Published online Mar 26, 2017. doi: 10.4330/wjc.v9.i3.207
Peer-review started: October 17, 2016
First decision: November 14, 2016
Revised: November 15, 2016
Accepted: December 7, 2016
Article in press: December 9, 2016
Published online: March 26, 2017
Processing time: 165 Days and 0.5 Hours
In the last few decades, the recommended treatment for coronary artery disease has been dramatically improved by percutaneous coronary intervention (PCI) and the use of balloon catheters, bare metal stents (BMSs), and drug-eluting stents (DESs). Catheter balloons were burdened by acute vessel occlusion or target-lesion re-stenosis. BMSs greatly reduced those problems holding up the vessel structure, but showed high rates of in-stent re-stenosis, which is characterized by neo-intimal hyperplasia and vessel remodeling leading to a re-narrowing of the vessel diameter. This challenge was overtaken by first-generation DESs, which reduced re-stenosis rates to nearly 5%, but demonstrated delayed arterial healing and risk for late in-stent thrombosis, with inflammatory cells playing a pivotal role. Finally, new-generation DESs, characterized by innovations in design, metal composition, surface polymers, and anti-proliferative drugs, finally reduced the risk for stent thrombosis and greatly improved revascularization outcomes. New advances include bioresorbable stents potentially changing the future of revascularization techniques as the concept bases upon the degradation of the stent scaffold to inert particles after its function expired, thus theoretically eliminating risks linked with both stent thrombosis and re-stenosis. Talking about DESs also dictates to consider dual antiplatelet therapy (DAPT), which is a fundamental moment in view of the good outcome duration, but also deals with bleeding complications. The better management of patients undergoing PCI should include the use of DESs and a DAPT finely tailored in consideration of the potentially developing bleeding risk in accordance with the indications from last updated guidelines.
Core tip: Percutaneous coronary intervention (PCI) has made progress in leaps and bounds in the last 20 years. Complications occurring with catheter balloons and bare metal stents have been overwhelmed by drug-eluting stents (DESs), especially the new-generation ones. They are characterized by innovations in design, metal composition, surface polymers, and anti-proliferative drugs, thus reducing the risk for stent thrombosis and greatly improving revascularization outcomes. DESs also need dual antiplatelet therapy (DAPT), but the latter implies bleeding complications, too. Patients undergoing PCI should be implanted with DESs and DAPT should be tailored on each patient considering the bleeding risk.
- Citation: Bonaventura A, Montecucco F, Liberale L. Coronary stenting: A matter of revascularization. World J Cardiol 2017; 9(3): 207-211
- URL: https://www.wjgnet.com/1949-8462/full/v9/i3/207.htm
- DOI: https://dx.doi.org/10.4330/wjc.v9.i3.207
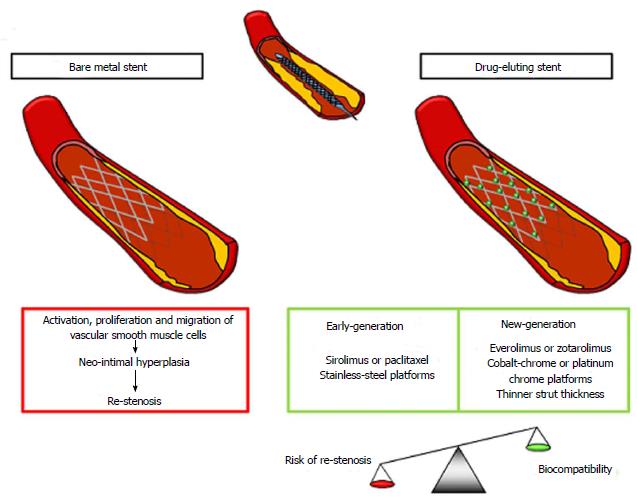
Since the 1990s, percutaneous coronary intervention (PCI) has brought a revolution in the field of coronary artery disease (CAD). Coronary stents have been found to efficiently halt dissection flaps and restore the round lumen in order to decrease the possibility of acute occlusion of the vessel. Bare metal stents (BMSs) have been demonstrated to limit early vessel recoil and late remodeling, justifying the lower rates of re-stenosis with respect to balloon angioplasty[1] and the favorable outcomes in terms of mortality, myocardial infarction, and stent thrombosis (ST) (Figure 1)[2]. Nonetheless, they also increased the formation of the neo-intima layer leading to re-stenosis, which was partially limited by the thinning of stent struts[3,4]. Hence, the development of drug-eluting stents (DESs) was required (Figure 1). Early DESs are characterized by a metallic structure coupled with anti-proliferative drugs, usually controlled by surface polymers demonstrating a lower risk of clinical re-stenosis compared to BMSs[2] and reduced angiographic target-vessel revascularization[5]. New-generation DESs featured durable or biodegradable polymer-coated, metallic, thin scaffold releasing anti-proliferative drugs, thus improving the post-PCI vessel injury and the healing response leading to neo-intimal hyperplasia[6]. In general, DESs naturally limiting healing processes can lead to an incomplete endothelization, which appears as a main contributor to ST resulting in acute myocardial infarction and mortality rates ranging from 20% to 40%[6].
