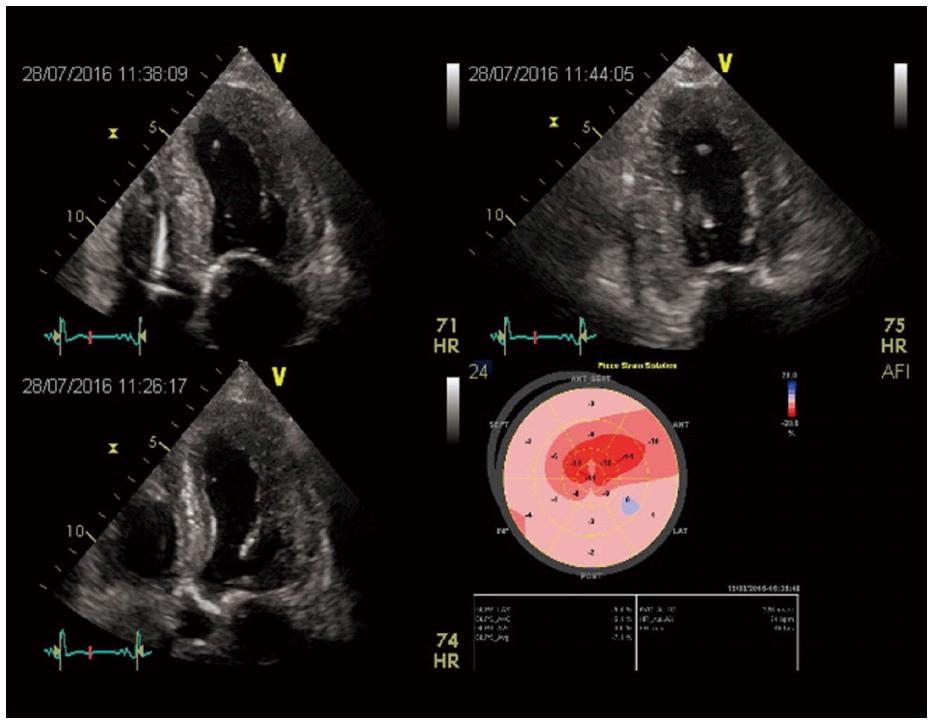
Furthermore an alteration in LV structure and function has been demonstrated in patients with severe aortic stenosis regardless a preserved LVEF and this phenomenon can be studied also with speckle tracking echocardiography, a relative new technique that provides non-Doppler evaluation of myocardial deformation as expression of systolic and diastolic dynamics[40]. In this context, in fact, a reduced GLS (global longitudinal score) has been documented with a more evident alteration in the basal LV segments and a value > -15.9% correlated with adverse prognosis[41,42].
In patients with severe aortic stenosis undergoing TAVR, LV reverse remodelling and improvement of longitudinal myocardial function assessed by speckle tracking echocardiography have been observed together with a decrease of aorto-valvular impedence and an improvement of atrial morphology and function[43]. In fact, our group evaluated 55 patients before and 6 mo after CoreValve implantation demonstrating a significant reduction in mean transaortic gradient, LV mass, LA volume index, and an improvement of ejection fraction (P < 0.0001). In addition, LV GLS and LA longitudinal strain significantly increased after TAVI and at the multiple logistic regression analysis, LV mass before TAVI (P < 0.001) and peak CK MB mass after TAVI (P < 0.0001) were powerful independent predictors of lower improvement of LV GLS. Moreover, LV mass index (P < 0.001) and LV GLS strain (P < 0.001) before TAVI was powerful independent predictor of LA longitudinal strain after TAVI (Figure 4).
Haemodynamically relevant mitral regurgitation is present in a substantial amount of patients with severe valvular aortic stenosis. It may have many different underlying mechanisms, both organic and functional. Functional mitral regurgitation may be also of ischemic nature, because of the common occurrence of coronary artery disease in these subjects. Moreover left ventricular systolic dysfunction and dilatation and concomitant aortic regurgitation may contribute to cause or aggravate mitral regurgitation[44].
In addition high grade mitral regurgitation may result in reduced transvalvular flow and lead to incorrect classification of stenosis severity, so it has to be taken in consideration in pre-procedural TTE for a comprehensive global assessment of aortic valve disease.
Interestingly in these subset of patients improvement of mitral insufficiency is reported in around 50%, more often in the case of secondary mitral regurgitation[45,46]. This finding was consistent with the results of a recently published meta-analysis which demonstrated that MR improvement was associated with pre-procedural grade and not with causative mechanism[47].
Pre-procedural TTE should include a comprehensive evaluation of right ventricular dimensions and function, in addition to estimation of pulmonary arterial systolic pressure (PAPs) from tricuspid regurgitation velocity.
Registries report that after TAVR moderate or severe tricuspid regurgitation is frequent (occurring in about 15%) and in most cases it is not improved after the procedure[48].
Pulmonary hypertension (PH) can be found in up to 25% of subjects affected by severe aortic stenosis, secondary to post-capillary increase of left ventricular filling pressure and the eventual presence of associated mitral regurgitation. PH is a predictor of worse prognosis following surgical aortic valve replacement and recently there is increasing evidence that it is a negative prognostic marker together with tricuspid regurgitation also in the setting of transcatheter aortic intervention[49].
Evidence from TAVR registries suggests that PH (estimated PAPs over 40 mmHg on TTE) does not negatively influence success rate, amount of complications in the early phase and 30-d survival, but a negative prognostic effect is present regarding 1 year mortality, which is raised up to 22% (or higher if estimated PAPs is above 60 mmHg)[50].
Transesophageal echocardiography (TEE) allows to better visualize aortic cusps, define etiology (bicuspid vs tricuspid) and directly measure aortic valve area by planimetry in doubt cases, when TTE is not conclusive. TEE can be used in association with other imaging techniques for optimal pre-procedural planning in the setting of TAVI.
Aortic valve annulus can be defined as a ring-shaped structure virtually identifiable at the level of basal attachment of aortic cusps measured in systole[46]. A correct measurement of annular size allows an appropriate delivery of aortic valve prosthesis and reduce the incidence of complications[51].
When aortic annulus is underestimated the delivery of a prosthesis too small can be followed by displacement or paraprosthetic regurgitation[4]. On the other hand prosthesis oversizing can cause insufficient expansion and valvular or paraprosthetic regurgitation or annular rupture. Optimal annular sizing aims to deliver a valve of an adequate dimension large enough to avoid paravalvular regurgitation, but not exceeding more than 20% the measured annular diameter, which increases risk of rupture.
In practice antero-posterior annular diameter is measured by TEE in mid-esophageal long axis view (120°-150°) in correspondence of basal hinge points of aortic cusps to aortic root.
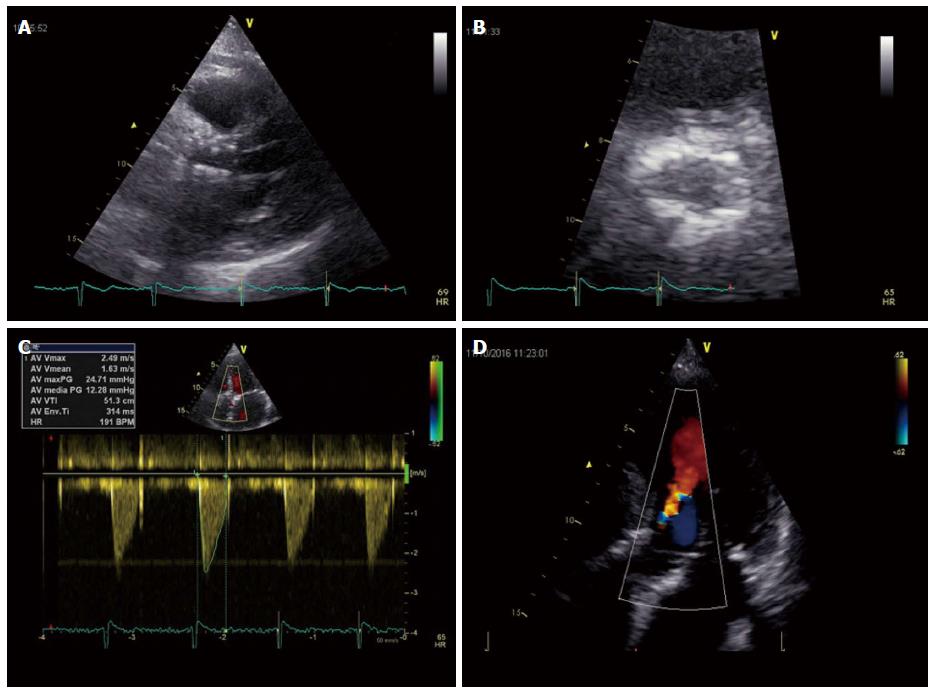
Three dimensional TEE allows to visualize the real shape of LVOT, which is oval in 90% of patients[52]. 3D-TEE has proved more effective in providing optimal annular measurement and was more useful in predicting paravalvular aortic regurgitation compared to 2D-TEE[53,54] (Figure 5).
