Published online Feb 26, 2017. doi: 10.4330/wjc.v9.i2.154
Peer-review started: October 17, 2016
First decision: November 14, 2016
Revised: November 25, 2016
Accepted: December 13, 2016
Article in press: December 15, 2016
Published online: February 26, 2017
Processing time: 132 Days and 9.6 Hours
To investigate the impact of timing of same-admission orthotopic heart transplant (OHT) after left ventricular assist device (LVAD) implantation on in-hospital mortality and post-transplant length of stay.
Using data from the Nationwide Inpatient Sample from 1998 to 2011, we identified patients 18 years of age or older who underwent implantation of a LVAD and for whom the procedure date was available. We calculated in-hospital mortality for those patients who underwent OHT during the same admission as a function of time from LVAD to OHT, adjusting for age, sex, race, household income, and number of comorbid diagnoses. Finally, we analyzed the effect of time to OHT after LVAD implantation on the length of hospital stay post-transplant.
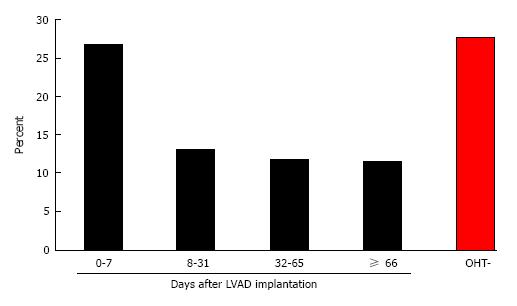
Two thousand and two hundred patients underwent implantation of a LVAD in this cohort. One hundred and sixty-four (7.5%) patients also underwent OHT during the same admission, which occurred on average 32 d (IQR 7.75-66 d) after LVAD implantation. Of patients who underwent OHT, patients who underwent transplantation within 7 d of LVAD implantation (“early”) experienced increased in-hospital mortality (26.8% vs 12.2%, P = 0.0483) compared to patients who underwent transplant after 8 d (“late”). There was no statistically significant difference in age, sex, race, household income, or number of comorbid diagnoses between the early and late groups. Post-transplant length of stay after LVAD implantation was also not significantly different between patients who underwent early vs late OHT.
In this cohort of patients who received LVADs, the rate of in-hospital mortality after OHT was lower for patients who underwent late OHT (at least 8 d after LVAD implantation) compared to patients who underwent early OHT. Delayed timing of OHT after LVAD implantation did not correlate with longer hospital stays post-transplant.
Core tip: The optimal timing of same-admission orthotopic heart transplantation (OHT) after the implantation of a left ventricular assist device (LVAD) is unknown. The need for clinical stability and time to recover from surgery is counterbalanced by the risk of LVAD complications and formation of adhesions and scarring, particularly when OHT is considered early after LVAD implantation. We reviewed adult patients in the Nationwide Inpatient Sample who underwent same-admission OHT after LVAD between 1998 and 2011. Compared to early transplantation after LVAD, OHT after 8 d of LVAD implantation was associated with decreased mortality risk without increased post-transplant length of stay.
- Citation: Gulati G, Ouyang D, Ha R, Banerjee D. Optimal timing of same-admission orthotopic heart transplantation after left ventricular assist device implantation. World J Cardiol 2017; 9(2): 154-161
- URL: https://www.wjgnet.com/1949-8462/full/v9/i2/154.htm
- DOI: https://dx.doi.org/10.4330/wjc.v9.i2.154
Heart failure (HF) affects an estimated 5.8 million people in the United States and contributes to over 300000 deaths every year[1,2]. It is the most common cause of hospital admission and readmission in people greater than 65 years of age, annually accounting for over 2.4 million hospitalizations[2,3] and $39 billion in healthcare costs[1,4]. Although most patients respond favorably to standard medical treatment, a considerable number of patients progress to end-stage HF refractory to medical therapy[5]. Currently, orthotopic heart transplant (OHT) is the gold standard therapy for these patients[6-8], but the number of donor hearts available for transplantation is far fewer than the number of patients on the transplant list. For this reason, left ventricular assist devices (LVADs), a class of electromechanical devices used for cardiac circulatory support, are increasingly being used to bridge patients to cardiac transplantation[5].