According to the 2014 European Society of Cardiology (ESC)/European Association for Cardio-Thoracic Surgery (EACTS) guidelines, revascularization by either PCI or coronary artery by-pass graft (CABG) is generally indicated in coronary stenoses leading to a reduced flow in order to limit myocardial ischemia, relieve symptoms, and improve the prognosis[7]. Several studies concluded that neither PCI nor CABG alone provided a definitive solution for the entire spectrum of stable CAD needing revascularization, which should be considered as a complementary to the medical therapy. We believe that an exhaustive discussion about PCI or CABG indications would deserve appropriate focus in systematic reviews, meta-analyses or position papers. Therefore, additional speculation appears out of the scope to the present editorial[7].
As stated by the 2014 ESC/EACTS guidelines, no indication for BMSs over new-generation DESs is stated, irrespective of patient and lesion subset[7]. Similarly, in randomized clinical trials, BMSs and DESs did not significantly differ in long-term rates of death or myocardial infarction[2,8]. DESs have been described to better prevent coronary re-stenosis[9]. New-generation DESs have been found to reduce ST rates[10-12], being safer and more efficient than early DESs[13,14]; finally, new-generation DESs were demonstrated to decrease the rates of death and myocardial infarction[10,11,15].
ST is a relatively rare complication (around 1% up to 3 years) characterized by angiographic or post-mortem evidence of a thrombus in a stented segment of the coronary tree[6]. The definition includes definite, probable, and possible ST according to the presence of a thrombus and the angiographic detection of an occlusion or not[16]. Moreover, ST can be divided between early (within the first 30 d from stent implantation) and late (beyond 30 d), with the former accounting for the great majority of the cases[17]. Recognized risk factors can be attributed to patient characteristics, such as diabetes, impaired left ventricular function, and premature antiplatelet disruption; stent features (BMS vs DES); and procedure-related problems, such as primary PCI, stent undersizing, and residual dissection or stenosis. The most important mean to prevent ST is represented by the prescription of an appropriate duration of a post-PCI dual antiplatelet therapy (DAPT).
Re-stenosis is defined as a re-narrowing of more than 50% of the vessel diameter when evaluated by angiography technique or as a re-narrowing of more than 75% of the reference vessel area in cross-section when measured by intravascular imaging techniques[6]. The pathophysiology starts with the vessel injury caused by BMS implantation, followed by neo-intimal hyperplasia, inflammation and remodeling of the coronary vessel[18]. Risk factors for in-stent re-stenosis can be considered as patient-dependent (such as diabetes and chronic renal disease), stent-dependent (such as BMS vs DES and early vs new-generation DESs), and procedure-related (such as small vessel, residual stenosis, longer stented segment, and bifurcation lesion)[6].
In light of new stent design and different scaffold composition, Bønaa et al[19] have evaluated new-generation DESs and new-generation BMSs in a randomized trial conducted in eight centers in Norway, named Norwegian Coronary Stent Trial (NORSTENT). Among more than 20000 patients undergoing PCI between 2008 and 2011, 12425 met the eligibility criteria and 9013 were randomly assigned to either DESs or BMSs. After a 5-five year follow-up period, no significant differences were found between groups for either the primary outcome (composite of death from any cause and non-fatal spontaneous myocardial infarction) or the secondary ones (death; fatal and non-fatal spontaneous and peri-procedural myocardial infarction and stroke; hospitalization for unstable angina pectoris). Interestingly, even if not considered as the primary end-point, new-generation DESs have shown better performances than BMSs in terms of rates of any revascularization (16.5% vs 19.8%, respectively, P < 0.001), target-lesion revascularization (5.3% vs 10.3%, respectively, P < 0.001) both for PCI and coronary artery bypass graft surgery, and definite ST (0.8% vs 1.2%, P = 0.0498).