3D-TEE has been directly compared with cardiac CT demonstrating the two imaging modalities were equally effective in predicting paraprosthetic aortic regurgitation[55], although annulus diameter and planimetric area determined by 3D-TEE tend to result smaller than those measured by cardiac CT, except for sagittal dimensions. Considering sagittal dimensions both diagnostic techniques were equally accurate in predicting prosthetic dimensions with good post-procedural results. In conclusion before TAVI, 3D-TEE can be considered a valuable alternative to cardiac CT in pre-procedural planning, especially in patients with chronic kidney disease.
Transesophageal Echocardiography is able to evaluate the distance of coronary arterial ostia from aortic annulus and to correlate this distance with aortic cusp length, in long axis view. If cusp length is longer than coronary-annular distance there is a risk of coronary occlusion after valve delivery, when aortic valve cusps are displaced by the prosthesis expansion.
In clinical practice in order to avoid coronary occlusion, coronary-annular distance should be higher than 10 mm[56,57]. Moreover aortic valve calcium burden should be always assessed and confronted with aortic sinus capacity. Although it is possible to measure coronary-annular distance with 2D-TEE, in the majority of patients it is necessary to use Multi Slice Computed Tomography (MSCT) or as an alternative 3D-TEE.
TEE allows visualization of calcium deposits, which are present in almost all subjects affected by degenerative aortic stenosis, and their distribution. The presence of extensive aortic valve calcifications may cause paravalvular regurgitation due to formation of gaps between prosthetic and native valve and increase the risk of coronary ostium obstruction after TAVI delivery[58]. In addition extensively calcified sino-tubular junction may impair the expansion at the aortic end of the prosthesis eventually causing ventricular displacement of the prosthetic valve during delivery[59,60]. Great amount of calcification, particularly in subvalvular region, is also associated with increased risk of periprocedural annular rupture or sinus rupture.
TEE examination provides an higher spatial and temporal resolution and in pre-procedural phase allows to evaluate the ascendingaorta and the descending thoracic tract in order to exclude the presence of extensive and soft atheromas which are associated with higher risk of peri-procedural ischemic stroke because they can be mobilized and hinder the passage of delivery system[61,62]. It remains a suboptimal tool for the assessment of the distal ascending aorta and the proximal arch (TEE “blind spot” due to tracheal air shadowing) as well as for the abdominal aorta. Finally TEE may show a significant basal septal hypertrophy that may lead to prosthesis displacement in periprocedural or postprocedural phase[63,64].
Coronary angiography represents an essential part of patient evaluation before planning a TAVR procedure. Significant coronary artery disease is commonly found in patients with indication to TAVI, however there is no universal agreement about if and how it should be treated[61,65]. Secondary left ventricular hypertrophy may cause myocardial ischemia irrespectively of the presence of obstructive atherosclerotic lesions in major coronary arteries, in fact manifestations of angina are reported also by patients without evidence of relevant coronary artery disease (CAD) on angiographic examination[66].
Moreover even though degenerative stenotic aortic valve disease has the same risk factors of CAD, there is substantial variability in CAD prevalence in aortic stenosis population between different studies, ranging from 34% to 75%[67,68]. A possible explanation for this inconsistency can be found in the definition adopted for significant CAD and which method is used for its diagnosis, usually angiographic examination, which shows relevant interobserver variability. Usually angiographic cut-off for coronary obstruction is considered ≥ 50%[69-72], but some authors use a cut off value of ≥ 70%[73-75].
Latest recommendations about myocardial revascularization released from European Society of Cardiology suggest PTCA for patients undergoing TAVI in the presence of coronary obstructive lesions of more than 70% (class IIa, level of evidence C), despite the impact on long term survival of obstructive CAD is controversial according to different TAVI registries[76-78]. In order to definite the prognostic benefit of percutaneous revascularization of anatomically relevant CAD in patients undergoing TAVR a randomized controlled trial, the ACTIVATION study, is ongoing[79].
In addition the burden of CAD in this setting fall in a broad spectrum going from a simple single lesion to multiple complex lesions, with different prognostic implications. Currently CAD treatment can be guided by coronary angiography and Anatomical Scoring Systems. Moreover in patients with borderline risk profile the assessment or the exclusion of coronary artery disease can induce the Heart Team to lean towards SAVR or TAVR.
According to angiographic data significant obstructive CAD is found in 40%-60% of TAVR patients, evaluated through quantitative coronary angiography (QCA). Khawaja et al[66] in a retrospective study “Coronary artery disease in patients undergoing TAVI- why not to treat” including 271 patients evaluated through QCA, reported an incidence of obstructive CAD of 34% (defined as a 70% or more stenosis of a major coronary artery or 50% or more in left main stem or a venous graft); 26.9% of them underwent revascularization before TAVR procedure. Moreover no significant increase in mortality for patients carrying obstructive CAD was found in this study, either at 30 d or at 1 year and among them, those treated by revascularization also did not show any significant prognostic improvement.
However QCA has several pitfalls: (1) eccentric and markedly calcific plaques are difficult to assess through this technique because of calcium pools projection by X-rays; and (2) extremely tortuous epicardial coronary arteries may cause mistakes in vessel measurement and thus in stenosis evaluation[80]. Alternatively markedly calcific and contorted lesions may be more reliably evaluated through optical coherence tomography or intravascular ultrasonography, but at present the use of these techniques has not been investigated in TAVR population.
Finally according to old fashioned studies using QCA of left coronary artery in aortic valve stenosis it was demonstrated a progressive increase in coronary vessel dimensions as aortic valvular stenosis progresses, such phenomenon was reverted by SAVR, so angiographic evaluation may not reliably predict CAD extension after TAVR procedure[81].
Anatomical scores are used to grade coronary artery disease extension in everyday clinical practice, among them the most frequently used is SYNTAX score[82]. Recently these scoring systems have been applied in small TAVR registries, taking in consideration the location and complexity of coronary lesions in order to estimate procedural risk of coronary revascularization[83,84].
In the previously cited retrospective analysis by Khawaja et al[66] a SYNTAX score > 33 (which defines an high risk according to SYNTAX study) had an higher rate of periprocedural complications during TAVR, whereas a SYNTAX score between 0 and 22 identified patients with a lower risk. Moreover a cut off value of 9 was a predictor of all-cause death at one month and at one year of follow-up so that revascularization may be indicated for patients with a SYNTAX score ≥ 9.
Furthermore comparing surgical aortic valve replacement with TAVR in the setting of low flow-low gradient aortic stenosis, which represent an higher risk population, the extent of CAD evaluated through SYNTAX score or remaining CAD severity assessed by residual SYNTAX score after revascularization were both predictors of worse prognosis and cardiovascular death after 1 year follow-up[85].
No methods are validated to assess ischemia in patient with severe aortic stenosis and also evaluation offunctional significance of coronary artery stenosis by fractional flow reserve (FFR) is not recommended in this population. In fact the mechanism of ischemia in severe aortic stenosis is more complex and due to multiple hemodynamic factors so that aortic pressure waveform and coronary blood flow regulation is altered by left ventricular hypertrophy leading to an impaired coronary flow reserve also in absence of coronary obstruction[86]. In addition, the increased left ventricular filling pressure will rise left ventricular diastolic wall stress, this phenomenon together with reduced diastolic time may contribute to impair diastolic coronary blood flow per se.
On the other hand the administration of vasodilator drugs, necessary to asses FFR, could induce critical fall in systemic arterial pressure with potentially hemodynamic instability.