The REMATCH trial in 2001 showed significant mortality reductions in patients placed on a pulsatile-flow LVAD compared to standard medical treatment[9]. Several subsequent studies since have confirmed the survival benefit of both the older pulsatile and newer continuous-flow LVADs[10-13]. Although LVADs have substantially reduced mortality in end-stage HF patients, the absolute mortality rates still remain high. A large portion of this mortality is attributable to complications and other occurrences during the patient’s stay in the hospital[14]. In-hospital mortality rates as high as 27% have been reported in patients after LVAD surgery[15-18].
As the rate of LVAD implantation in the United States continues to increase[19-22], effective recommendations for the in-hospital management of LVAD implantation are needed. Although the majority of cardiac transplants performed after LVAD implantation occur after a patient has been discharged from hospital, there is an important cohort of patients who cannot be discharged from hospital post-LVAD implant due to severe right ventricular failure, arrhythmias refractory to oral therapy, and infectious complications. Patients bridged to OHT with a LVAD achieve similar survival rates as patients who undergo direct heart transplant[14], but there is little data to guide clinicians on the optimal timing of same-admission OHT after LVAD implantation. Though patients receiving LVADs may be considered for OHT while still inpatients, some have argued that performing OHT early after LVAD placement poses an increased risk of morbidity and mortality to patients.
Past studies on the appropriate use and outcomes of LVADs have been mostly limited to institutional experience and case series of select populations. While such descriptive investigations are useful, they are often limited by small sample size and variation between institutions and comparison groups. We used the Nationwide Inpatient Sample (NIS), the largest national database of hospitalizations in the United States with data from over 36 million hospitalizations, to assess the optimal timing of OHT after LVAD implantation. The NIS dataset complements the UNOS database and INTERMACS dataset with additional information on patient comorbidities, additional same-hospitalization procedures, hospital and center characteristics, and markers of patient’s socioeconomic status including insurance provider and regional income quartiles. In addition, the NIS dataset contains data on both LVAD and inpatient OHT, which are not simultaneously available in the UNOS or INTERMACS databases.
We analyzed a patient cohort who had OHT performed during the same admission after LVAD implantation. We hypothesized that early OHT after LVAD implantation would be associated with higher mortality than late OHT, and that the hospital length of stay (LOS) after early OHT would be less than LOS after late OHT.
The NIS, from the Healthcare Cost and Utilization Project sponsored by the Agency for Healthcare Research and Quality is the largest database of all-payer inpatient discharge information, sampling approximately 20% of all non-federal United States hospitals and including approximately 9 million hospital admissions each year. It contains discharge data from over 5000 hospitals located across 45 states, of which approximately 1200 hospitals are sampled each year to create a stratified sample of United States hospitals. Each NIS entry includes all diagnosis and procedure codes of activity during the patient’s hospitalization at the time of discharge, as well as patient demographics, hospital characteristics, and short-term complications of the hospitalization.
This was a retrospective cross-sectional study using the NIS between 1998 and 2011. We identified all hospitalizations from 1998 to 2011 of patients 18 years of age or older who underwent LVAD implantation and for whom the hospital day of each procedure was available. Procedures during the hospitalization in addition to LVAD placement, including OHT, extracorporeal membrane oxygenation, intubation, hemodialysis, invasive hemodynamic monitoring, and surgical revision were identified by associated ICD9 codes (Supplementary Table 1). Additionally, hospital mortality and perioperative morbidity such as post-operative infections, cardiopulmonary complications, and hemorrhagic complications requiring endoscopy were identified.