This trial is worth being considered not only for its results, but also because it is well-designed, correctly powered, and especially not sponsored by industry. Results are very interesting as they partly oppose to those who claimed that there is no longer a role for BMSs in PCI because of the larger superiority of DESs; these conclusions are surely derived from studies which were underpowered, only observational or from meta-analyses pooling results[15,20,21]. Moreover, results from the NORSTENT trial are important because they are not centered on death or recurrent myocardial infarction, certainly reduced also by lifestyle modifications and appropriate drugs, but rather on reduction of need for revascularization and ST. Indeed, in the latter case, the result in absolute terms is very encouraging, but between-group difference is to be considered to the extreme limit of the statistical significance (P = 0.0498).
As things stand at present, new-generation DESs are to be preferred in the majority of clinical situations. Recent recommendations from the American College of Cardiology/American Heart Association allow a shorter duration of DAPT to 6 mo in patients developing a high risk of bleeding[22]. If this possibility is considered, the choice of DESs with respect to BMSs becomes surely more attractive. Moreover, several trials have demonstrated that a prolonged DAPT (over 12 or 24 mo) did not add benefits in terms of major cardiovascular events, including ST, across a median 2- or 5-year follow-up period, but rather increased the frequency of bleeding complications[23,24]. These data have also been confirmed in a meta-analysis by Valgimigli et al[25], who did not find any significant difference in ischemic end-points, such as cardiac death, myocardial infarction with or without stroke, and death from any or cardiovascular causes. Indeed, in spite of the poor number of ST, prolonged DAPT duration has not been shown to provide a decrease in the definite or probable ST when compared to shorter DAPT duration. Moreover, clear evidence for prolonged DAPT for 1 year or more was found for major bleeding events and the risk of stroke[25]. Recently, Helft et al[26] partially confirmed the same results in the OPTImal DUAL antiplatelet therapy trial showing no reduction in adverse clinical events in the prolonged DAPT group and no apparent difference in the major bleeding rate, even if the reduced trial power has to be considered in this case; interestingly, STs were rare with no between-group differences.
Anyway, it is important to underline that BMSs might be recommended in patients with a large vessel diameter, and they show low re-stenosis rates, with poor compliance to DAPT or need for non-cardiac surgery, with reimbursement problems, and with increased risk of bleeding (such as patients with recent bleeding or under concurrent anticoagulation therapy), as indicated by Morice et al[27]. The latter study showed how the choice of BMSs seems to be guided mainly by the concern of bleeding or poor compliance, considering only 1 mo of DAPT for BMSs compared to 6-12 mo for DESs according to European or American guidelines, and neglecting the potential, future need for revascularization, which is accordingly an ineluctable matter to be considered in terms of costs and quality of life.