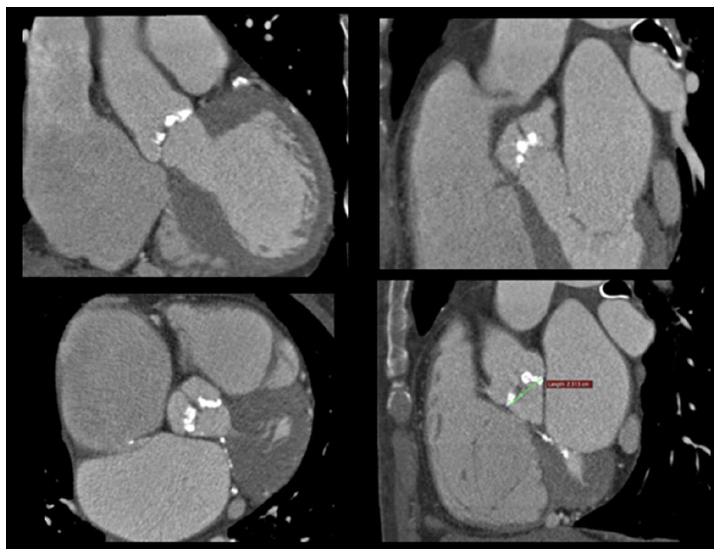
Among the imaging modalities, computed tomography (CT) plays a central role in the evaluation of patients with severe aortic stenosis prior to TAVR since it allows to study anatomical details in order to choice the best fitting prosthesis, evaluate the morphology of the access path, select the best fluoroscopic projection angles and detect other relevant comorbidities (Figure 6).
Multidetector scanners allow multiplanar reformation and 3-dimensional reconstruction of aortic root, ascending tract, arch and descending segments of aorta. Novel technological advances in CT result in higher imagine quality with substantially reduced scan duration, contrast volume and radiation exposure. CT provide an accurate measurement of anatomic AVA by a cross-sectional view of the aortic valve derived from left sagittal and left coronal oblique views[52]. Moreover this modality gives precise measurements diameters, expressed also as mean value between different planar reliefs, area and perimeter of aortic annulus which are essential information for a correct prosthesis choice. The annulus size is larger when measured by MSCT than by 2D transthoracic or transoesophageal echocardiography with an absolute difference ≤ 1.52 ± 1.1 mm. Comparing the measurements of aortic annulus size as obtained by CT angiography and 2-dimensional transesophageal echocardiography with direct surgical measurement in patients undergoing surgical valve replacement, CTA overestimates aortic annulus diameter in 72.2% of cases, with 46.3% > 1 TAVI valve-size (> 3 mm) overestimations, whereas TEE underestimated aortic annulus diameter in 51.1% of cases, with 16.7% > 1 valve-size underestimations[87,88].
MSCT allows also to give precise measurements of the distance between annulus and coronary ostia and represents the gold standard for this purpose,providing a more comprehensive assessment, showing an average annular-right coronary artery distance of 13.6 ± 2.8 mm and annular-left coronary artery distance of 13.4 ± 3.2 mm[89,90]. The distance between the aortic valve annular plane and the coronary ostia should be at least of ≥ 10-11 mm for both type of most used prosthesis (Corevalve and Edwards). It is important also to evaluate the dimensions of ascending aorta at 45 mm above the annulus plane when the strategy foresees the implantation of a Corevalve prosthesis as this value should not exceed 40 mm for the 26-mm valve and 43 mm for the 29-mm and 31-mm.
This technique is useful to make many reconstructions with adjunctive information about calcification severity, plaque burden and prohibitive risk findings as dissections and complex atheroma of aorta[91].
CT permits to calculate aortic valve calcium scoring. In severe aortic annular calcification the protrusion of calcium into the lumen > 4 mm can lead to an undersizing of the prosthesis valve and predict a post procedural paravalvular regurgitation[92]. Furthermore a high calcium score can help to distinguish between severe and pseudosevere aortic stenosis in patients with low left ventricle ejection fraction. Different cutoff values of calcium score in aortic stenosis have been described for men (≥ 2000 AU or ≥ 480 AU/cm2) and women (≥ 1200 AU or ≥ 290 AU/cm2) to identify severe AS[93]. In risk stratification, mostly in asymptomatic or paucisymptomatic patients, the aortic valve calcium load assessed by MSCT is a powerful predictor of rapid stenosis progression and of cardiac events[94].
Invasive coronary angiography remains the gold standard diagnostic modality for the detection of significant CAD in patients with severe aortic stenosis. The role of coronary computed tomography angiography (CTA) in selection of patients for TAVR until now remains not established mainly because there are few data regarding on its diagnostic accuracy in this contest. In a large unselected cohort of patients with severe aortic stenosis, the identification of significant CAD has been limited by feasibility and an overall moderate accuracy (driven by the high rate of false-positive observations) so that this test cannot be used instead of invasive coronary angiography[95]. In fact, also in patients without arrhythmias, high heart rate and coronary stents up to 25% of the CTA images were found to be not fully evaluable representing coronary calcification the major confounding factor[96-98]. On the other hand, CTA has shown a good sensitivity (97%) and negative predictive value (97%) so that it can be reasonably be used as a rule-out test in some selected cases mostly inpatients without prior known CAD and little calcifications[95].
Computed tomography as a part of pre-TAVR diagnostic work-up is often able to detect other concomitant pathologies with important influence on outcome and sometimes questioning the indication to the procedure as in case of detection of potentially malignant diseases with poor prognosis. In fact, during CT aortography, images are acquired throughout the thorax and abdomen, and potentially significant incidental findings can be found. Until today patients candidates to TAVR tend to be elderly and it has been shown a very high overall incidence of incidental pathological findings in this population (more than 50%) and in 18.1% of cases a clinical signification has been documented[99].
Appropriate approach selection is crucial for a good results of TAVR and is based on minimal aorto-femoral tract diameter detected by projection aortography or CTA. In addition to conventional angiography (XA), CTA provides more detailed 3D imagines including calcification and tortuosity and allows to exclude a transfemoral access in patients with poor vessel quality or small diameter in aorto-femoral tract considering that the 18 Fr sheath requires a minimal arterial diameter of 6 mm of the aorto-femoral tract for prosthesis delivery[100,101]. It is important to know that the semi-automated CTA diameter measurement of the aorta-iliac tract resulted statistically significantly smaller compared to XA-based measurements.
Patients not suitable for transfemoral TAVR should be considered for transapical implantation or conventional surgery[102].
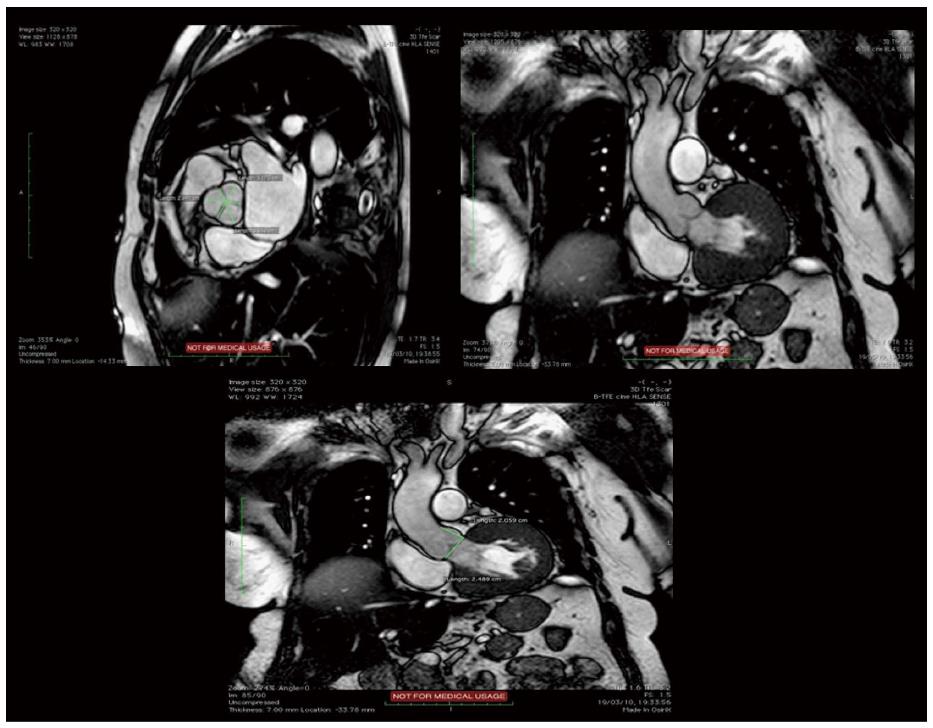
Cardiovascular magnetic resonance (CMR) is an emergent modality for evaluation of patients before TAVR and it is expected to gain more and more space in this setting, mostly in patients with contraindications to contrast medium. As MSCT, this technique provides precise measurements of aortic valve, annulus, aortic root, coronary ostia,definition of the thoraco-abdominal aorta and luminal caliber of the iliofemoral branches (Table 2)[103]. Moreover it is able to study LV function with the advantage of not using ionizing radiation (Figure 7).
| Three-plane localizer | To localize aortic valve plane |
| Axial SSFP non ECG gated without contrast | To identify potential ascending aorta and subclavian access sites |
| To determinae size, calcification, and presence of aneurysmal dilatation of aorta | |
| Breath held free breathing 2D ECG gated SSFP | To evaluate aortic annulus,aortic valve structure, and sinus higher |
| Coronal aorta, LVOT and aortic root | Planimetry valve orifice area |
| SSFP ECG gated images:short axis stak | To calculate ejection fraction, ventricular volumes and mass |
| Breath held free breathing phase contrast at aortic orifice | Calculate blood flow velocity, pressure gradient, and flow volume across the aortic valve |
| Calculate Aortic regurgitant volume | |
| 3D Navigator assisted SSFP | Coronary ostia height |
| Aortic diameter | |
| T2 black blood | Useful in presence of susceptibility artifacts from sternal wires of prosthetic valves |
Non contrast MR should have an important role in preoperative evaluation in selected groups of patients with aortic stenosis: (1) patients affected by severe renal function impairment with GFR < 30 mL/min/mq; (2) patients with inadequate acoustic window mostly in the contest of low gradient detection and/or reduced left ventricle ejection fraction; (3) discrepancy between parameters obtained by echocardiography and symptoms; and (4) History of allergic reactions to iodated contrast medium.
MRI technique are influenced by some limitations. In the first place multiple breath holds, claustrophobia and the presence of arrhythmias can interfere with an adequate acquisition. Aneurism clips, carotid vascular clamp, neurostimulator devices, insulin or infusion pumps, ear implant and ocular foreign bodies represent absolute contraindications.
MRI is able to provide accurate measurements of aortic annulus that in terms of capacity to predict the presence and the severity of post-implantation aortic regurgitation is similar to MSCT[104]. A good concordance between MSCT, CMR and echocardiography has been documented for aortic valve morphology definition and aortic valve area measurements[105]. In fact MRI is able to provide the planimetry of aortic valve opening area which is similar to other diagnostic modality as 3D TEE and flow-derived area calculation by catheterization using the Gorlin equation or by Doppler echocardiography using the continuity equation[106]. Although anatomic planimetry of aortic stenosis and assessment of valvular anatomy and motion is possible with MRI, this became less than optimal in patients with severe calcifications mostly in the presence of non-planar orifices. Furthermore assessment of severity of aortic stenosis can be completed by velocity-encoded cine MRI with other standard measures as peak anterograde velocity and pressure gradient but it is necessary to know that velocities and gradients are usually underestimated if compared with Doppler echocardiography[107].
MRI can be an alternative to 3D imaging modality for the measurement of aortic annulus (minor an major diameters, area and perimeter) having showed a good agreement with CT in this context also in presence of oval shape of the structure in which, after adequate plane orientation and 3 dimensional reconstruction, generally the coronal diameter is larger than the sagittal one[108]. As for MSCT, MRI diameters were found to be larger than those measured by 2D TEE modality.
Measurements of sinus of Valsalva diameters and definition of aortic root orientation are also possible with this approach but conversely this doesn’t represent a good modality to thoracic aorta plaque burden definition as calcifications cause signal voids[103].
The concordance with CT has been documented also for the assessment of the distance between the annulus and the ostium of the left coronaryartery in relation to the length of the left coronary leaflet but at the moment more studies are needed to determine whether a strategy based on a different imaging method could achieve better results. A3-D SSFP free breathing stack in late diastole with a respiratory navigator allows to measure the height of coronary ostia from the annular plane.
Moreover magnetic resonance angiography can characterize aorto-ilio-femoral arteries in order to plan the more adequate access[109].
MRI provides quantitative evaluation of left ventricle volumes and function and late gadolinium enhancement at T1-weighted sequences allows to detect myocardial fibrosis which is more often localized in mid-wall of myocardium, like in pressure-overload cardiomyopathies, and represents a predictor of poor prognosis[110]. Fibrosis represents one of the most important factors implicated in progression of hypertrophy towards heart failure and an early detection can be useful in risk profile definition mostly in asymptomatic patients or in case of borderlines parameters at conventional echocardiography evaluation. Advanced fibrosis replacement of left ventricle myocardium predicts a lack of improvement in LV systolic function after aortic valve replacement and is an independent predictor of all-cause mortality[111,112].
An emergent role in pre-TAVR evaluation is attributable to positron emission tomography (PET)/CT with the advantage of combining the anatomic definition derived from CT and the functional and metabolic characterization gained from PET[15]. 18F-sodium fluoride (18F-NaF) is a tracer used to detect calcification and in aortic stenosis the amount of uptake correlates with disease severity and is able to predict the progression of the disease[113-115]. On the other hand 18F-fluorodeoxyglucose uptake, representing the burden of inflammation, is higher in patients with mild or moderate aortic stenosis and decrease with stenosis progression.
Patient selection for TAVR should be based not only on accurate assessment of aortic stenosis morphology, but also on several clinical and functional data. The Heart Team is key in the overall risk evaluation of this population. Multi-Imaging modalities are preferred for assessing the anatomy and the dimensions of the aortic annulus before TAVI (Table 3). In any case, we should tailor our patient selection and prosthesis selection on a case-to-case basis.
| Technique | Principal advantages | Disadvantages |
| Transthoracic echocardiography | Widespread availability First line diagnostic tool | Poor acoustic window Frequent discrepancy between different parameters |
| Transesophageal echocardiography | Good spatial resolution | Suboptimal for distal ascending aorta and arch |
| 3 D reconstruction | Semi-invasive exam | Anatomic definition and annulus measurement |
| Multislice computed tomography | Multiplanar reconstruction Quantification of calcium score Evaluation of aorto-femoral tract | Potential nephrotoxicity of contrast medium Radiations exposition Controlled heart rate |
| Magnetic resonance imaging | Tissue characterization Multiplanar reconstruction Evaluation of aorto-femoral tract Controlled heart rate | Reduced availability Poor evaluation of calcifications Contraindicated in metallic devices wearers |
| Positron emission tomography | Evaluation of calcification and inflammation | Poor spatial resolution |
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Avanzas P, Shehada SE, Tang JM S- Editor: Qi Y L- Editor: A E- Editor: Wu HL
| 1. | Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597-1607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5086] [Cited by in RCA: 5501] [Article Influence: 366.7] [Reference Citation Analysis (1)] |
| 2. | Nagaraja V, Raval J, Eslick GD, Ong AT. Transcatheter versus surgical aortic valve replacement: a systematic review and meta-analysis of randomised and non-randomised trials. Open Heart. 2014;1:e000013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, Tuzcu EM, Miller DC, Herrmann HC. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med. 2016;374:1609-1620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3232] [Cited by in RCA: 3815] [Article Influence: 423.9] [Reference Citation Analysis (0)] |
| 4. | Piazza N, de Jaegere P, Schultz C, Becker AE, Serruys PW, Anderson RH. Anatomy of the aortic valvar complex and its implications for transcatheter implantation of the aortic valve. Circ Cardiovasc Interv. 2008;1:74-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 427] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 5. | Webb JG, Doshi D, Mack MJ, Makkar R, Smith CR, Pichard AD, Kodali S, Kapadia S, Miller DC, Babaliaros V. A Randomized Evaluation of the SAPIEN XT Transcatheter Heart Valve System in Patients With Aortic Stenosis Who Are Not Candidates for Surgery. JACC Cardiovasc Interv. 2015;8:1797-1806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 87] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 6. | Généreux P, Head SJ, Wood DA, Kodali SK, Williams MR, Paradis JM, Spaziano M, Kappetein AP, Webb JG, Cribier A. Transcatheter aortic valve implantation 10-year anniversary: review of current evidence and clinical implications. Eur Heart J. 2012;33:2388-2398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 7. | Généreux P, Head SJ, Wood DA, Kodali SK, Williams MR, Paradis JM, Spaziano M, Kappetein AP, Webb JG, Cribier A. Transcatheter aortic valve implantation: 10-year anniversary part II: clinical implications. Eur Heart J. 2012;33:2399-2402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Osswald BR, Gegouskov V, Badowski-Zyla D, Tochtermann U, Thomas G, Hagl S, Blackstone EH. Overestimation of aortic valve replacement risk by EuroSCORE: implications for percutaneous valve replacement. Eur Heart J. 2009;30:74-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 145] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 9. | Green P, Woglom AE, Genereux P, Daneault B, Paradis JM, Schnell S, Hawkey M, Maurer MS, Kirtane AJ, Kodali S. The impact of frailty status on survival after transcatheter aortic valve replacement in older adults with severe aortic stenosis: a single-center experience. JACC Cardiovasc Interv. 2012;5:974-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 378] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 10. | Vahanian A, Alfieri OR, Al-Attar N, Antunes MJ, Bax J, Cormier B, Cribier A, De Jaegere P, Fournial G, Kappetein AP. Transcatheter valve implantation for patients with aortic stenosis: a position statement from the European Association of Cardio-Thoracic Surgery (EACTS) and the European Society of Cardiology (ESC), in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur J Cardiothorac Surg. 2008;34:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 175] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 11. | Gada H, Kirtane AJ, Wang K, Lei Y, Magnuson E, Reynolds MR, Williams MR, Kodali S, Vahl TP, Arnold SV. Temporal Trends in Quality of Life Outcomes After Transapical Transcatheter Aortic Valve Replacement: A Placement of AoRTic TraNscathetER Valve (PARTNER) Trial Substudy. Circ Cardiovasc Qual Outcomes. 2015;8:338-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Badiani S, Bhattacharyya S, Lloyd G. Role of Echocardiography Before Transcatheter Aortic Valve Implantation (TAVI). Curr Cardiol Rep. 2016;18:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Hahn RT, Pibarot P, Stewart WJ, Weissman NJ, Gopalakrishnan D, Keane MG, Anwaruddin S, Wang Z, Bilsker M, Lindman BR. Comparison of transcatheter and surgical aortic valve replacement in severe aortic stenosis: a longitudinal study of echocardiography parameters in cohort A of the PARTNER trial (placement of aortic transcatheter valves). J Am Coll Cardiol. 2013;61:2514-2521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 189] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 14. | Dimitrova NA, Dimitrov GV. Effect of electrical stimulus parameters on the development and propagation of action potentials in short excitable fibres. Electroencephalogr Clin Neurophysiol. 1988;70:453-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1918] [Cited by in RCA: 1865] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 15. | Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Guyton RA, O’Gara PT, Ruiz CE, Skubas NJ, Sorajja P. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:e57-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Briand M, Dumesnil JG, Kadem L, Tongue AG, Rieu R, Garcia D, Pibarot P. Reduced systemic arterial compliance impacts significantly on left ventricular afterload and function in aortic stenosis: implications for diagnosis and treatment. J Am Coll Cardiol. 2005;46:291-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 381] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 17. | Dumesnil JG, Pibarot P, Carabello B. Paradoxical low flow and/or low gradient severe aortic stenosis despite preserved left ventricular ejection fraction: implications for diagnosis and treatment. Eur Heart J. 2010;31:281-289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 252] [Cited by in RCA: 237] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 18. | Hachicha Z, Dumesnil JG, Bogaty P, Pibarot P. Paradoxical low-flow, low-gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation. 2007;115:2856-2864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 698] [Cited by in RCA: 712] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 19. | Herrmann S, Störk S, Niemann M, Lange V, Strotmann JM, Frantz S, Beer M, Gattenlöhner S, Voelker W, Ertl G. Low-gradient aortic valve stenosis myocardial fibrosis and its influence on function and outcome. J Am Coll Cardiol. 2011;58:402-412. [PubMed] |
| 20. | Hachicha Z, Dumesnil JG, Pibarot P. Usefulness of the valvuloarterial impedance to predict adverse outcome in asymptomatic aortic stenosis. J Am Coll Cardiol. 2009;54:1003-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 267] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 21. | Herrmann HC, Pibarot P, Hueter I, Gertz ZM, Stewart WJ, Kapadia S, Tuzcu EM, Babaliaros V, Thourani V, Szeto WY. Predictors of mortality and outcomes of therapy in low-flow severe aortic stenosis: a Placement of Aortic Transcatheter Valves (PARTNER) trial analysis. Circulation. 2013;127:2316-2326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 350] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 22. | Lancellotti P, Lebois F, Simon M, Tombeux C, Chauvel C, Pierard LA. Prognostic importance of quantitative exercise Doppler echocardiography in asymptomatic valvular aortic stenosis. Circulation. 2005;112:I377-I382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 168] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 23. | Maréchaux S, Hachicha Z, Bellouin A, Dumesnil JG, Meimoun P, Pasquet A, Bergeron S, Arsenault M, Le Tourneau T, Ennezat PV. Usefulness of exercise-stress echocardiography for risk stratification of true asymptomatic patients with aortic valve stenosis. Eur Heart J. 2010;31:1390-1397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 193] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 24. | Lancellotti P, Magne J, Donal E, O’Connor K, Dulgheru R, Rosca M, Pierard LA. Determinants and prognostic significance of exercise pulmonary hypertension in asymptomatic severe aortic stenosis. Circulation. 2012;126:851-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 153] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 25. | Maréchaux S, Ennezat PV, LeJemtel TH, Polge AS, de Groote P, Asseman P, Nevière R, Le Tourneau T, Deklunder G. Left ventricular response to exercise in aortic stenosis: an exercise echocardiographic study. Echocardiography. 2007;24:955-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Fryearson J, Edwards NC, Doshi SN, Steeds RP. The role of TTE in assessment of the patient before and following TAVI for AS. Echo Res Pract. 2016;3:R19-R34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, Iung B, Otto CM, Pellikka PA, Quiñones M. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr. 2009;22:1-23; quiz 101-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1287] [Cited by in RCA: 1375] [Article Influence: 85.9] [Reference Citation Analysis (0)] |
| 28. | Minners J, Allgeier M, Gohlke-Baerwolf C, Kienzle RP, Neumann FJ, Jander N. Inconsistencies of echocardiographic criteria for the grading of aortic valve stenosis. Eur Heart J. 2008;29:1043-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 290] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 29. | Thaden JJ, Nkomo VT, Lee KJ, Oh JK. Doppler Imaging in Aortic Stenosis: The Importance of the Nonapical Imaging Windows to Determine Severity in a Contemporary Cohort. J Am Soc Echocardiogr. 2015;28:780-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 30. | Kadem L, Dumesnil JG, Rieu R, Durand LG, Garcia D, Pibarot P. Impact of systemic hypertension on the assessment of aortic stenosis. Heart. 2005;91:354-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 121] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 31. | Baumgartner H, Stefenelli T, Niederberger J, Schima H, Maurer G. “Overestimation” of catheter gradients by Doppler ultrasound in patients with aortic stenosis: a predictable manifestation of pressure recovery. J Am Coll Cardiol. 1999;33:1655-1661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 207] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 32. | Chiam PT, Chao VT, Tan SY, Koh TH, Lee CY, Tho VY, Sin YK, Chua YL. Percutaneous transcatheter heart valve implantation in a bicuspid aortic valve. JACC Cardiovasc Interv. 2010;3:559-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 33. | Mylotte D, Lefevre T, Søndergaard L, Watanabe Y, Modine T, Dvir D, Bosmans J, Tchetche D, Kornowski R, Sinning JM. Transcatheter aortic valve replacement in bicuspid aortic valve disease. J Am Coll Cardiol. 2014;64:2330-2339. [PubMed] |
| 34. | Himbert D, Pontnau F, Messika-Zeitoun D, Descoutures F, Détaint D, Cueff C, Sordi M, Laissy JP, Alkhoder S, Brochet E. Feasibility and outcomes of transcatheter aortic valve implantation in high-risk patients with stenotic bicuspid aortic valves. Am J Cardiol. 2012;110:877-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 35. | Zegdi R, Ciobotaru V, Noghin M, Sleilaty G, Lafont A, Latrémouille C, Deloche A, Fabiani JN. Is it reasonable to treat all calcified stenotic aortic valves with a valved stent? Results from a human anatomic study in adults. J Am Coll Cardiol. 2008;51:579-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 185] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 36. | Delgado V, Tops LF, Schuijf JD, de Roos A, Brugada J, Schalij MJ, Thomas JD, Bax JJ. Assessment of mitral valve anatomy and geometry with multislice computed tomography. JACC Cardiovasc Imaging. 2009;2:556-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 114] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 37. | Phan K, Wong S, Phan S, Ha H, Qian P, Yan TD. Transcatheter Aortic Valve Implantation (TAVI) in Patients With Bicuspid Aortic Valve Stenosis--Systematic Review and Meta-Analysis. Heart Lung Circ. 2015;24:649-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 38. | Kochman J, Huczek Z, Scisło P, Dabrowski M, Chmielak Z, Szymański P, Witkowski A, Parma R, Ochala A, Chodór P. Comparison of one- and 12-month outcomes of transcatheter aortic valve replacement in patients with severely stenotic bicuspid versus tricuspid aortic valves (results from a multicenter registry). Am J Cardiol. 2014;114:757-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 39. | Elmariah S, Palacios IF, McAndrew T, Hueter I, Inglessis I, Baker JN, Kodali S, Leon MB, Svensson L, Pibarot P. Outcomes of transcatheter and surgical aortic valve replacement in high-risk patients with aortic stenosis and left ventricular dysfunction: results from the Placement of Aortic Transcatheter Valves (PARTNER) trial (cohort A). Circ Cardiovasc Interv. 2013;6:604-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 135] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 40. | Pibarot P, Dumesnil JG. Aortic stenosis: look globally, think globally. JACC Cardiovasc Imaging. 2009;2:400-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 41. | Lafitte S, Perlant M, Reant P, Serri K, Douard H, DeMaria A, Roudaut R. Impact of impaired myocardial deformations on exercise tolerance and prognosis in patients with asymptomatic aortic stenosis. Eur J Echocardiogr. 2009;10:414-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 152] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 42. | Lancellotti P, Donal E, Magne J, Moonen M, O’Connor K, Daubert JC, Pierard LA. Risk stratification in asymptomatic moderate to severe aortic stenosis: the importance of the valvular, arterial and ventricular interplay. Heart. 2010;96:1364-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 243] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 43. | D’Andrea A, Padalino R, Cocchia R, Di Palma E, Riegler L, Scarafile R, Rossi G, Bianchi R, Tartaglione D, Cappelli Bigazzi M. Effects of transcatheter aortic valve implantation on left ventricular and left atrial morphology and function. Echocardiography. 2015;32:928-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 44. | Nombela-Franco L, Ribeiro HB, Urena M, Allende R, Amat-Santos I, DeLarochellière R, Dumont E, Doyle D, DeLarochellière H, Laflamme J. Significant mitral regurgitation left untreated at the time of aortic valve replacement: a comprehensive review of a frequent entity in the transcatheter aortic valve replacement era. J Am Coll Cardiol. 2014;63:2643-2658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 151] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 45. | Toggweiler S, Boone RH, Rodés-Cabau J, Humphries KH, Lee M, Nombela-Franco L, Bagur R, Willson AB, Binder RK, Gurvitch R. Transcatheter aortic valve replacement: outcomes of patients with moderate or severe mitral regurgitation. J Am Coll Cardiol. 2012;59:2068-2074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 162] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 46. | Bedogni F, Latib A, De Marco F, Agnifili M, Oreglia J, Pizzocri S, Latini RA, Lanotte S, Petronio AS, De Carlo M. Interplay between mitral regurgitation and transcatheter aortic valve replacement with the CoreValve Revalving System: a multicenter registry. Circulation. 2013;128:2145-2153. [PubMed] |
| 47. | Chakravarty T, Van Belle E, Jilaihawi H, Noheria A, Testa L, Bedogni F, Rück A, Barbanti M, Toggweiler S, Thomas M. Meta-analysis of the impact of mitral regurgitation on outcomes after transcatheter aortic valve implantation. Am J Cardiol. 2015;115:942-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 48. | Barbanti M, Binder RK, Dvir D, Tan J, Freeman M, Thompson CR, Cheung A, Wood DA, Leipsic J, Webb JG. Prevalence and impact of preoperative moderate/severe tricuspid regurgitation on patients undergoing transcatheter aortic valve replacement. Catheter Cardiovasc Interv. 2015;85:677-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 49. | Luçon A, Oger E, Bedossa M, Boulmier D, Verhoye JP, Eltchaninoff H, Iung B, Leguerrier A, Laskar M, Leprince P. Prognostic implications of pulmonary hypertension in patients with severe aortic stenosis undergoing transcatheter aortic valve implantation: study from the FRANCE 2 Registry. Circ Cardiovasc Interv. 2014;7:240-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 50. | Lindman BR, Zajarias A, Maniar HS, Miller DC, Suri RM, Arnold SV, Webb J, Svensson LG, Kodali S, Xu K. Risk stratification in patients with pulmonary hypertension undergoing transcatheter aortic valve replacement. Heart. 2015;101:1656-1664. [PubMed] |
| 51. | Chin D. Echocardiography for transcatheter aortic valve implantation. Eur J Echocardiogr. 2009;10:i21-i29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 52. | Kempfert J, Van Linden A, Lehmkuhl L, Rastan AJ, Holzhey D, Blumenstein J, Mohr FW, Walther T. Aortic annulus sizing: echocardiographic versus computed tomography derived measurements in comparison with direct surgical sizing. Eur J Cardiothorac Surg. 2012;42:627-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 53. | Saitoh T, Shiota M, Izumo M, Gurudevan SV, Tolstrup K, Siegel RJ, Shiota T. Comparison of left ventricular outflow geometry and aortic valve area in patients with aortic stenosis by 2-dimensional versus 3-dimensional echocardiography. Am J Cardiol. 2012;109:1626-1631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 54. | Messika-Zeitoun D, Serfaty JM, Brochet E, Ducrocq G, Lepage L, Detaint D, Hyafil F, Himbert D, Pasi N, Laissy JP. Multimodal assessment of the aortic annulus diameter: implications for transcatheter aortic valve implantation. J Am Coll Cardiol. 2010;55:186-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 329] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 55. | Santos N, de Agustín JA, Almería C, Gonçalves A, Marcos-Alberca P, Fernández-Golfín C, García E, Hernández-Antolín R, de Isla LP, Macaya C. Prosthesis/annulus discongruence assessed by three-dimensional transoesophageal echocardiography: a predictor of significant paravalvular aortic regurgitation after transcatheter aortic valve implantation. Eur Heart J Cardiovasc Imaging. 2012;13:931-937. [PubMed] [DOI] [Full Text] |
| 56. | Husser O, Holzamer A, Resch M, Endemann DH, Nunez J, Bodi V, Schmid C, Riegger GA, Gössmann H, Hamer O. Prosthesis sizing for transcatheter aortic valve implantation--comparison of three dimensional transesophageal echocardiography with multislice computed tomography. Int J Cardiol. 2013;168:3431-3438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 57. | Tops LF, Krishnàn SC, Schuijf JD, Schalij MJ, Bax JJ. Noncoronary applications of cardiac multidetector row computed tomography. JACC Cardiovasc Imaging. 2008;1:94-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 58. | Rivard AL, Bartel T, Bianco RW, O’Donnell KS, Bonatti J, Dichtl W, Cury RC, Feuchtner GM. Evaluation of aortic root and valve calcifications by multi-detector computed tomography. J Heart Valve Dis. 2009;18:662-670. [PubMed] |
| 59. | Delgado V, Kapadia S, Schalij MJ, Schuijf JD, Tuzcu EM, Bax JJ. Transcatheter aortic valve implantation: implications of multimodality imaging in patient selection, procedural guidance, and outcomes. Heart. 2012;98:743-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 60. | John D, Buellesfeld L, Yuecel S, Mueller R, Latsios G, Beucher H, Gerckens U, Grube E. Correlation of Device landing zone calcification and acute procedural success in patients undergoing transcatheter aortic valve implantations with the self-expanding CoreValve prosthesis. JACC Cardiovasc Interv. 2010;3:233-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 200] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 61. | Haensig M, Lehmkuhl L, Rastan AJ, Kempfert J, Mukherjee C, Gutberlet M, Holzhey DM, Mohr FW. Aortic valve calcium scoring is a predictor of significant paravalvular aortic insufficiency in transapical-aortic valve implantation. Eur J Cardiothorac Surg. 2012;41:1234-1240; discussion 1234-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 62. | Kurra V, Lieber ML, Sola S, Kalahasti V, Hammer D, Gimple S, Flamm SD, Bolen MA, Halliburton SS, Mihaljevic T. Extent of thoracic aortic atheroma burden and long-term mortality after cardiothoracic surgery: a computed tomography study. JACC Cardiovasc Imaging. 2010;3:1020-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 63. | Ghanem A, Müller A, Nähle CP, Kocurek J, Werner N, Hammerstingl C, Schild HH, Schwab JO, Mellert F, Fimmers R. Risk and fate of cerebral embolism after transfemoral aortic valve implantation: a prospective pilot study with diffusion-weighted magnetic resonance imaging. J Am Coll Cardiol. 2010;55:1427-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 265] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 64. | Bloomfield GS, Gillam LD, Hahn RT, Kapadia S, Leipsic J, Lerakis S, Tuzcu M, Douglas PS. A practical guide to multimodality imaging of transcatheter aortic valve replacement. JACC Cardiovasc Imaging. 2012;5:441-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 143] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 65. | Stefanini GG, Stortecky S, Wenaweser P, Windecker S. Coronary artery disease in patients undergoing TAVI: why, what, when and how to treat. EuroIntervention. 2014;10 Suppl U:U69-U75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 66. | Khawaja MZ, Redwood SR, Thomas M. Coronary artery disease in patients undergoing TAVI--why not to treat. EuroIntervention. 2014;10 Suppl U:U76-U83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 67. | Julius BK, Spillmann M, Vassalli G, Villari B, Eberli FR, Hess OM. Angina pectoris in patients with aortic stenosis and normal coronary arteries. Mechanisms and pathophysiological concepts. Circulation. 1997;95:892-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 90] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 68. | Khawaja MZ, Asrress KN, Haran H, Arri S, Nadra I, Bolter K, Wilson K, Clack L, Hancock J, Young CP. The effect of coronary artery disease defined by quantitative coronary angiography and SYNTAX score upon outcome after transcatheter aortic valve implantation (TAVI) using the Edwards bioprosthesis. EuroIntervention. 2015;11:450-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 69. | Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187-2198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4547] [Cited by in RCA: 4931] [Article Influence: 352.2] [Reference Citation Analysis (0)] |
| 70. | Van Mieghem NM, van der Boon RM, Faqiri E, Diletti R, Schultz C, van Geuns RJ, Serruys PW, Kappetein AP, van Domburg RT, de Jaegere PP. Complete revascularization is not a prerequisite for success in current transcatheter aortic valve implantation practice. JACC Cardiovasc Interv. 2013;6:867-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 71. | Abdel-Wahab M, Mostafa AE, Geist V, Stöcker B, Gordian K, Merten C, Richardt D, Toelg R, Richardt G. Comparison of outcomes in patients having isolated transcatheter aortic valve implantation versus combined with preprocedural percutaneous coronary intervention. Am J Cardiol. 2012;109:581-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 116] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 72. | Pasic M, Dreysse S, Unbehaun A, Buz S, Drews T, Klein C, D’Ancona G, Hetzer R. Combined elective percutaneous coronary intervention and transapical transcatheter aortic valve implantation. Interact Cardiovasc Thorac Surg. 2012;14:463-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 73. | Gasparetto V, Fraccaro C, Tarantini G, Buja P, D’Onofrio A, Yzeiraj E, Pittarello D, Isabella G, Gerosa G, Iliceto S. Safety and effectiveness of a selective strategy for coronary artery revascularization before transcatheter aortic valve implantation. Catheter Cardiovasc Interv. 2013;81:376-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 74. | Gautier M, Pepin M, Himbert D, Ducrocq G, Iung B, Dilly MP, Attias D, Nataf P, Vahanian A. Impact of coronary artery disease on indications for transcatheter aortic valve implantation and on procedural outcomes. EuroIntervention. 2011;7:549-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 75. | Abramowitz Y, Banai S, Katz G, Steinvil A, Arbel Y, Havakuk O, Halkin A, Ben-Gal Y, Keren G, Finkelstein A. Comparison of early and late outcomes of TAVI alone compared to TAVI plus PCI in aortic stenosis patients with and without coronary artery disease. Catheter Cardiovasc Interv. 2014;83:649-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 76. | Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Jüni P. 2014 ESC/EACTS guidelines on myocardial revascularization. EuroIntervention. 2015;10:1024-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 198] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 77. | Ludman PF, Moat N, de Belder MA, Blackman DJ, Duncan A, Banya W, MacCarthy PA, Cunningham D, Wendler O, Marlee D. Transcatheter aortic valve implantation in the United Kingdom: temporal trends, predictors of outcome, and 6-year follow-up: a report from the UK Transcatheter Aortic Valve Implantation (TAVI) Registry, 2007 to 2012. Circulation. 2015;131:1181-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 241] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 78. | Masson JB, Lee M, Boone RH, Al Ali A, Al Bugami S, Hamburger J, John Mancini GB, Ye J, Cheung A, Humphries KH. Impact of coronary artery disease on outcomes after transcatheter aortic valve implantation. Catheter Cardiovasc Interv. 2010;76:165-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 120] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 79. | Mohr FW, Morice MC, Kappetein AP, Feldman TE, Ståhle E, Colombo A, Mack MJ, Holmes DR, Morel MA, Van Dyck N. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet. 2013;381:629-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1214] [Cited by in RCA: 1233] [Article Influence: 102.8] [Reference Citation Analysis (0)] |
| 80. | Tu S, Xu L, Ligthart J, Xu B, Witberg K, Sun Z, Koning G, Reiber JH, Regar E. In vivo comparison of arterial lumen dimensions assessed by co-registered three-dimensional (3D) quantitative coronary angiography, intravascular ultrasound and optical coherence tomography. Int J Cardiovasc Imaging. 2012;28:1315-1327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 81. | Villari B, Hess OM, Meier C, Pucillo A, Gaglione A, Turina M, Krayenbuehl HP. Regression of coronary artery dimensions after successful aortic valve replacement. Circulation. 1992;85:972-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 31] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 82. | Khawaja MZ, Wang D, Pocock S, Redwood SR, Thomas MR. The percutaneous coronary intervention prior to transcatheter aortic valve implantation (ACTIVATION) trial: study protocol for a randomized controlled trial. Trials. 2014;15:300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 83. | Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ, Ståhle E, Feldman TE, van den Brand M, Bass EJ. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360:961-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2982] [Cited by in RCA: 2994] [Article Influence: 187.1] [Reference Citation Analysis (0)] |
| 84. | Généreux P, Palmerini T, Caixeta A, Rosner G, Green P, Dressler O, Xu K, Parise H, Mehran R, Serruys PW. Quantification and impact of untreated coronary artery disease after percutaneous coronary intervention: the residual SYNTAX (Synergy Between PCI with Taxus and Cardiac Surgery) score. J Am Coll Cardiol. 2012;59:2165-2174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 290] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 85. | O’Sullivan CJ, Englberger L, Hosek N, Heg D, Cao D, Stefanini GG, Stortecky S, Gloekler S, Spitzer E, Tüller D. Clinical outcomes and revascularization strategies in patients with low-flow, low-gradient severe aortic valve stenosis according to the assigned treatment modality. JACC Cardiovasc Interv. 2015;8:704-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 86. | Rajappan K, Rimoldi OE, Dutka DP, Ariff B, Pennell DJ, Sheridan DJ, Camici PG. Mechanisms of coronary microcirculatory dysfunction in patients with aortic stenosis and angiographically normal coronary arteries. Circulation. 2002;105:470-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 236] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 87. | Wang H, Hanna JM, Ganapathi A, Keenan JE, Hurwitz LM, Vavalle JP, Kiefer TL, Wang A, Harrison JK, Hughes GC. Comparison of aortic annulus size by transesophageal echocardiography and computed tomography angiography with direct surgical measurement. Am J Cardiol. 2015;115:1568-1573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 88. | Willson AB, Webb JG, Freeman M, Wood DA, Gurvitch R, Thompson CR, Moss RR, Toggweiler S, Binder RK, Munt B. Computed tomography-based sizing recommendations for transcatheter aortic valve replacement with balloon-expandable valves: Comparison with transesophageal echocardiography and rationale for implementation in a prospective trial. J Cardiovasc Comput Tomogr. 2012;6:406-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 89. | Holmes DR, Mack MJ, Kaul S, Agnihotri A, Alexander KP, Bailey SR, Calhoon JH, Carabello BA, Desai MY, Edwards FH. 2012 ACCF/AATS/SCAI/STS expert consensus document on transcatheter aortic valve replacement. J Am Coll Cardiol. 2012;59:1200-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 566] [Cited by in RCA: 563] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 90. | Narang A, Guerrero M, Feldman T, Pursnani A. Computed tomography assessment for transcatheter mitral valve interventions. J Cardiovasc Surg (Torino). 2016;57:360-371. [PubMed] |
| 91. | Barbanti M1, Immè S, Ohno Y, Gulino S, Todaro D, Sgroi C, Tamburino C2, Patanè M, Pilato G, Capodanno D. Prosthesis choice for transcatheter aortic valve replacement: Improved outcomes with the adoption of a patient-specific transcatheter heart valve selection algorithm. Int J Cardiol. 2016;203:1009-1010. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 92. | Feuchtner G, Plank F, Bartel T, Mueller S, Leipsic J, Schachner T, Müller L, Friedrich G, Klauser A, Grimm M. Prediction of paravalvular regurgitation after transcatheter aortic valve implantation by computed tomography: value of aortic valve and annular calcification. Ann Thorac Surg. 2013;96:1574-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 93. | Aggarwal SR, Clavel MA, Messika-Zeitoun D, Cueff C, Malouf J, Araoz PA, Mankad R, Michelena H, Vahanian A, Enriquez-Sarano M. Sex differences in aortic valve calcification measured by multidetector computed tomography in aortic stenosis. Circ Cardiovasc Imaging. 2013;6:40-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 198] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 94. | Clavel MA, Pibarot P, Messika-Zeitoun D, Capoulade R, Malouf J, Aggarval S, Araoz PA, Michelena HI, Cueff C, Larose E. Impact of aortic valve calcification, as measured by MDCT, on survival in patients with aortic stenosis: results of an international registry study. J Am Coll Cardiol. 2014;64:1202-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 364] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 95. | Opolski MP, Kim WK, Liebetrau C, Walther C, Blumenstein J, Gaede L, Kempfert J, Van Linden A, Walther T, Hamm CW. Diagnostic accuracy of computed tomography angiography for the detection of coronary artery disease in patients referred for transcatheter aortic valve implantation. Clin Res Cardiol. 2015;104:471-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 96. | Gilard M, Cornily JC, Pennec PY, Joret C, Le Gal G, Mansourati J, Blanc JJ, Boschat J. Accuracy of multislice computed tomography in the preoperative assessment of coronary disease in patients with aortic valve stenosis. J Am Coll Cardiol. 2006;47:2020-2024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 93] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 97. | Meijboom WB, Mollet NR, Van Mieghem CA, Kluin J, Weustink AC, Pugliese F, Vourvouri E, Cademartiri F, Bogers AJ, Krestin GP. Pre-operative computed tomography coronary angiography to detect significant coronary artery disease in patients referred for cardiac valve surgery. J Am Coll Cardiol. 2006;48:1658-1665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 164] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 98. | Stagnaro N, Della Latta D, Chiappino D. Diagnostic accuracy of MDCT coronary angiography in patients referred for heart valve surgery. Radiol Med. 2009;114:728-742. [PubMed] |
| 99. | Lindsay AC, Sriharan M, Lazoura O, Sau A, Roughton M, Jabbour RJ, Di Mario C, Davies SW, Moat NE, Padley SP. Clinical and economic consequences of non-cardiac incidental findings detected on cardiovascular computed tomography performed prior to transcatheter aortic valve implantation (TAVI). Int J Cardiovasc Imaging. 2015;31:1435-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 100. | Achenbach S, Delgado V, Hausleiter J, Schoenhagen P, Min JK, Leipsic JA. SCCT expert consensus document on computed tomography imaging before transcatheter aortic valve implantation (TAVI)/transcatheter aortic valve replacement (TAVR). J Cardiovasc Comput Tomogr. 2012;6:366-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 497] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 101. | Wiegerinck EM, Marquering HA, Oldenburger NY, Elattar MA, Planken RN, De Mol BA, Piek JJ, Baan J. Imaging for approach selection of TAVI: assessment of the aorto-iliac tract diameter by computed tomography-angiography versus projection angiography. Int J Cardiovasc Imaging. 2014;30:399-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 102. | Dewey TM, Bowers B, Thourani VH, Babaliaros V, Smith CR, Leon MB, Svensson LG, Tuzcu EM, Miller DC, Teirstein PS. Transapical aortic valve replacement for severe aortic stenosis: results from the nonrandomized continued access cohort of the PARTNER trial. Ann Thorac Surg. 2013;96:2083-2089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 103. | Chaturvedi A, Hobbs SK, Ling FS, Chaturvedi A, Knight P. MRI evaluation prior to Transcatheter Aortic Valve Implantation (TAVI): When to acquire and how to interpret. Insights Imaging. 2016;7:245-254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 104. | Jabbour A, Ismail TF, Moat N, Gulati A, Roussin I, Alpendurada F, Park B, Okoroafor F, Asgar A, Barker S. Multimodality imaging in transcatheter aortic valve implantation and post-procedural aortic regurgitation: comparison among cardiovascular magnetic resonance, cardiac computed tomography, and echocardiography. J Am Coll Cardiol. 2011;58:2165-2173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 157] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 105. | Pouleur AC, le Polain de Waroux JB, Pasquet A, Vanoverschelde JL, Gerber BL. Aortic valve area assessment: multidetector CT compared with cine MR imaging and transthoracic and transesophageal echocardiography. Radiology. 2007;244:745-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 97] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 106. | Paelinck BP, Van Herck PL, Rodrigus I, Claeys MJ, Laborde JC, Parizel PM, Vrints CJ, Bosmans JM. Comparison of magnetic resonance imaging of aortic valve stenosis and aortic root to multimodality imaging for selection of transcatheter aortic valve implantation candidates. Am J Cardiol. 2011;108:92-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 107. | Eichenberger AC, Jenni R, von Schulthess GK. Aortic valve pressure gradients in patients with aortic valve stenosis: quantification with velocity-encoded cine MR imaging. AJR Am J Roentgenol. 1993;160:971-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 63] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 108. | Koos R, Altiok E, Mahnken AH, Neizel M, Dohmen G, Marx N, Kühl H, Hoffmann R. Evaluation of aortic root for definition of prosthesis size by magnetic resonance imaging and cardiac computed tomography: implications for transcatheter aortic valve implantation. Int J Cardiol. 2012;158:353-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 109. | Auerbach EG, Martin ET. Magnetic resonance imaging of the peripheral vasculature. Am Heart J. 2004;148:755-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 110. | Dweck MR, Joshi S, Murigu T, Alpendurada F, Jabbour A, Melina G, Banya W, Gulati A, Roussin I, Raza S. Midwall fibrosis is an independent predictor of mortality in patients with aortic stenosis. J Am Coll Cardiol. 2011;58:1271-1279. [PubMed] |
| 111. | Weidemann F, Herrmann S, Störk S, Niemann M, Frantz S, Lange V, Beer M, Gattenlöhner S, Voelker W, Ertl G. Impact of myocardial fibrosis in patients with symptomatic severe aortic stenosis. Circulation. 2009;120:577-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 564] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 112. | Azevedo CF, Nigri M, Higuchi ML, Pomerantzeff PM, Spina GS, Sampaio RO, Tarasoutchi F, Grinberg M, Rochitte CE. Prognostic significance of myocardial fibrosis quantification by histopathology and magnetic resonance imaging in patients with severe aortic valve disease. J Am Coll Cardiol. 2010;56:278-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 415] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 113. | Irkle A, Vesey AT, Lewis DY, Skepper JN, Bird JL, Dweck MR, Joshi FR, Gallagher FA, Warburton EA, Bennett MR. Identifying active vascular microcalcification by (18)F-sodium fluoride positron emission tomography. Nat Commun. 2015;6:7495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 356] [Cited by in RCA: 378] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 114. | Dweck MR, Jenkins WS, Vesey AT, Pringle MA, Chin CW, Malley TS, Cowie WJ, Tsampasian V, Richardson H, Fletcher A. 18F-sodium fluoride uptake is a marker of active calcification and disease progression in patients with aortic stenosis. Circ Cardiovasc Imaging. 2014;7:371-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 206] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 115. | Dweck MR, Jones C, Joshi NV, Fletcher AM, Richardson H, White A, Marsden M, Pessotto R, Clark JC, Wallace WA. Assessment of valvular calcification and inflammation by positron emission tomography in patients with aortic stenosis. Circulation. 2012;125:76-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 254] [Article Influence: 18.1] [Reference Citation Analysis (0)] |