| 0-7 d (n = 41) | 8-31 d (n = 38) | 32-65 d (n = 42) | ≥ 66 d (n = 43) | No OHT (n = 2036) | |
| Length of stay, mean ± SD | 39.3 ± 33.2 | 48.9 ± 25.6 | 85.8 ± 40.1 | 151.2 ± 52.6 | 37.1 ± 34.6 |
| Length of stay after OHT, mean ± SD | 23.8 ± 21.4 | 21.7 ± 15.8 | 27.6 ± 37.1 | 27.1 ± 22.8 | NA |
| Mortality, n (%) | 11 (26.8) | 5 (13.2) | 5 (11.9) | 5 (11.6) | 564 (27.3) |
| Age, mean ± SD | 50.6 ± 12.6 | 48.6 ± 12.7 | 47.4 ± 15.3 | 46.3 ± 13.1 | 55.4 ± 13.2 |
| Sex, n (%) | |||||
| Male | 33 (80.5) | 32 (84.2) | 35 (83.3) | 34 (79.1) | 1525 (74.9) |
| Female | 8 (19.5) | 6 (15.8) | 7 (16.7) | 9 (20.9) | 511 (25.1) |
| Race, n (%) | |||||
| White | 25 (61.0) | 19 (50.0) | 23 (54.8) | 22 (51.2) | 1185 (58.2) |
| Black | 3 (7.3) | 5 (13.2) | 8 (19.0) | 6 (14.0) | 330 (16.2) |
| Hispanic | 3 (7.3) | 7 (18.4) | 2 (4.8) | 5 (11.6) | 125 (6.1) |
| Asian/Pacific Islander | 2 (4.9) | 0 (0.0) | 1 (2.4) | 4 (9.3) | 44 (2.2) |
| Native American | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 5 (0.2) |
| Other or unknown | 8 (19.5) | 7 (18.4) | 8 (19.0) | 6 (14.0) | 347 (17.0) |
| Median household income, n (%) | |||||
| $1-24999 | 4 (9.8) | 8 (21.1) | 8 (19.0) | 8 (18.6) | 447 (22.0) |
| $25000-34999 | 10 (24.4) | 10 (26.3) | 10 (23.8) | 7 (16.3) | 454 (22.3) |
| $35000-44999 | 12 (29.3) | 8 (21.1) | 10 (23.8) | 13 (30.2) | 509 (25.0) |
| $45000 or more | 129 (29.3) | 12 (31.6) | 14 (33.3) | 14 (32.6) | 579 (28.4) |
| Unknown | 3 (7.3) | 0 (0.0) | 0 (0.0) | 1 (2.3) | 47 (2.3) |
| Comorbidities | |||||
| Diabetes | 8 (19.5) | 5 (13.2) | 4 (9.5) | 2 (4.7) | 373 (18.3) |
| Hyperlipidemia | 5 (12.2) | 2 (5.3) | 3 (7.1) | 3 (7.0) | 297 (14.6) |
| Hypertension | 5 (12.2) | 1 (2.6) | 2 (4.8) | 2 (4.7) | 291 (14.3) |
| History of smoking | 5 (12.2) | 2 (5.3) | 0 (0.0) | 0 (0.0) | 137 (6.7) |
| BMI ≥ 30 kg/m2 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 96 (4.7) |
| No. of comorbid diagnoses, mean ± SD | 11.9 ± 3.1 | 12.3 ± 3.0 | 12.5 ± 3.2 | 12.5 ± 3.2 | 12.8 ± 2.9 |
| Location of hospital, n (%) | |||||
| Rural | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 17 (0.8) |
| Urban | 41 (100.0) | 38 (100.0) | 42 (100.0) | 43 (100.0) | 2017 (99.1) |
| Unknown | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (0.1) |
| Size of hospital, n (%) | |||||
| Small | 4 (9.8) | 0 (0.0) | 0 (0.0) | 2 (4.7) | 32 (1.6) |
| Medium | 7 (17.0) | 6 (15.8) | 5 (11.9) | 0 (0.0) | 211 (10.4) |
| Large | 30 (73.2) | 32 (84.2) | 37 (88.1) | 41 (95.3) | 1791 (88.0) |
| Unknown | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (0.1) |
| Teaching status of hospital, n (%) | |||||
| Nonteaching | 1 (2.4) | 1 (2.6) | 2 (4.8) | 1 (2.3) | 160 (7.9) |
| Teaching | 40 (97.6) | 37 (97.4) | 40 (95.2) | 42 (97.7) | 1874 (92.0) |
| Unknown | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (0.1) |
The statistical methods of this study were reviewed by Dr. David Ouyang from the Stanford University Department of Medicine. Python 2.7 (Python Software Foundation, http://www.python.org) and R 2.13 (R Foundation, http://www.r-project.org) were used for statistical analysis. P-values for numerical and count data were calculated by two-sided t-tests and χ2 tests, respectively, with significance thresholds of 0.05. The multivariate linear model evaluating post-LVAD OHT mortality was performed using a generalized linear model with input variable selection by Bayesian Information Criteria (BIC). The dependent variable was in-hospital mortality. Independent variables of age, gender, median income, race, number of comorbidities, LVAD era, and timing of OHT were evaluated in the model.