In conclusion, DESs have to be considered as the first choice in patients undergoing PCI both in stable CAD and in acute coronary syndrome as they demonstrated to reach better outcomes in terms of mortality, recurrent myocardial infarction, and revascularization. The DAPT duration is still debated depending on bleeding and ischemic risks following stent implantation both changing over time. The known rule of one-year DAPT duration cannot be applied to all patients, but rather the therapy should be tailored for each patient according to the latest guidelines. For example, those treated with new-generation DES for stable coronary disease can be administered DAPT for 6 mo or 3 mo in case of bleeding risk, while for those treated for acute coronary syndrome the choice should be at least 12 mo, which can be reduced to 6 mo in case of developing a high risk bleeding.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): E
P- Reviewer: Puddu PE, Schoenhagen P, Wang CH S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Brophy JM, Belisle P, Joseph L. Evidence for use of coronary stents. A hierarchical bayesian meta-analysis. Ann Intern Med. 2003;138:777-786. [PubMed] |
| 2. | Stettler C, Wandel S, Allemann S, Kastrati A, Morice MC, Schömig A, Pfisterer ME, Stone GW, Leon MB, de Lezo JS. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet. 2007;370:937-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1154] [Cited by in RCA: 1095] [Article Influence: 60.8] [Reference Citation Analysis (0)] |
| 3. | Kastrati A, Mehilli J, Dirschinger J, Dotzer F, Schühlen H, Neumann FJ, Fleckenstein M, Pfafferott C, Seyfarth M, Schömig A. Intracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR-STEREO) trial. Circulation. 2001;103:2816-2821. [PubMed] |
| 4. | Pache J, Kastrati A, Mehilli J, Schühlen H, Dotzer F, Hausleiter J, Fleckenstein M, Neumann FJ, Sattelberger U, Schmitt C. Intracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR-STEREO-2) trial. J Am Coll Cardiol. 2003;41:1283-1288. [PubMed] |
| 5. | Mehilli J, Pache J, Abdel-Wahab M, Schulz S, Byrne RA, Tiroch K, Hausleiter J, Seyfarth M, Ott I, Ibrahim T. Drug-eluting versus bare-metal stents in saphenous vein graft lesions (ISAR-CABG): a randomised controlled superiority trial. Lancet. 2011;378:1071-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 6. | Byrne RA, Joner M, Kastrati A. Stent thrombosis and restenosis: what have we learned and where are we going? The Andreas Grüntzig Lecture ESC 2014. Eur Heart J. 2015;36:3320-3331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 337] [Cited by in RCA: 421] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 7. | Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Jüni P. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014;35:2541-2619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3295] [Cited by in RCA: 3380] [Article Influence: 307.3] [Reference Citation Analysis (0)] |
| 8. | Kirtane AJ, Gupta A, Iyengar S, Moses JW, Leon MB, Applegate R, Brodie B, Hannan E, Harjai K, Jensen LO. Safety and efficacy of drug-eluting and bare metal stents: comprehensive meta-analysis of randomized trials and observational studies. Circulation. 2009;119:3198-3206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 424] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 9. | Stefanini GG, Holmes DR. Drug-eluting coronary-artery stents. N Engl J Med. 2013;368:254-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 546] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 10. | Sabate M, Cequier A, Iñiguez A, Serra A, Hernandez-Antolin R, Mainar V, Valgimigli M, Tespili M, den Heijer P, Bethencourt A. Everolimus-eluting stent versus bare-metal stent in ST-segment elevation myocardial infarction (EXAMINATION): 1 year results of a randomised controlled trial. Lancet. 2012;380:1482-1490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 349] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 11. | Sabaté M, Brugaletta S, Cequier A, Iñiguez A, Serra A, Jiménez-Quevedo P, Mainar V, Campo G, Tespili M, den Heijer P. Clinical outcomes in patients with ST-segment elevation myocardial infarction treated with everolimus-eluting stents versus bare-metal stents (EXAMINATION): 5-year results of a randomised trial. Lancet. 2016;387:357-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 155] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 12. | Jensen LO, Thayssen P, Christiansen EH, Maeng M, Ravkilde J, Hansen KN, Hansen HS, Krusell L, Kaltoft A, Tilsted HH. Safety and Efficacy of Everolimus- Versus Sirolimus-Eluting Stents: 5-Year Results From SORT OUT IV. J Am Coll Cardiol. 