We identified 2200 patients greater than 18 years of age between 1998 and 2011 who underwent LVAD implantation and for whom hospital day of procedure was listed (66.4% of all LVAD patients in the NIS database 1998-2011). Comparison of baseline characteristics between this study sample and all LVAD patients in the NIS 1998-2011 database confirmed that our study sample is representative of the entire patient population. The two groups were well matched based on age, sex, household income, prevalence of comorbidities, length of stay, and number of comorbidities, however there were more patients without documented race in the overall group (Supplementary Table 2). The mean age of all patients was 53.4 years (SD = 13.7, range = 18-92 years). Baseline patient demographics, patient comorbidities, and hospital characteristics were well matched between LVAD patients with and without same-admission OHT (Table 1). Most LVAD implantations were performed in large (87.8%), urban (99.1%), teaching hospitals (92.4%). The most common comorbidities were diabetes (17.8%), disorders of lipid metabolism (14.1%), hypertension (13.7%), history of or current use of tobacco (6.5%), and BMI ≥ 30 kg/m2 (4.4%). The mean day of LVAD implantation was 9.4 d (SD = 12.5 d) into the hospitalization. The overall in-hospital mortality rate was 26.8%, with respiratory failure, cardiac dysrhythmias, right HF, and renal failure among the most frequent in-hospital complications immediately following LVAD implantation (Table 2).
| Early OHT (n = 41) | Late OHT (n = 123) | OHT- (n = 2036) | Total (n = 2200) | |
| Acute renal failure | 24 (58.5) | 64 (52.0) | 963 (47.3) | 1051 (47.8) |
| Reoperation | 28 (68.3) | 87 (70.7) | 803 (39.4) | 918 (41.7) |
| Bleeding requiring transfusion | 7 (17.1) | 30 (24.4) | 780 (38.3) | 817 (37.1) |
| Acute respiratory failure | 8 (19.5) | 37 (30.1) | 518 (25.4) | 563 (25.6) |
| Sepsis | 2 (4.9) | 17 (13.8) | 233 (11.4) | 252 (11.5) |
| Postoperative cardiac complication | 7 (17.1) | 15 (12.2) | 234 (11.5) | 256 (11.6) |
| Acute liver failure | 3 (7.3) | 9 (7.3) | 224 (11.0) | 236 (10.7) |
| Device failure | 0 (0.0) | 4 (3.3) | 62 (3.0) | 66 (3.0) |
| Stroke | 1 (2.4) | 1 (0.8) | 53 (2.6) | 55 (2.5) |
Our dataset includes patients from both the pulsatile-flow era (1998-2005) and the continuous-flow era (2006- 2011) of mechanical support (Table 3). Comparing the two eras, there was significantly less mortality in the continuous-flow era compared to the pulsatile-flow era (20.4% vs 43.0%; P < 0.001) even as patients were older (55.4 years vs 53.2 years; P < 0.001) and suffering more comorbid diagnoses (13.5 vs 10.6; P < 0.001). During the continuous-flow era, fewer patients received OHT during the same admission as LVAD implantation (3.8% vs 17.3%; P < 0.001), and mechanical support was more frequently initiated in large (88.8% vs 85.1%; P = 0.002), teaching (94.4% vs 87.1%; P < 0.001) institutions. Median household income quartile and race distribution also were different between the two eras, although there was no difference in gender ratio of patients.
| All LVADs (n = 2200) | 1998-2005 (n = 589) | 2006-2011 (n = 1611) | P-valuea | |
| Mortality, n (%) | 590 (26.5) | 253 (43.0) | 329 (20.4) | < 0.001 |
| Same admission OHT, n (%) | 164 (7.5) | 102 (17.3) | 62 (3.8) | < 0.001 |
| Early same admission OHT, n (%) | 41 (25.0) | 22 (21.6) | 19 (30.6) | 0.373 |
| Early same admission OHT mortality, n (%) | 11 (26.8) | 8 (36.4) | 3 (15.8) | 0.319 |
| Length of stay after early OHT, mean ± SD | 23.8 ± 21.4 | 30.9 ± 26.0 | 17.6 ± 14.3 | 0.054 |
| Late same admission OHT, n (%) | 123 (75.0) | 80 (78.4) | 43 (69.4) | 0.849 |
| Late same admission OHT mortality, n (%) | 15 (12.2) | 11 (13.8) | 4 (9.3) | 0.774 |
| Length of stay after late OHT, mean ± SD | 25.6 ± 26.9 | 26.1 ± 22.9 | 25.4 ± 29.0 | 0.883 |
| Length of stay, mean ± SD | 40.5 ± 38.9 | 44.7 ± 48.6 | 39.0 ± 34.6 | 0.008 |
| Age, mean ± SD | 53.4 ± 13.7 | 53.2 ± 13.4 | 55.4 ± 13.4 | < 0.001 |
| Sex, n (%) | ||||
| Male | 1659 (75.4) | 433 (73.5) | 1226 (76.1) | 0.23 |
| Female | 541 (24.6) | 156 (26.5) | 385 (23.9) | |
| Race, n (%) | < 0.001 | |||
| White | 1274 (57.9) | 327 (55.5) | 947 (58.8) | |
| Black | 352 (16.0) | 62 (10.5) | 290 (18.0) | |
| Hispanic | 142 (6.5) | 28 (4.8) | 114 (7.1) | |
| Asian/Pacific Islander | 51 (2.3) | 13 (2.2) | 38 (2.4) | |
| Native American | 5 (0.2) | 1 (0.2) | 4 (0.2) | |
| Other or unknown | 376 (17.1) | 143 (24.3) | 148 (9.2) | |
| Median household income, n (%) | < 0.001 | |||
| $1-24999 | 475 (21.6) | 88 (14.9) | 387 (24.0) | |
| $25000-34999 | 491 (22.3) | 126 (21.4) | 365 (22.7) | |
| $35000-44999 | 552 (25.1) | 141 (23.9) | 411 (25.5) | |
| $45000 or more | 631 (28.7) | 214 (36.3) | 417 (25.9) | |
| Unknown | 51 (2.3) | 20 (3.4) | 31 (2.4) | |
| Comorbidities | ||||
| Diabetes | 391 (17.8) | 91 (15.4) | 300 (18.6) | 0.097 |
| Hyperlipidemia | 310 (14.1) | 61 (10.4) | 249 (15.5) | 0.003 |
| Hypertension | 309 (14.0) | 88 (14.9) | 221 (13.7) | 0.508 |
| History of smoking | 131 (6.0) | 29 (4.9) | 102 (6.3) | 0.257 |
| BMI ≥ 30 kg/m2 | 96 (4.4) | 12 (2.0) | 84 (5.2) | 0.002 |
| No. of comorbid diagnosis, mean ± SD | 12.7 ± 2.9 | 10.6 ± 2.9 | 13.5 ± 2.5 | < 0.001 |
| Location of hospital, n (%) | 0.73 | |||
| Rural | 17 (0.8) | 5 (0.8) | 12 (0.7) | |
| Urban | 2181 (99.1) | 583 (99.0) | 1598 (99.2) | |
| Unknown | 2 (0.1) | 1 (0.2) | 1 (0.1) | |
| Size of hospital, n (%) | 0.002 | |||
| Small | 38 (1.7) | 20 (3.4) | 18 (1.1) | |
| Medium | 229 (10.4) | 67 (11.4) | 162 (10.1) | |
| Large | 1931 (87.8) | 501 (85.1) | 1430 (88.8) | |
| Unknown | 2 (0.1) | 1 (0.2) | 1 (0.1) | |
| Teaching status of hospital, n (%) | < 0.001 | |||
| Nonteaching | 165 (7.5) | 75 (12.7) | 90 (5.6) | |
| Teaching | 2033 (92.4) | 513 (87.1) | 1520 (94.4) | |
| Unknown | 2 (0.1) | 1 (0.2) | 1 (0.1) |
Of the patients who underwent LVAD implantation, 164 (7.5%) also underwent OHT during the same admission. OHT occurred a median of 32 d (IQR 7.75-66 d) after LVAD implantation. Patients who underwent OHT at least 8 d after LVAD implantation experienced significantly lower mortality compared to patients who underwent OHT earlier (26.8% vs 12.2%; P = 0.048; Table 1 and Figure 1). Baseline patient demographics, patient comorbidities, and hospital characteristics were similar between the early and late OHT groups. LVAD patients who underwent late OHT also had lower mortality compared to LVAD patients who were not transplanted (12.2% vs 27.0%; P < 0.001). However, LVAD patients who underwent early transplant did not experience a similar mortality benefit (26.8% vs 27.0%; P = 0.946). The reduced mortality trend with delayed OHT post-LVAD was observed in both the pulsatile-flow (13.8% vs 36.4%; P = 0.081) and continuous-flow eras (9.3% vs 15.8%; P = 0.672), although due to small sample numbers in each subgroup, the differences were not statistically significant (Table 2). Multivariate linear model also confirmed the strong association between early OHT after LVAD and in-hospital mortality, independent of patient age, LVAD era, comorbidities, and demographics (Table 4).
| Regression coefficient | Standard error | P-value | |
| Age | 0.003 | 0.002 | 0.158 |
| Female sex | 0.071 | 0.075 | 0.342 |
| Caucasian race | -0.01 | 0.027 | 0.695 |
| Median household income | 0.013 | 0.027 | 0.638 |
| Number of comorbidities | 0.006 | 0.010 | 0.518 |
| Years 1998-2005 | 0.096 | 0.060 | 0.113 |
| Early OHT | 0.2 | 0.067 | 0.004a |
Comparing the quartiles of post-LVAD OHT transplant times, there was no statistically significant difference in post-transplant length of stay (23.8 ± 21.4 d for the first quartile, 21.7 ± 15.8 d for the second quartile, 27.6 ± 37.1 d for the third quartile, 27.1 ± 22.8 d for the fourth quartile; P = 0.6571 comparing first quartile to other quartiles; Table 1). However, as expected, patients who waited longer after LVAD implantation for OHT had longer overall hospital stays (39.3 ± 33.2 d for the first quartile, 48.87 ± 25.6 d for the second quartile, 85.8 ± 40.1 d for the third quartile, 151.2 ± 52.6 d for the fourth quartile; P < 0.001 comparing first quartile to other quartiles; Table 1).
Our study addresses the difficult question of timing of same-admission OHT after LVAD implantation. Using the inpatient data on procedure timing from the NIS 1998-2011, we show that mortality risk significantly decreases in patients who undergo OHT at least 8 d after LVAD implantation. We also report that post-transplant length of stay is independent of the timing of OHT after LVAD.
For patients who receive an LVAD for bridge to transplant therapy (BTT), the optimal timing of post-LVAD OHT is controversial. The need for clinical stability and time to recover from major surgery is counterbalanced by the risk of LVAD complications and the formation of adhesions and scarring, particularly when OHT is considered early after LVAD implantation.
The high failure rate of the early, pulsatile-flow LVADs had in part led to the initial 1999 UNOS allocation algorithm giving LVAD patients 30 d of IA status on the transplant list. The elective nature of the 30-d IA status allows for optimization of management prior to transplant and suggests the time period immediately post-mechanical support is often not the optimal time for transplant. Our data showing that delaying post-LVAD transplant can lead to superior outcomes is consistent with the excellent long term outcomes of BTT mechanical support, pushing some groups to question the justification of elective IA status[23].
Our study, using a large national database, solidifies and extends previous findings that early transplantation after initiation of BTT mechanical support is associated with worse outcomes. In the pulsatile-flow era of LVAD, John et al[24] (2010) had shown that cardiac transplants done less than 6 wk after LVAD confer higher mortality risk in patients, and Gammie et al[25] (2003) and Ashton et al[26] (1996) have similarly reported optimal timing to be 2 wk after LVAD implantation. With the advantage of procedural timing data of patients who underwent same admission LVAD implantation and transplant, we add to those findings by showing there is an increased mortality associated with early same-admission transplant after LVAD in the continuous flow era.
During the study period between 1998 and 2011, there was a significant increase in the number of LVAD implantations, but patient characteristics of this population - including timing of LVAD, usage of invasive hemodynamic monitoring, and timing of post-LVAD OHT - has remained relatively unchanged. Our sample patient population is representative of LVAD patients studied in other databases with regards to age, gender, race, and other demographic characteristics and also mortality trends between the pulsatile and continuous-flow eras. Without randomized control trials to better characterize the optimal management and timing of transplant after LVAD, our study describes representative clinical practice and trends in outcomes associated with changing practice patterns.
Our study has a few limitations. First, the NIS is a deidentified administrative database dependent on the appropriate coding of individual ICD-9-CM codes. Studies using such databases are susceptible to errors related to coding such as undercoding complications or variation in the application of diagnostic codes. This database also lacks many details available in registries, and unmeasured confounders cannot be excluded. Additionally, the NIS only captures events during the hospitalization, so complications and adverse events after discharge are not recorded. This limitation is counterbalanced by the larger sample size relative to other studies and the absence of reporting bias as compared to studies relying upon the institutional experiences from a few specialized centers. Additionally, patients who undergo LVAD implantation have long hospital stays that capture most, if not all, of the acute complications causing morbidity and mortality. Finally, the ability of the NIS to capture detailed LVAD implantation and OHT data provided advantages in answering our central question over either the INTERMACS or UNOS databases, which capture largely LVAD or transplant data, respectively.
It is important to note that our cohort only assessed outcomes of OHT after LVAD placement in hospitalized patients. This represents a minority of patients (7.5%) in practice, as most institutions prefer to wait 2-3 mo after LVAD implantation to list patients for cardiac transplantation. Nevertheless, there will continue to be patients in the future who receive same-admission OHT after LVAD implantation, and our study provides meaningful guidelines on the timing of such OHT.
In conclusion, our analysis suggests that delayed same-admission OHT after LVAD implantation decreases mortality risk without increasing post-transplant length of stay, and, therefore, may be the preferred option in such a clinical setting. This new understanding of the optimal timing of same-admission OHT after LVAD implantation can greatly improve patient outcomes, although prospective data will be needed to enhance the validity of our findings.
Heart failure (HF) affects an estimated 5.8 million people in the United States and contributes to over 300000 deaths every year. Although most patients respond favorably to standard medical treatment, a considerable number of patients progress to end-stage HF refractory to medical therapy. Orthotopic heart transplant (OHT) is currently the gold standard therapy for these patients, but the number of donor hearts available for transplantation is far fewer than the number of patients on the transplant list. For this reason, left ventricular assist devices (LVADs), a class of electromechanical devices used for cardiac circulatory support, are increasingly being used to bridge patients to OHT. The optimal timing of when patients with LVADs should be bridged to OHT is an important consideration for patient care and has yet to be characterized.
As the rate of LVAD implantation in the United States continues to increase, effective recommendations on the in-hospital management of LVAD implantation are needed. The optimal timing of when to bridge patients with LVADs to OHT remains controversial and is an active area of research.
Few groups have studied the impact of timing of same-admission OHT after LVAD on patient outcomes. Past studies on the appropriate use and outcomes of LVADs have been mostly limited to institutional experience and case series of select populations. The authors used the Nationwide Inpatient Sample (NIS), the largest national database of hospitalizations in the United States with data from over 36 million hospitalizations, to assess the optimal timing of OHT after LVAD implantation. It has been suggested that performing OHT early after LVAD placement confers an increased risk to patient. The study corroborates these claims and concludes that early OHT after LVAD placement (less than 8 d) is associated with increased in-hospital mortality. Therefore, depending on the clinical scenario, it might be reasonable for physicians to defer OHT immediately after LVAD placement.
This study offers recommendations for cardiologists and cardiac surgeons on the optimal timing of same-admission OHT after LVAD implantation. It also summarizes the demographics and characteristics of LVAD and post-LVAD OHT patients in the United States.
Left ventricular assist device (LVAD): A class of electromechanical devices that help the left ventricle pump blood to the rest of the body; Orthotopic heart transplant (OHT): A procedure in which the patient’s heart is removed and replaced with a donor heart.
Very interesting and clinically relevant question with novel use of the NIS database. Overall well written with interesting findings.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Everitt MD, Puddu PE, Zielinski TA S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol. 2011;8:30-41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1550] [Cited by in RCA: 1417] [Article Influence: 101.2] [Reference Citation Analysis (0)] |
| 2. | Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:e46-e215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2338] [Cited by in RCA: 2613] [Article Influence: 174.2] [Reference Citation Analysis (0)] |
| 3. | Blecker S, Paul M, Taksler G, Ogedegbe G, Katz S. Heart failure–associated hospitalizations in the United States. J Am Coll Cardiol. 2013;61:1259-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 301] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 4. | Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1935] [Cited by in RCA: 2122] [Article Influence: 176.8] [Reference Citation Analysis (0)] |
| 5. | Friedrich EB, Böhm M. Management of end stage heart failure. Heart. 2007;93:626-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Fanaroff AC, DeVore AD, Mentz RJ, Daneshmand MA, Patel CB. Patient selection for advanced heart failure therapy referral. Crit Pathw Cardiol. 2014;13:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Carabello BA. Modern management of mitral stenosis. Circulation. 2005;112:432-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Taylor DO, Stehlik J, Edwards LB, Aurora P, Christie JD, Dobbels F, Kirk R, Kucheryavaya AY, Rahmel AO, Hertz MI. Registry of the International Society for Heart and Lung Transplantation: Twenty-sixth Official Adult Heart Transplant Report-2009. J Heart Lung Transplant. 2009;28:1007-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 320] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 9. | Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, Long JW, Ascheim DD, Tierney AR, Levitan RG. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345:1435-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3136] [Cited by in RCA: 2963] [Article Influence: 123.5] [Reference Citation Analysis (0)] |
| 10. | Takeda K, Takayama H, Kalesan B, Uriel N, Colombo PC, Jorde UP, Yuzefpolskaya M, Mancini DM, Naka Y. Outcome of cardiac transplantation in patients requiring prolonged continuous-flow left ventricular assist device support. J Heart Lung Transplant. 2015;34:89-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | McIlvennan CK, Magid KH, Ambardekar AV, Thompson JS, Matlock DD, Allen LA. Clinical outcomes after continuous-flow left ventricular assist device: a systematic review. Circ Heart Fail. 2014;7:1003-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Nativi JN, Drakos SG, Kucheryavaya AY, Edwards LB, Selzman CH, Taylor DO, Hertz MI, Kfoury AG, Stehlik J. Changing outcomes in patients bridged to heart transplantation with continuous- versus pulsatile-flow ventricular assist devices: an analysis of the registry of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2011;30:854-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | John R, Kamdar F, Liao K, Colvin-Adams M, Boyle A, Joyce L. Improved survival and decreasing incidence of adverse events with the HeartMate II left ventricular assist device as bridge-to-transplant therapy. Ann Thorac Surg. 2008;86:1227-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 195] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 14. | Deo SV, Sung K, Daly RC, Shah IK, Altarabsheh SE, Stulak JM, Joyce LD, Boilson BA, Kushwaha SS, Park SJ. Cardiac transplantation after bridged therapy with continuous flow left ventricular assist devices. Heart Lung Circ. 2014;23:224-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Lietz K, Long JW, Kfoury AG, Slaughter MS, Silver MA, Milano CA, Rogers JG, Naka Y, Mancini D, Miller LW. Outcomes of left ventricular assist device implantation as destination therapy in the post-REMATCH era: implications for patient selection. Circulation. 2007;116:497-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 545] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 16. | La Francesca S, Palanichamy N, Kar B, Gregoric ID. First use of the TandemHeart percutaneous left ventricular assist device as a short-term bridge to cardiac transplantation. Tex Heart Inst J. 2006;33:490-491. [PubMed] |
| 17. | Naidu SS. Novel percutaneous cardiac assist devices: the science of and indications for hemodynamic support. Circulation. 2011;123:533-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Kar B, Adkins LE, Civitello AB, Loyalka P, Palanichamy N, Gemmato CJ, Myers TJ, Gregoric ID, Delgado RM. Clinical experience with the TandemHeart percutaneous ventricular assist device. Tex Heart Inst J. 2006;33:111-115. [PubMed] |
| 19. | Lampropulos JF, Kim N, Wang Y, Desai MM, Barreto-Filho JA, Dodson JA, Dries DL, Mangi AA, Krumholz HM. Trends in left ventricular assist device use and outcomes among Medicare beneficiaries, 2004-2011. Open Heart. 2014;1:e000109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 20. | Hasin T, Marmor Y, Kremers W, Topilsky Y, Severson CJ, Schirger JA, Boilson BA, Clavell AL, Rodeheffer RJ, Frantz RP. Readmissions after implantation of axial flow left ventricular assist device. J Am Coll Cardiol. 2013;61:153-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 188] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 21. | Terracciano CM, Miller LW, Yacoub MH. Contemporary use of ventricular assist devices. Annu Rev Med. 2010;61:255-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Miller LW. Left ventricular assist devices are underutilized. Circulation. 2011;123:1552-1558; discussion 1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 23. | Dardas T, Mokadam NA, Pagani F, Aaronson K, Levy WC. Transplant registrants with implanted left ventricular assist devices have insufficient risk to justify elective organ procurement and transplantation network status 1A time. J Am Coll Cardiol. 2012;60:36-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | John R, Pagani FD, Naka Y, Boyle A, Conte JV, Russell SD, Klodell CT, Milano CA, Rogers J, Farrar DJ. Post-cardiac transplant survival after support with a continuous-flow left ventricular assist device: impact of duration of left ventricular assist device support and other variables. J Thorac Cardiovasc Surg. 2010;140:174-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 131] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 25. | Gammie JS, Edwards LB, Griffith BP, Pierson RN, Tsao L. Optimal timing of cardiac transplantation after ventricular assist device implantation. J Thorac Cardiovasc Surg. 2004;127:1789-1799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Ashton RC, Goldstein DJ, Rose EA, Weinberg AD, Levin HR, Oz MC. Duration of left ventricular assist device support affects transplant survival. J Heart Lung Transplant. 1996;15:1151-1157. [PubMed] |