2016;67:751-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 109] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 13. | Stefanini GG, Byrne RA, Serruys PW, de Waha A, Meier B, Massberg S, Jüni P, Schömig A, Windecker S, Kastrati A. Biodegradable polymer drug-eluting stents reduce the risk of stent thrombosis at 4 years in patients undergoing percutaneous coronary intervention: a pooled analysis of individual patient data from the ISAR-TEST 3, ISAR-TEST 4, and LEADERS randomized trials. Eur Heart J. 2012;33:1214-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 313] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 14. | Park KW, Kang SH, Velders MA, Shin DH, Hahn S, Lim WH, Yang HM, Lee HY, Van Boven AJ, Hofma SH. Safety and efficacy of everolimus- versus sirolimus-eluting stents: a systematic review and meta-analysis of 11 randomized trials. Am Heart J. 2013;165:241-50.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 15. | Palmerini T, Benedetto U, Biondi-Zoccai G, Della Riva D, Bacchi-Reggiani L, Smits PC, Vlachojannis GJ, Jensen LO, Christiansen EH, Berencsi K. Long-Term Safety of Drug-Eluting and Bare-Metal Stents: Evidence From a Comprehensive Network Meta-Analysis. J Am Coll Cardiol. 2015;65:2496-2507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 378] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 16. | Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, Steg PG, Morel MA, Mauri L, Vranckx P. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344-2351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4265] [Cited by in RCA: 4693] [Article Influence: 260.7] [Reference Citation Analysis (0)] |
| 17. | Kimura T, Morimoto T, Kozuma K, Honda Y, Kume T, Aizawa T, Mitsudo K, Miyazaki S, Yamaguchi T, Hiyoshi E. Comparisons of baseline demographics, clinical presentation, and long-term outcome among patients with early, late, and very late stent thrombosis of sirolimus-eluting stents: Observations from the Registry of Stent Thrombosis for Review and Reevaluation (RESTART). Circulation. 2010;122:52-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 193] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 18. | Costa MA, Simon DI. Molecular basis of restenosis and drug-eluting stents. Circulation. 2005;111:2257-2273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 365] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 19. | Bønaa KH, Mannsverk J, Wiseth R, Aaberge L, Myreng Y, Nygård O, Nilsen DW, Kløw NE, Uchto M, Trovik T. Drug-Eluting or Bare-Metal Stents for Coronary Artery Disease. N Engl J Med. 2016;375:1242-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 395] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 20. | Bangalore S, Kumar S, Fusaro M, Amoroso N, Attubato MJ, Feit F, Bhatt DL, Slater J. Short- and long-term outcomes with drug-eluting and bare-metal coronary stents: a mixed-treatment comparison analysis of 117 762 patient-years of follow-up from randomized trials. Circulation. 2012;125:2873-2891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 466] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 21. | Valgimigli M, Sabaté M, Kaiser C, Brugaletta S, de la Torre Hernandez JM, Galatius S, Cequier A, Eberli F, de Belder A, Serruys PW. Effects of cobalt-chromium everolimus eluting stents or bare metal stent on fatal and non-fatal cardiovascular events: patient level meta-analysis. BMJ. 2014;349:g6427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 22. | Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, Granger CB, Lange RA, Mack MJ, Mauri L. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2016;68:1082-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 919] [Cited by in RCA: 1065] [Article Influence: 118.3] [Reference Citation Analysis (0)] |
| 23. | von Birgelen C, Basalus MW, Tandjung K, van Houwelingen KG, Stoel MG, Louwerenburg JH, Linssen GC, Saïd SA, Kleijne MA, Sen H. A randomized controlled trial in second-generation zotarolimus-eluting Resolute stents versus everolimus-eluting Xience V stents in real-world patients: the TWENTE trial. J Am Coll Cardiol. 2012;59:1350-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 206] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 24. | Valgimigli M, Campo G, Monti M, Vranckx P, Percoco G, Tumscitz C, Castriota F, Colombo F, Tebaldi M, Fucà G. Short- versus long-term duration of dual-antiplatelet therapy after coronary stenting: a randomized multicenter trial. Circulation. 2012;125:2015-2026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 530] [Cited by in RCA: 543] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 25. | Valgimigli M, Park SJ, Kim HS, Park KW, Park DW, Tricoci P, Ferrante G. Benefits and risks of long-term duration of dual antiplatelet therapy after drug-eluting stenting: a meta-analysis of randomized trials. Int J Cardiol. 2013;168:2579-2587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Helft G, Le Feuvre C, Georges JL, Carrie D, Leclercq F, Eltchaninoff H, Furber A, Prunier F, Sebagh L, Cattan S. Efficacy and safety of 12 versus 48 months of dual antiplatelet therapy after implantation of a drug-eluting stent: the OPTImal DUAL antiplatelet therapy (OPTIDUAL) trial: study protocol for a randomized controlled trial. Trials. 2013;14:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Morice MC, Urban P, Greene S, Schuler G, Chevalier B. Why are we still using coronary bare-metal stents? J Am Coll Cardiol. 2013;61:1122-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |