Published online Feb 26, 2017. doi: 10.4330/wjc.v9.i2.109
Peer-review started: October 19, 2016
First decision: November 30, 2016
Revised: December 2, 2016
Accepted: January 2, 2017
Article in press: January 3, 2017
Published online: February 26, 2017
Processing time: 130 Days and 13.5 Hours
Cardiovascular magnetic resonance (CMR) imaging uniquely characterizes myocardial and microvascular injury in acute myocardial infarction (AMI), providing powerful surrogate markers of outcomes. The last 10 years have seen an exponential increase in AMI studies utilizing CMR based endpoints. This article provides a contemporary, comprehensive review of the powerful role of CMR imaging in the assessment of outcomes in AMI. The theory, assessment techniques, chronology, importance in predicting left ventricular function and remodelling, and prognostic value of each CMR surrogate marker is described in detail. Major studies illustrating the importance of the markers are summarized, providing an up to date review of the literature base in CMR imaging in AMI.
Core tip: Cardiovascular magnetic resonance (CMR) imaging uniquely characterizes myocardial and microvascular injury in acute myocardial infarction (AMI). Contrast-enhanced CMR offers robust, validated and reproducible surrogate markers, providing an accurate representation of pathophysiology, assessment of myocardial function and injury, and predictive value for medium to long-term LV function, remodelling and prognosis following primary percutaneous coronary intervention for STEMI. These qualities significantly increase the statistical power of studies using CMR endpoints and has resulted in an exponential increase in AMI studies utilizing CMR based endpoints. An understanding of the role of CMR in the assessment of outcomes in AMI is of key importance not only to interventional and imaging cardiologists, but to the cardiology community as a whole.
- Citation: Khan JN, McCann GP. Cardiovascular magnetic resonance imaging assessment of outcomes in acute myocardial infarction. World J Cardiol 2017; 9(2): 109-133
- URL: https://www.wjgnet.com/1949-8462/full/v9/i2/109.htm
- DOI: https://dx.doi.org/10.4330/wjc.v9.i2.109
Cardiovascular magnetic resonance (CMR) imaging uniquely characterises myocardial and microvascular injury in acute myocardial infarction (AMI), providing powerful surrogate markers of outcomes. The last 10 years have seen an exponential increase in studies utilising CMR based endpoints in patients with AMI undergoing primary percutaneous intervention. This article provides a contemporary, comprehensive review of the powerful role of CMR imaging in the assessment of outcomes in AMI. The theory, assessment techniques, chronology, importance in predicting left ventricular function and remodelling, and prognostic value of each CMR surrogate marker is described in detail. Major studies illustrating the importance of the markers are summarised, providing an up to date review of the literature base in CMR imaging in AMI.
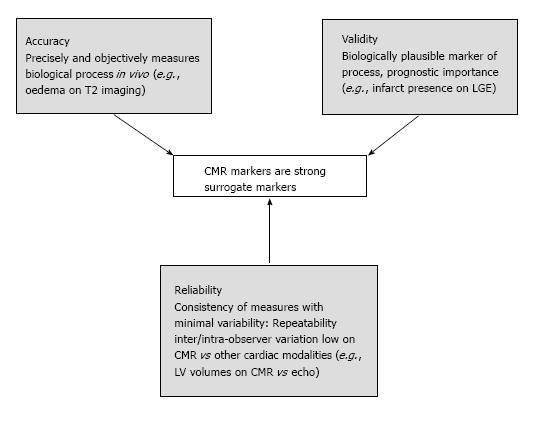
Prognostic studies using clinical outcomes, in particular mortality require large sample sizes. Surrogate biomarkers of outcome are directly measured alternative endpoints used as a substitute for biological processes and clinical outcomes[1,2]. CMR imaging uniquely characterises myocardial and microvascular injury in AMI due to its accuracy, reliability and validity (Figure 1)[2-4]. This significantly increases the statistical power of studies, allowing sample size requirements to be reduced. CMR data are strong surrogate markers of outcome following primary percutaneous coronary intervention (PPCI) in acute ST-segment elevation MI.
In the medium-term following STEMI, LV end-diastolic volume (LVEDV) increases, LV end-systolic volume (LVESV) decreases[5-7] and there can be compensatory hypertrophy of remote myocardium[8,9] in order to preserve stroke volume and ejection fraction (LVEF). Adverse remodelling results from an inability of the heart to maintain geometry post MI in the context of large infarcts and increased wall stresses[10,11]. An increase in LVEDVI > 20%[12,13] and increase in LVESVI > 15%[14] at follow-up are the most commonly used criteria for adverse remodelling.
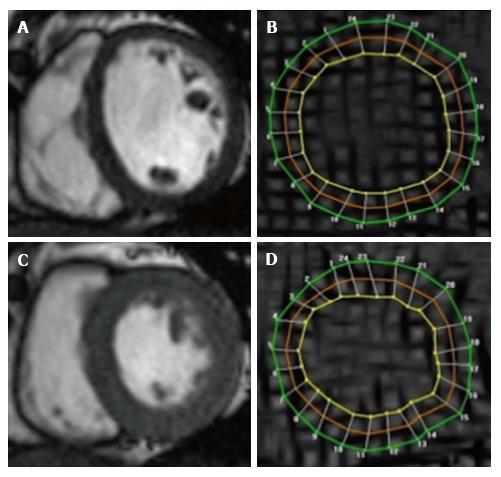
CMR is the gold standard modality for the assessment of ventricular function and volumes. It has higher spatial resolution than single-photon emission computed tomography (SPECT) (approximately 1.8 mm × 1.8 mm × 8 mm vs 10 mm × 10 mm × 10 mm)[15], and suffers from little subjectivity or reliance on patient body habitus[16].
Volumes and mass are assessed on analysis of a 3D cine stack of short-axis biventricular contiguous slices. Modern cine sequences use breath-hold, electrocardiographic-gated, segmented steady-state free precession (SSFP) to produce high spatial resolution images with excellent myocardium-blood contrast. Regional systolic function can alternatively be assessed using wall motion scoring[17].
CMR studies have demonstrated that recovery of LVEF occurs relatively early post STEMI. Ripa showed that improvement in LVEF and systolic wall thickening occurred by 1 mo, with no further change at 6 mo[5]. The majority of improvement in LVEF occurred between day 2 and 1 wk in the study by Mather[18], with a final increase by 3 mo. Beek showed that 55% of segments with initially impaired systolic wall thickness improved at 13-wk[19]. Ganame et al[20] and Dall’Armelina et al[21] however showed that LVEF underwent no significant change by 6 and 12 mo post PPCI respectively. This may be because their subjects sustained less myocardial damage, represented by relatively preserved LVEF and thus lower potential for improvement[21].
Volumetric changes occur more slowly. Ripa et al[5] showed a continued increase in LVEDV and reduction in LVESV until 6 mo. Engblom et al[7] demonstrated similar sequelae to 12-mo. Ganame showed progressive significant changes in LVEDV and LVESV and resulting LV sphericity at all timepoints to 12 mo[20]. These studies have important implications for optimising timing of follow-up CMR studies assessing remodelling.
The degree of impairment of LVEF and changes in volume depend on a number of CMR-based markers including infarct size (IS)[22], microvascular obstruction (MVO)[23,24], intramyocardial haemorrhage (IMH)[25] and myocardial salvage [non-infarcted proportion of ischaemic area at risk (AAR)][26,27]. Anterior STEMI results in larger IS and lower LVEF due to the greater ischaemic AAR[28].
Norris et al[29] and White et al[30] first illustrated the prognostic importance of LVEF (strongest independent predictor of survival at 3.5 years) and LVESV (only independent predictor of long-term mortality at 6 years) respectively, using invasive ventriculography. Burns first demonstrated the prognostic importance of LVEF and LV volumes and their strong correlation with each other, using radionucletide analysis[31].
A large evidence base has emerged for the prognostic impact of impaired systolic function based on reduced CMR-derived LVEF (Table 1).
| Ref. | Year | n | CMR time | Main findings | Follow-up |
| El Aidi et al[32] | 2014 | 25497 | N/A | Meta analysis of prognostic value of CMR surrogate markers. LVEF was only IP for MACE (HR 1.05 per -5%) | N/A |
| Husser et al[33] | 2012 | 304 | 7 d | LVEF was IP for MACE (HR 0.95 for each +1% LVEF) | 140 wk |
| Eitel et al[34] | 2011 | 208 | 3 d | LVEF was IP for MACE (HR 0.95 for each +1% LVEF) | 18.5 mo |
| Amabile et al[35] | 2010 | 114 | 6 d | LVEF was IP for MACE (HR 0.96 for each +1% LVEF) | 12 mo |
| de Waha et al[36] | 2010 | 438 | 3 d | LVEF was IP for MACE (OR 1.63) and all-cause mortality (OR 2.51) | 19 mo |
| Cochet et al[37] | 2009 | 127 | 3-7 d | LVEF of < 40% was IP for MACE (OR 1.20) | 12 mo |
| Hombach et al[6] | 2005 | 110 | 6 d | LVEF was IP for 9 mo MACE (P = 0.006) | 225 d |
In addition to LVEF-based global systolic function, Bodi demonstrated that the number of dysfunctional segments on CMR at 1-wk post STEMI was an independent predictor of combined MACE at a median follow-up of 553 d[38]. The evidence base for the prognostic importance of LV volumes is largely historical, based on large echocardiographic and radionucleotide studies, demonstrating the negative prognostic impact of ventricular dilatation and remodelling as summarised in Table 2.
| Ref. | Year | n | Modality | Main findings | Follow-up |
| Ahn et al[13] | 2013 | 135 | Echo | Adverse LV remodelling (> 20% inc. LVEDV) at 6 mo was IP 3 yr MACE. MACE rate approximately 25% in patients with adverse LV remodelling vs approximately 6% in non-remodelled patients | 981 d |
| Hombach et al[6] | 2005 | 110 | CMR | Baseline LVEDV was IP for MACE (P = 0.038) | 225 d |
| St John Sutton et al[39] | 2003 | 512 | Echo | Percentage change in LV area (surrogate for LV volume) between baseline echo and follow-up at 12 mo was IP for ventricular ectopy and VT | 24 mo |
| Bolognese et al[12] | 2002 | 284 | Echo | Baseline LVESV was IP for cardiac death and MACE. Components of MACE higher in patients with adverse remodelling (> 20% inc. LVEDV: Mortality 14% vs 5%, MACE 18% vs 10%) | 5 yr |
| Otterstad et al[40] | 2001 | 712 | Echo | Increase in LVESV between acute scan at 7 d and echo at 3 mo strongest IP for MACE | 24 mo |
| St John Sutton et al[41] | 1994 | 512 | Echo | LV end-diastolic area (RR 1.1) and LV end-systolic area (RR 1.1) on baseline echo, and %-change in LV area at 12 mo echo (RR 1.55) were strongest IPs for MACE | 12 mo |
| White et al[30] | 1987 | 605 | LV gram | LVESV of LV gram at 4 wk was strongest IP of long-term mortality (P < 0.0001) | 78 mo |
Negative LV remodelling has demonstrated prognostic importance in two studies, based on the cut-off of LVEDVI dilation of > 20% at 6-mo follow-up[12,13].
Recently, left ventricular global performance index has been proposed as a CMR marker of cardiac performance, incorporating LVEF, LV volumes and mass. It has been assessed in one study in STEMI and correlated strongly with IS, MSI, MVO and IMH extent, and had incremental prognostic value to LVEF in predicting 12-mo MACE[42]. Further work is needed to investigate its prognostic value in STEMI.
CMR-measured myocardial strain (tissue deformity) is the gold standard non-invasive measure of systolic and diastolic myocardial function[43]. Circumferential strain (Ecc) describes shortening of fibres (contraction) in a short-axis plane tangential to the epicardium; longitudinal strain (Ell) describes shortening in the long axis, and radial strain (Err) describes lengthening (thickening) of fibres towards the centre of the ventricle. Torsion is wringing of the ventricle caused by clockwise rotation at the base, and anticlockwise at the apex.
Strain offers greater accuracy in detecting myocardial dysfunction compared with global (LVEF) and regional (visual wall-motion scoring, segmental wall thickening)[44] measures.
In 1989, Axel et al[45] developed a T1 spoiled gradient echo sequence, creating “tags” formed by saturation of thin myocardial lines running in perpendicular directions in-plane to form a myocardial grid. These lines act as tissue markers, tracking myocardial deformation as shown in Figure 2. Peak systolic strain and peak diastolic strain rate (relaxation rate of strain) provide very sensitive measures of systolic and diastolic function respectively. Its accuracy has been validated on comparison with sonomicrometry[46,47]. Harmonic Phase Analysis (HARP) is currently the most widely used CMR strain method[48].
Feature tracking (FT) has been introduced as an alternative method to tagging for assessing strain on CMR. FT tracks anatomical features of interest along contour lines on routinely acquired SSFP cine images analogous to echocardiographic Speckle Tracking, obviating the need for additional tagging sequences[49]. FT-derived strain has been compared to tagging in acute STEMI and shown greater feasibility, accuracy and observer agreement[50] and remains an exciting prospect.
Strain could improve our understanding of the mechanics underlying LV dysfunction associated with prognostic CMR surrogate markers of myocardial damage in STEMI (e.g., MVO, IMH, oedema).
Systolic function is also in remote (non-infarcted) segments, and LV mechanics outside of the infarct zone are also affected during infarction and contribute to remodelling[44,51,52]. MVO had the highest predictive value for persistent dysfunction on circumferential strain at 7-mo post STEMI and may result in systolic dysfunction due to direct mechanical effects (myocardial stiffness)[53]. Baseline segmental circumferential strain was the strongest predictor of segmental functional recovery at 3-mo in a model containing infarct transmurality and MVO[54]. FT-derived global circumferential strain assessed acutely post PPCI was recently shown to correlated strongly with acute IS on late gadolinium enhancement (LGE) imaging (r = 0.75) and final LVEF at 6 mo (r = -0.71). Global circumferential strain was a stronger predictor of functional recovery (LVEF > 50%) at 6 mo than global longitudinal strain, age, diabetes and baseline LVEF, and was of similar predictive value to acute IS [AUC 0.86 (Ecc) vs 0.92 (IS)][55].
The evidence base for the prognostic importance of LV strain post STEMI is currently based on echocardiographic studies demonstrating that global longitudinal predicts medium and long-term using Speckle Tracking analysis as summarised in Table 3.
| Ref. | Year | n | Modality | Main findings | Follow-up |
| Ersbøll et al[56] | 2014 | 1048 | TTE | (E-prime divided by peak early diastolic strain rate) strongest IP of MACE and death | 29 mo |
| Ersbøll et al[57] | 2013 | 849 | TTE | GLS was IP of MACE | 30 mo |
| Hung et al[58] | 2010 | 610 | TTE | GLS and strain-rate, and GCS and strain-rate IPs for MACE in model with WMS, LVEF | 25 mo |
| Antoni et al[59] | 2010 | 659 | TTE | GLS (HR 1.2) was IP of mortality. LVEF, wall-motion score and Tissue Doppler mitral valve inflow not | 21 mo |
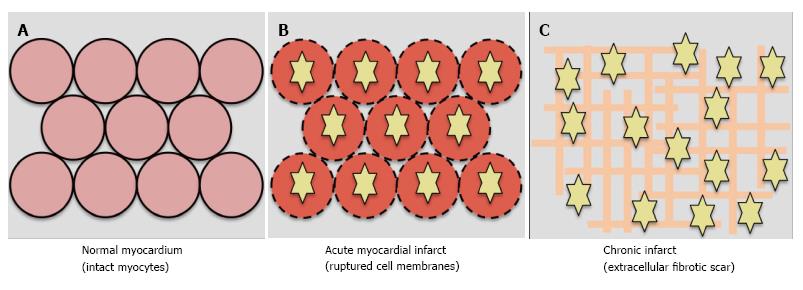
The “ischaemic cascade” is the sequence of pathophysiological effects developing immediately following coronary occlusion. Aerobic respiration loses efficiency resulting in cellular oedema. With increasing ischaemic time, cell membranes rupture. Following healing, necrotic cells are replaced by extracellular collagen deposition (scar). The acute and chronic phases are characterised by increased myocardial extracellular volume[60-62].
Gadolinium contrast agents are large extracellular molecules (Figure 3). Infarct can be visualised on T1-weighted imaging approximately 10 min after intravenous contrast administration, known as LGE imaging.
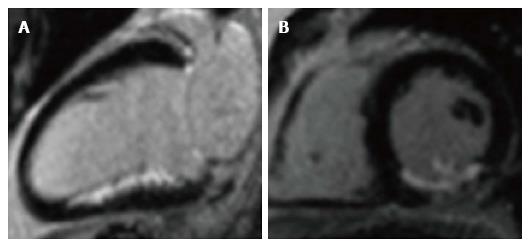
In acute infarct, LGE results from gadolinium entering ruptured cell membranes. In chronic infarction, LGE results from increased extracellular space due to collagen deposition and prolonged washout due to reduced capillary density within myocardium[60,63]. Gadolinium shortens T1, causing infarcted myocardium to appear bright, and normal myocardium to appears black (Figure 4)[63,64]. Normal myocardium is progressively nulled using the appropriate inversion time to provide optimal contrast between infarct and normal myocardium.
Typically, a high spatial resolution of approximately 1.4 mm × 1.6 mm × 6-8 mm is achieved[15]. IS is typically expressed as a percentage of total LV mass. Delineation of infarct can be performed visually (manual quantification)[6,9,22], however most groups use semi-automated methods to reduce observer variability. These include enhancing myocardium exceeding a pre-defined signal intensity (SI) threshold, typically > 2-6 standard deviations above that of remote (non-infarcted) myocardium[2,65]. Currently, the semi-automated full-width at half-maximum (FWHM) method is commonly used[66-70], defining infarct as myocardium with SI > 50% of the peak SI in the infarct core. Amado demonstrated that FWHM had the highest interobserver agreement and closest correlation with TTC-stained infarct in a dog model of acute infarction (r2 = 0.94), compared with standard deviation methods[66]. This may be because FWHM is less prone to IS overestimation in the presence of oedema, and partial volume effects giving rise to intermediate signal intensities[18,71]. Comparing techniques in STEMI patients showed that FWHM quantification had the lowest intraobserver and interobserver variability, and greatest agreement with LVEF[72].
CMR measurement of IS on LGE is well validated[63,64]. Kim demonstrated that IS in dog myocardium on ex-vivo CMR corresponded closely with IS derived from tetrazolium (TTC) staining (r = 0.99)[15,64]. LGE has higher sensitivity for infarct detection compared with SPECT. In an experimental model of MI, CMR LGE detected 92% of all segments with subendocardial infarction (< 50% transmurality) compared with only 28% with SPECT[15]. In patients with MI, SPECT only detects approximately 50% of the infarcts seen on LGE. The superior sensitivity is due to the increased spatial resolution and reproducibility of CMR[60].
Since gadolinium is distributed throughout the extracellular space, gadolinium contrast agents are not specific to necrosis. Acutely, the area of LGE detects not only necrotic cells but also the increased (oedematous) interstitium surrouding viable cells, and thus can overestimate true IS. Studies of IS chronology in humans corroborate this (Table 4). Indeed, severely dysfunctional segments with minimal myocardial salvage early post STEMI can show significant functional improvement at follow-up[73].
| Ref. | Year | n | CMR times post STEMI | Relative LGE IS reduction | LGE method | Main findings |
| Carrick et al[74] | 2016 | 30 | 8 h → 3 d → 10 d → 7 mo | 26% | Automated | Significant decrease d3 to d10 (20% ± 13% to 14% ± 10% LV mass). No change at 7 mo |
| Dall’Armelina et al[21] | 2011 | 30 | 2 d → 6 mo | 22% | > 2SD | IS reduced at times from 27% ± 15% LV mass 24 h post PPCI, to 21% ± 11% at 6 mo |
| Mather et al[18] | 2011 | 48 | 2 d → 1 wk → 30 d → 3 mo | 37% | > 2SD | 27% IS drop between d2 and d7 post PPCI, no change at 3 mo |
| Ganame et al[20] | 2011 | 58 | 3 d → 4 mo → 12 mo | 45% | Manual | 33% decrease IS d3 and 4 mo then no further decrease at 12 mo |
| Ibrahim et al[9] | 2010 | 17 | 1 d → 1 wk → 1 mo → 6 mo | 37% | Manual | 34% reduction in IS from d2 to 1 wk, then no further change at 1 and 6 mo |
| Engblom et al[7] | 2009 | 22 | 1 d → 1 wk → 12 mo | 40% | Automated | 28% reduction in IS between d1 and 1 wk |
| Ripa et al[5] | 2007 | 58 | 2 d → 1 mo → 6 mo | 30% | Manual | 14% % reduction in IS from d2 to 1 mo |
| Hombach et al[6] | 2005 | 110 | 6 d → 9 mo | 28% | Manual | 28% reduction in IS from d6 to 9 mo |
The majority of IS reduction occurs relatively early post STEMI, particularly by 1 wk. Indeed IS assessed at 1 wk has been shown to closely correlate with final IS[7,9,18]. Overestimation of necrosis by LGE-derived IS early post STEMI is due to a combination of oedema, infarct resorption and partial volume effects. Oedema results in an overestimation of LGE IS due to increased extracellular water content and thus volume of distribution of contrast agent[66,75].
Infarct resorption results from the healing process where collagenous scar tissue is produced to provide stability and tensile strength to necrotic myocardium[7,11]. This was confirmed in a canine model where a 3.4-fold decrease in infarct volume was seen between day 3 and 8-wk post infarct on ex-vivo LGE and TTC-stained slices[64]. The degree of infarct resorption has been shown to be proportional to initial IS (r = 0.65) and presence of LV remodelling (r = 0.41)[10]. The greater degree of infarct resorption relative to total myocardial mass and volume results in an inability to maintain LV geometry in light of mechanical stresses post STEMI, resulting in adverse LV remodelling and sphericity[10,76].
Factors known to affect IS include AAR extent[77-79]; collateral flow to the AAR[79,80]; MVO[81]; time to reperfusion[82] and hyperglycaemia[83].
Segmental function: Kim illustrated in stable patients awaiting revascularisation, that LGE transmurality strongly predicted recovery of systolic function in dysfunctional segments. Only 2% of segments with > 75% transmurality improved after revascularisation[84]. Segmental extent of LGE has also been shown to negatively predict functional recovery in dysfunctional segments following PPCI for acute STEMI, as summarised in Table 5.
| Ref. | Year | n | LGE method | Cutoff (LGE) | Main findings | Time of CMR 1 | Time of CMR 2 |
| Khan et al[85] | 2016 | FWHM | 50% SEE | SEE strong predictor or segmental functional improvement (AUC 0.840) and normalisation (AUC 0.887) | 2 d | 9 mo | |
| Wong et al[54] | 2014 | 45 | FWHM | 50% SEE | Inverse relationship between TEE and likelihood of functional recovery on WMS at 24 wk (area under curve 0.68) | 8 d | 13 wk |
| Natale et al[86] | 2011 | 46 | 2SD | 50% TEE | Inverse relationship TEE and likelihood of functional recovery on SWT (93% sens, 75% spec) | 5 d | 20 wk |
| Engblom et al[7] | 2008 | 22 | Manual | 50% TEE | Inverse relationship between TEE and functional recovery on WMS | 7 d | 24 wk |
| Shapiro et al[87] | 2007 | 17 | Manual | 50% SEE | Inverse relationship between TEE and likelihood of functional recovery on WMS at 26 wk. Odds-ratio of functional recovery 0.2 with each SEE quartile | 6 d | 26 wk |
| Kitagawa et al[88] | 2007 | 18 | 2SD | 50% TEE | Inverse relationship between TEE and functional recovery. 31% segments > 50% TEE still improved | 5 d | 39 wk |
| Janssen et al[89] | 2006 | 67 | Manual | 50% TEE | Inverse relationship between TEE and functional recovery on WMS at 12w (51%-75%: 39% segments improved, 76%+: 21% improved) | 4 d | 12 wk |
| Motoyasu et al[90] | 2004 | 23 | 2SD | 50% TEE | Inverse relationship between SEE and functional recovery on SWT | 25 d | 24 wk |
| Beek et al[19] | 2003 | 30 | 6SD | 50% SEE | Inverse relationship between SEE and functional recovery on WMS | 7 d | 13 wk |
Global function: IS is a powerful independent predictor of global LV function and adverse LV remodelling in the medium to long-term post STEMI as summarised in Table 6.
| Ref. | Year | n | LGE method | Main findings | Time post STEMI of predictive CMR | Follow-up |
| Ahn et al[13] | 2013 | 135 | Manual | IS strongest IP of LVR in model with LVEF and MI location | 7 d | 6 mo (echocardiogram) |
| Husser et al[33] | 2012 | 304 | > 2SD | IS IP of LVR in model incl. LVEF, IS, LV vols, MVO | 6 d | 189 d |
| Monmeneu et al[91] | 2012 | 118 | > 2SD | No. segments > 50% transmurality IP for LVR | 6 d | 6 mo |
| Ezekowicz et al[92] | 2010 | 64 | Manual | IS strongest IP of LVEF in model with MVO, troponins | 7 d | 3 mo |
| Ganame et al[25] | 2009 | 98 | Manual | IS strongest IP of LVR (>> MVO, AAR, Troponin-I) | 2 d | 6 mo |
| Bodi et al[93] | 2009 | 214 | > 2SD | Extent of transmural necrosis (no. segments > 50% TEE) strongest IP for LV recovery (+ > 5% LVEF) | 7 d | 6 mo |
| Wu et al[94] | 2008 | 122 | Manual | IS extent only IP for LVEF and LVR | 2 d | 4 mo |
| Hombach et al[6] | 2005 | 110 | Manual | IS extent IP of LVR in model with MVO, % transmurality | 6 d | 225 d |
The goal of STEMI management is early reperfusion in order to minimise IS and thus maximise myocardial salvage[95]. There is a strong evidence base for the prognostic importance of CMR-derived IS post STEMI, as summarised in Table 7. IS strongly predicts medium to long-term clinical outcomes.
| Ref. | Year | n | LGE method | Main findings | CMR timepoint | Follow-up |
| Husser et al[96] | 2013 | 250 | > 2SD | Extent of transmural infarction (no. of segments > 50% transmurality) only IP for MACE at 6 mo | 7 d | 163 wk |
| Izquierdo et al[97] | 2013 | 440 | > 2SD | IS was IP for AACEs (arrhythmic cardiac events: Sudden death, VT, VF, ICD shock) in model including LVEF, hypertension | 7 d | 123 wk |
| Eitel et al[34] | 2011 | 208 | > 5SD | IS was IP of MACE at 19 mo in model including MVO, LVEF, MSI, Killip, TIMI post-PPCI | 3 d | 18.5 mo |
| Miszalski-Jamka et al[98] | 2010 | 77 | Manual | LV transmurality index IP (HR 1.03) and IS (HR 1.03) IPs for MACE in a model containing RVEF and RV IS | “3-5 d” | 1150 d |
| Larose et al[67] | 2010 | 103 | FWHM | IS strongest IP for MACE (HR 1.36) in model containing LVEF, CK. LGE > 23% had HR 6.1 for MACE | 4.5 h | 2 yr |
| Bodi et al[38] | 2009 | 214 | > 2SD | Extent of transmural infarction (no. of segments > 50% transmurality) IP for MACE (HR 1.35 if > 5 segs) | 7 d | 553 d |
| Wu et al[99] | 2008 | 122 | Manual | IS only IP of 2 yr MACE in model containing LVEF, LVESVI | 2 d | 538 d |
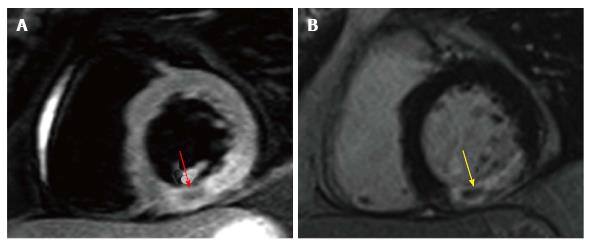
Despite prompt IRA recanalization, perfusion of the microcirculatory bed does not always ensue. Histopathogical studies have demonstrated that the infarct core (endocardial) perishes first as necrosis spreads transmurally towards the epicardium. This is known as the “wavefront theory”[100]. At the infarct core, necrosis occurs rapidly with myocardial and capillary endothelial cells perishing simultaneously. Capillaries can become obstructed by cellular debris, resulting in non-perfusion of the infarct core, despite IRA patency[101]. This is known as MVO and can be indicated at angiography, as “no reflow”[101].
Three CMR methods demonstrate MVO (Figure 5). MVO extent is typically expressed as a percentage of LV mass: (1) Qualitative first-pass rest perfusion. A modified version involves quantification of myocardial blood flow (SI-time curve) and time to 50% of maximal SI[102,103]; (2) Hypoperfusion on inversion recovery images between 1-3 min post contrast. A fixed inversion time of approximately 440 ms nulls MVO and retains intermediate signal in normal myocardium. This is known as “early MVO (E-MVO)”[28,104]; and (3) Hypointensity within infarct core on LGE due to absence contrast perfusion, known as “late MVO (L-MVO)”. L-MVO occurs in upto 60% of patients on CMR within the first week post STEMI[5,6,18,20]. This is the preferred method of MVO demonstration in contemporary clinical practice and research.
L-MVO extent is maximal at 48 h post infarct[8,18], and then decreases. It exists for at least 1 wk, and for up to 1 mo[8,18] and then resolves in the medium-term in humans (Table 8). Animal models corroborate these findings[105,106].
| Ref. | Year | n | CMR timepoints | LGE method | Main findings |
| Carrick et al[74] | 2016 | 30 | 8 h → 3 d → 10 d → 7 mo | Auto | L-MVO in 20%, peaked early at 8 h and stable at d3. Decreased by d10, absent at 7 mo |
| Mather et al[18] | 2011 | 48 | 2 d → 1 wk → 30 d → 3 mo | > 2SD | L-MVO in 60%, peak at d2. Decrease at subsequent points. L-MVO absent at 3 mo |
| Ganame et al[20] | 2011 | 58 | 3 d → 4 mo → 12 mo | Manual | L-MVO in 64%. L-MVO absent at 4 mo |
| Ripa et al[5] | 2007 | 58 | 2 d → 6 mo | Manual | L-MVO in 42%. L-MVO absent at 6 mo |
| Hombach et al[6] | 2005 | 110 | 6 d → 9 mo | Manual | 46% had L-MVO (2.8% LV mass, 16% of IS) on acute CMR. L-MVO absent at 6 mo |
The extent of MVO on CMR has been shown to correlate with IS[82,94,107,108], oedema, IMH, TIMI-flow pre PCI[35,109] and time to reperfusion[35,82,110].
L-MVO is a strong independent predictor of medium-term LV function and adverse remodelling (Table 9). It is likely that this is because L-MVO reflects more severe microvascular and myocardial damage than E-MVO[28,36]. In most studies demonstrating the independent predictive value of L-MVO on LV function and remodelling, E-MVO was not a predictor[103,111,112]. L-MVO was a predictor independent of baseline IS[6,20,92,111-113]. Monocyte recruitment, crucial in cellular debris removal and scar formation, is impaired in areas of L-MVO in rat myocardium and may contribute to the adverse remodelling[114].
| Ref. | Year | n | LGE method | Main findings | Time post STEMI of predictive CMR | Follow-up |
| Kidambi et al[115] | 2013 | 39 | > 2SD | L-MVO only IP of impaired infarct strain. Model with IS, TIMI flow, diabetes, transmurality | 3 d | 3 mo |
| Wong et al[103] | 2012 | 40 | Manual | L-MVO extent only IP for LVEF at 3 mo in model including E-MVO, IS and myocardial blood flow on perfusion | 3 d | 3 mo |
| Ezekowitz et al[92] | 2010 | 64 | Manual | L-MVO extent was IP of LVEF in model with IS and NT-proBNP | 7 d | 4 mo |
| Weir et al[112] | 2010 | 100 | Manual | L-MVO extent was only IP of LVR in model with TIMI post PCI, E-MVO, IS | 4 d | 6 mo |
| Ganame et al[25] | 2009 | 98 | Manual | L-MVO extent was IP of LVR in model with IS, troponin-I, TTR | 2 d | 6 mo |
| Nijveldt et al[111] | 2008 | 60 | Manual | L-MVO presence strongest IP of LVEF change and LVR in model with TTR, IS, LVEF, E-MVO | 5 d | 4 mo |
| Hombach et al[6] | 2005 | 110 | Manual | L-MVO extent IP for LVR in model with baseline IS, infarct transmurality | 6 d | 225 d |
An increasing evidence base demonstrates the strong medium-term prognostic value of L-MVO following STEMI, independent of IS and LVEF[6,36,37,116] (Table 10). The 2 studies featuring both L-MVO and E-MVO showed that L-MVO was a stronger prognostic indicator[36,37]. Regenfus et al[117] demonstrated that L-MVO was the strongest IP of long-term combined MACE at 6 years follow-up in a model including CMR-assessed LVEF and IS (HR 3.9), providing incremental prognostic value over traditional CMR markers of myocardial damage. A meta-analysis[118] (8 studies, n = 1025) demonstrated that L-MVO presence was the strongest independent predictor of medium-term combined MACE (HR 3.7) and cardiovascular death (HR 13.2) at 2 years independent of IS and LV volumes.
| Ref. | Year | n | LGE method | Main findings | Time of prognostic CMR post STEMI | Follow-up |
| Regenfus et al[117] | 2015 | 249 | Manual | L-MVO extent strongest IP for MACE in model including IS, LVEF, TIMI pre and post PPCI and no. diseased vessels | 3.7 d | 72 mo |
| Eitel et al[119] | 2014 | 738 | > 5SD | Largest multicentre study of L-MVO in PPCI. L-MVO > 1.4% LVM and TIMI risk score only IPs of combined MACE. Adding L-MVO to model with clinical predictors, LVEF and IS increased c-statistic | 7 d | 6 mo |
| de Waha et al[120] | 2012 | 438 | Manual | L-MVO extent IP for combined MACE in model including IS, LV volumes (only other IP was LVEF). L-MVO/IS strongest IP in model including L-MVO extent, LVEF, IS, LV volumes | 3 d | 19 mo |
| de Waha et al[36] | 2010 | 438 | Manual | Presence and extent of L-MVO were strongest IPs for MACE and mortality in models with IS, LVEF, ST-res, TIMI-flow post PCI. E-MVO was not an IP | 3 d | 19 mo |
| Cochet et al[37] | 2009 | 184 | Manual | L-MVO strongest IP for MACE, in models including GRACE score, IS, LVEF. L-MVO stronger IP than E-MVO (OR 8.7 vs 2.5) | “3-7 d” | 12 mo |
| Bruder et al[116] | 2008 | 143 | Manual | Only extent of L-MVO > 0.5% LV mass was IP for MACE; model included IS, LVEF, age, DM, sex | 4.5 d | 12 mo |
| Hombach et al[6] | 2005 | 110 | Manual | L-MVO IP for MACE (P = 0.04) in model including LV end-diastolic volume and LVEF | 6 d | 268 d |
The strong adverse prognostic value of L-MVO may be due to its negative effects on LV function, wall thickness and stiffness, and remodelling, and subsequent risk of heart failure and arrhythmias[6,20,92,111-113].
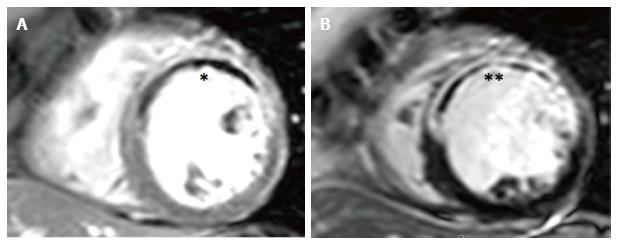
IMH is a reperfusion injury occurring when restored blood flow into damaged capillaries extravasates erythrocytes into myocardium[121,122]. CMR-derived IMH was first described in reperfused canine myocardium on ex-vivo T2-weighted spin-echo (T2w-TSE) imaging with excellent agreement with histology (r = 0.96 for IMH extent)[123].
Paramagnetic haemoglobin breakdown products shorten T2 relaxation times[123,124]. IMH is seen as hypointense zones within hyperintense oedematous myocardium on T2w-TSE sequences. It shows good histological correlation in canine myocardium (ex-vivo MRI, r = 0.96)[123] and in an human autopsy case series (in-vivo MRI, r = 0.97)[124]. IMH occurs exclusively in areas of L-MVO (r2 for co-localisation approximately 0.9) (Figure 6)[25,33,125,126].
Newer sequences based on direct quantification of T2 and T2*[74,126-129] allow IMH to be quantified without the limitations of T2w-TSE imaging. Initial studies have been promising and shown that these sequences are reproducible and appear more sensitive and accurate than T2w-TSE for IMH detection[126,130,131]. O’Regan et al[126] showed that T2* had 100% sensitivity for IMH detection compared to 90% for T2w-TSE, where the “gold standard” was co-localisation with L-MVO. In canines, T2* in haemorrhagic infarcts closely correlates with iron levels on spectrometry, and T2*-detected IMH co-localises with iron deposition on Perl’s staining[132] and extravasated erythrocytes on Haematoxylin-Eosin staining[128]. In pigs, regions of IMH on T2* imaging showed vessel degeneration and iron deposition[8].
There is a paucity of data on temporal changes in CMR-detected IMH. Mather et al[18] showed that IMH on T2w-TSE was present in 33% of patients, with maximal extent at 48 h post PPCI and progressively resolution by 3 mo. Carrick et al[74] recently demonstrated that the incidence and extent of IMH on T2* increased between 8 h and 3 d post PPCI. Its extent was significantly lower at 10 d and was seen in only 13% of patients at 7 mo. The authors also found that MVO was present in all patients with IMH, and its extent peaked earlier at 8 h suggesting that IMH is an ensuing reperfusion injury in regions of MVO.
There is a small evidence base demonstrating that IMH is a strong univariate predictor of medium-term impaired LV function and remodelling, however multivariate analysis reveals mixed results, with some studies suggesting no incremental predictive value of IMH over MVO and IS (Table 11).
| Ref. | Year | n | IMH CMR method | Main findings | CMR time post MI | Mean/median F/U CMR |
| Carrick et al[74] | 2016 | 245 | T2* | IMH strongest IP for LVR. IMH associated with lower LVEF and greater volumes | 3 d | 7 mo |
| Kidambi et al[115] | 2013 | 39 | T2w-TSE and T2* | IMH associated with attenuation of follow-up infarct strain | 3 d | 3 mo |
| Husser et al[33] | 2012 | 304 | T2w-TSE | IMH strongest IP for LVR in model with LVEF, IS, LV vol, L-MVO | 6 d | 189 d |
| Mather et al[131] | 2011 | 48 | T2w-TSE and T2* | IMH strongest IP of LVR in model with IS, LVEF, LVESV, E-MVO, MSI | 2 d | 3 mo |
| Beek et al[24] | 2010 | 45 | T2w-TSE | IMH was a univariate predictor of LVEF. However no prognostic significance beyond baseline LVEF and MVO in predicting final LVEF | 5 d | 4 mo |
| Bekkers et al[121] | 2010 | 90 | T2w-TSE | Acute MSI and LVEF increase at follow-up lowest if IMH present. But IMH no prognostic significance beyond MVO in predicting LVEF | 5 d | 103 d |
| O’Regan et al[126] | 2010 | 50 | T2* | IMH presence univariate predictor of LVEF and LV volumes. However only IS independently predicted LVEF | 3 d | N/A |
| Ganame et al[25] | 2009 | 98 | T2w-TSE | IMH extent strongest IP of LVR in model with IS, E-MVO, Troponin-I, AAR, TTR, IS | 2 d | 4 mo |
Multivariate analyses including IMH as a prognostic indicator also show mixed results. Amabile et al[133] demonstrated that IMH on T2w-TSE at 4 d post STEMI was the strongest independent predictor of MACE at 1-year (HR 2.8) in a model including LVEF, ST-resolution and L-MVO. Husser et al[33] showed that only LVEF and IMH extent on T2w-TSE independently predicted MACE at 140 wk follow-up in a model containing LV volumes, AAR, IS and L-MVO. However IMH and MVO extent showed strong correlation (r = 0.95) and adding T2w imaging to a model containing LGE and cine imaging did not improve the predictive power for MACE, supporting a strong concordance of IMH and MVO. Eitel et al[125] demonstrated that IMH presence on T2w-TSE and LVEF < 53% were the only CMR independent predictors of MACE at 6 mo in a model with lone MVO. Carrick et al[74] recently demonstrated that IMH on T2* mapping was the strongest independent predictor of cardiac death and heart failure hospitalisation at 830 d follow-up. In their multivariate model, L-MVO was not a predictor suggesting that IMH reflects extreme microvascular injury.
Oedema is seen in acute cardiac inflammation. In STEMI, it signifies reversible myocardial injury in the ischaemic cascade. The area of oedematous myocardium defines the ischaemic AAR supplied by the occluded IRA[61,134].
The T2 (transverse) relaxation time is increased by regional water content[135]. T2w-TSE sequences illustrate oedema as hyperintensity[134] and are currently the mainstay of CMR oedema imaging. Most commonly used is the black-blood T2-weighted short-tau inversion-recovery sequence (T2w-STIR). This uses two initial inversion pulses to null moving blood. This is followed by a third inversion pulse, which nulls tissues with short T1 times (fat) to provide high contrast between blood (nulled) and myocardium[134,136]. T2w imaging of myocardial oedema is well-validated in animal studies assessing myocardial water volume on histological assessment[137] and fluorescent microspheres[77]. T2w oedema assessment is well-validated with SPECT[138-140] and angiographic markers of AAR (BARI[141], APPROACHp[142] scoring). AAR on T2w can be assessed accurately for upto 1-wk post-PPCI unlike SPECT, which requires radionucleotide administration during coronary occlusion and has higher spatial resolution and thus ability to detect subendocardial injury[138].
However T2w-TSE imaging has inherent disadvantages that can compromise image quality and oedema detection. Upto 30% of datasets are non-analysable in studies[24,143,144]. New T2w sequences have been studied, with encouraging results (Figure 7).
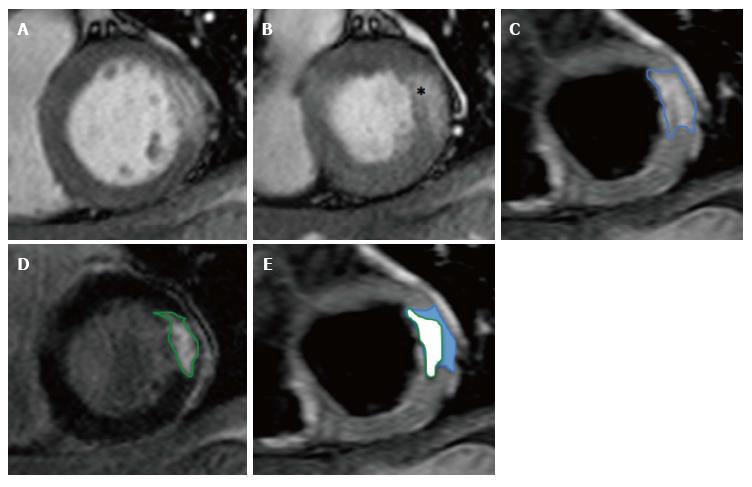
The aim of prompt reperfusion is to limit IS by minimizing the conversion of reversibly injured myocardial cells (AAR) into necrotic, infarcted tissue (IS)[95,156]. Anterior STEMI typically results in larger IS due to the larger coronary bed supplied by the left anterior descending artery[14,80,82]. Hence a more accurate assessment of revascularisation strategies can be provided by adjusting IS for the AAR. The resulting myocardial salvage index (MSI) defines the proportion of reversibly injured tissue (AAR) that does not progress to infarction (IS, Equation 1, Figure 8). MSI is expressed as percentage of the initial AAR [0% is no salvage, 100% is complete salvage (aborted STEMI)][157].
Equation 1: Myocardial salvage index (MSI, %) = 100 × [(AAR-IS)/(AAR)].
Desch showed excellent intraobserver and interobserver agreement for MSI assessment using T2w-STIR and LGE (coefficients of variation approximately 5.0%) and excellent test-retest reproducibility in a study of 20 acute STEMI patients[158].
Other determinants of AAR include TTR[91,130,159-162], extent of collateralised IRA territory flow[5,80,159,163], TIMI-flow pre PPCI, LAD IRA and diabetes[91].
Studies of the chronology of oedema suggest that it occurs very early in the ischaemic cascade. Abdel-Aty confirmed the presence of transmural oedema in canines on in-vivo T2w imaging at 28 min post LAD occlusion at which point LGE and troponin release were absent, indicating reversible injury[164]. Fernández-Jiménez et al[165] however recently demonstrated a bimodal pattern of AAR extent in pigs with T2-mapping CMR and histological water quantification. They showed peak values at 2 h thought to be a direct result of reperfusion, followed by a return to baseline at 2 d and then progressive increase towards peak values at 7 d, with the latter peak felt due to water replacement of cleared cellular debris. Studies of temporal changes in AAR and MSI in humans are summarised in Table 12. Correct timing of oedema imaging is crucial in accurate calculation of AAR and MSI.
| Ref. | Year | n | CMR timepoints post STEMI | AAR, IS method | Main findings |
| Mather et al[18] | 2011 | 48 | 2 d → 1 wk → 30 d → 3 mo | > 2SD STIR, > 2SD LGE | AAR reduction at successive timepoints, 1-3 mo (-75%). No change MSI at d2 or 1 wk as IS and AAR decreased proportionally |
| Dall’Armelina et al[21] | 2011 | 30 | 2 d → 1 wk → 2 wk → 6 mo | > 2SD T2p-BB, > 2SD LGE | 100% had oedema at d2. AAR stable over 1st week (37% vs 39% LVM). Decreased by 2 wk and nearly resolved at 6 mo |
| Carlsson et al[138] | 2009 | 16 | 1 d → 1 wk → 6 wk → 6 mo | Manual STIR, and LGE | AAR at all timepoints. AAR stable in 1st week, correlated with 1 wk SPECT. Decrease by 1 mo (10% LVM), nearly gone by 6 mo |
| Ripa et al[5] | 2007 | 58 | 2 d → 1 mo → 6 mo | Manual STIR and LGE | All had oedema at d2. AAR decreased at all time points. No data on MSI in this study |
The near-resolution of oedema by 6 mo[5,18,21,91,138] allows distinction between acute and chronic infarcts when combined with LGE imaging.
Myocardial salvage is a strong univariate predictor of medium-term LV function[14,166,167] and adverse LV remodelling post STEMI[14,27,91,161]. Multivariate analysis demonstrates mixed results. MSI independently predicted LV remodelling in the work of Mather[131] (Table 13). However MSI was not a predictor once IS was added into multivariate models in studies by Monmeneu[91] and Masci[14]. This, in conjunction with the correlation between MSI and IS, and AAR and IS[26] questions whether MSI and IS are truly independent of each other in predicting LV remodelling and prognosis post STEMI. It could be argued that since MSI adjusts IS for the extent of AAR, it may have less inherent variability than IS. Since up to 30% of AAR datasets have been deemed non-diagnostic in previous studies[24,143,144], this may impact on the robustness of MSI quantification whereas IS datasets are exceptionally rarely excluded based on image quality. It is not clear currently whether IS or MSI is the better measure of revascularisation success post PPCI.
| Ref. | Year | n | AAR, IS method | Main findings | CMR timepoint post STEMI | Follow-up |
| Mather et al[131] | 2011 | 48 | > 2SD STIR, > 2SD LGE | MSI was IP for LVR (OR 0.95) in model including LV volumes, LVEF, IS, IMH, MVO | 2 d | 3 mo |
| Monmeneu et al[91] | 2012 | 118 | > 2SD STIR, > 2SD LGE | MSI univariate predictor of LVR and final LVEF. However not IP of LVR in model with LVESVI, IS, no. transmural segs | 6 d | 6 mo |
| Masci et al[14] | 2011 | 260 | > 2SD STIR, > 5SD LGE | MSI strong univariate predictor of LVR and final LVEF. However not IP in model including IS, MVO | 1 wk | 4 mo |
| Masci et al[26] | 2010 | 137 | > 2SD STIR, > 5SD LGE | MSI strongest IP for LVR However IS and MSI (r = -0.72) and IS and AAR (r = 0.85) correlated | 1 wk | 4 mo |
Historically, the prognostic value of MSI was demonstrated using SPECT. Ndrepapa first showed that MSI was the strongest independent predictor of 6-mo mortality[168]. MSI was an independent prognostic indicator in the medium term post STEMI in two studies. Although both studies were from the same patient cohort, they have both been included in Table 14 due to their differing primary findings.
| Ref. | Year | n | AAR, IS method | Main findings | CMR timepoint post STEMI | Follow-up |
| Eitel et al[34] | 2011 | 208 | > 2SD -STIR, > 5SD LGE | MSI was only CMR-based IP of mortality in model with age, IS, MVO, LVEF, TIMI- post PPCI, diabetes, age (IS not IP). MSI not IP of MACE (only IS, LVEF, age were) | 3 d | 19 mo |
| Eitel et al[161] | 2010 | 208 | > 2SD STIR, > 5SD LGE | MSI was only IP for MACE and mortality in model including LVEF, MVO, IS, ST-resolution and TIMI-grade post PCI | 3 d | 6 mo |
The current mainstay of LGE and T2w techniques for the detection of infarct and oedema rely on semi-quantitative threshold-based quantification methods using arbitrary SI cut-offs compared to user-defined regions of interest, automated algorithms or are based on manual planimetry. There is currently no consensus on the optimal quantification method for IS or AAR using these sequences. This can lead to subjectivity and dependence upon optimal nulling of normal myocardium and thus potential for error. In addition, commonly used T2w-TSE sequences suffer from non-diagnostic image quality in upto 30% of patients[24,143,144].
T1, T2 and T2* quantification present an exciting and complementary approach to LGE and T2w imaging. Developed by Messroghli et al[169] in 2003, their use in MI research has accelerated over the last 5 years. They allow not only the location and extent of infarction, oedema, MVO and IMH to be determined from subsequent parametric myocardial maps, but also the severity of these pathologies to be assessed through the magnitude of values obtained[170,171]. These methods are not reliant on reference regions of interest and do not suffer from T2w-TSE artefacts.
T1 relaxation curves allow calculation of the T1 time (time taken for recovery of 63% of longitudinal magnetization). The currently used curve-fitting sequences used include MOLLI (Modified Look-Locker Inversion Recovery), ShMOLLI (Shortened MOLLI), SASHA (SAturation recovery single-SHot Acquisition) and SAPPHIRE (SAturation Pulse Prepared Heart rate independent Inversion REcovery)[172]. Infarcted and oedematous myocardium demonstrate prolonged pre-contrast T1 values and reduced post-contrast T1 values compared with normal myocardium, allowing infarct visualisation and quantification[169,170,173,174]. Messroghli showed that this technique had high test-retest reproducibility[175], was stable within the range of heart rates commonly seen in clinical practice and showed comparable sensitivity for IS quantification compared with LGE[169,173,176]. T1 values within in the infarct core were recently shown to demonstrate a strong inverse correlation with L-MVO extent, incidence of LV remodelling and all-cause mortality at 2.5 years[177].
T2w images are generated using a T2-SSFP sequence with log-transformed curve-fitting T2 quantification, with different T2 preparation (TE) times. T2 mapping has shown excellent reproducibility and no effect of slow-flow, through-plane movement, SI loss, or effects of coil SI inhomogeneities[151,178]. T2 mapping accurately assessed oedema in 96% of patients (good image quality in 100%), whereas T2w-STIR detected oedema in only 67% of patients (15% non-diagnostic 15%)[151]. High observer agreement and close agreement between T1 (r2 = 0.94) and T2 maps (r2 = 0.96), and fluorescent microspheres for AAR detection was seen in canine myocardium[179].
T2* mapping allows visualisation and quantification of IMH due to the presence of paramagnetic haemoglobin breakdown products. A cut-off value of < 20 ms has been used to define the presence of IMH[180]. Although the evidence base for T2* mapping in assessing IMH is currently limited, O’Regan demonstrated that it has greater sensitivity than T2w-STIR imaging (100% vs 90%) for IMH. Kali showed good correlation between in-vivo T2* and histological assessment of IMH and iron levels in canine myocardium[127,128]. T2* mapping may improve the specificity of IMH detected on CMR[131].
T1, T2 and T2* surrogate markers hold promise for improving the accuracy of detection of infarct, oedema and IMH respectively, and further improving statistical power of STEMI studies. However, due to the importance of protocol standardisation, these techniques are rarely used in multicentre studies at present.
CMR is the gold standard imaging modality for the assessment of right ventricular (RV) volumes, function, oedema[181] and infarction (RVI)[182]. CMR identifies RVI with greater sensitivity than echocardiography, ECG (V4R ST-segment elevation) and clinical examination[183,184] and demonstrates RV L-MVO[185,186]. There is good interobserver and intraobserver agreement for the identification of RV oedema (κ = 0.62, κ = 0.62, respectively) and very good agreement for RVI (κ = 0.70, κ = 0.70, respectively)[181]. The high MSI in RVI often > 90%[187,188] is thought to be due the relatively low RV nutrient needs, direct endocardial oxygen diffusion and good collateral blood supply[188,189].
RVI confers adverse short-term prognosis, with a large meta-analysis (n = 7136) demonstrating that RVI on ECG, echocardiography or radionucleotide imaging predicted 30-d mortality and in-hospital MACE[190]. Shah demonstrated the prognostic importance of right ventricular infarction on imaging, where RVEF < 38% on radionucleotide ventriculography post STEMI was a strong independent predictor of 1-year mortality[191]. Right ventricular infarction is a strong independent predictor of medium to long-term prognosis in a small number of CMR studies (Table 15).
| Ref. | Year | n | RV LGE analysis method | Main findings | CMR timepoint post STEMI | Follow-up |
| Jensen et al[184] | 2010 | 50 | Manual | RVI only IP of MACE in model with age, sex, LVEF, LV IS | 3 d | 32 mo |
| Miszalski-Jamka et al[98] | 2010 | 99 | Manual | RVEF (HR 1.46) and RVI extent (HR 1.50) IP for MACE | “3-5 d” | 1150 d |
| Grothoff et al[187] | 2012 | 450 | Manual | RVI was IP of MACE (HR 6.70) | “1-4 d” | 20 mo |
In acute STEMI, IS, AAR and MSI are best imaged at 7 d post PPCI due to overestimation of necrosis on LGE, and IS at 7 d best predicts final IS, LV remodelling and function and prognosis[5-7,9,18,20,21]. Human studies suggest that AAR is stable during the first week[21,138]. Although Fernández-Jiménez et al[165] demonstrated a bimodal AAR peak in pigs, their drop in AAR extent on T2w CMR at 2 d post-reperfusion may be due to a high incidence of IMH in pigs and peak IMH extent at 2 d[74]. Indeed the drop in AAR extent on the gold standard of histological water analysis in their study at 2 d was much less pronounced, and at 7 d AAR extent had returned to stable peak levels. In addition, studies demonstrating close agreement between T2w-derived AAR and the reference non-invasive modality of SPECT[138,139] were undertaken at 7 d post STEMI. MVO and IMH extent peak at 48 h then decrease[18] but are present at 7 d[9,18]. Although undertaking CMR at 7-d may potentially underestimate MVO and IMH extent[9,18,74], this may be minimised by expressing MVO and IMH extent as a proportion of IS rather than LV mass, to correct for the corresponding reduction in IS. Thus, acutely post STEMI for the assessment of IS, MSI, MVO and IMH, imaging at 7 d may provide the best compromise in relation to their temporal changes[5-7,9,18,20,21] for accurate quantification and prediction of LV function, remodelling and prognosis. This needs to be balanced with contemporary clinical practice where patients are typically discharged at 3-4 d post-PPCI, and the risk of early attrition. Using final IS at follow-up as a primary outcome risks underestimating potential differences in treatment strategies due to greater infarct resorption with the larger infarcts.
Data on the chronology of IS suggests that infarct resorption is essentially complete by 3 mo post MI[9,18,20,74]. However a key objective of follow-up CMR is to assess LV geometry and remodelling and hence must allow the relatively slower adaptations of ventricular volumes (approximately 12 mo), compared with changes in IS and LVEF to complete. LVEF shows no significant change after 1-mo post STEMI. Follow-up CMR at 3 and 6-mo may fail to provide an accurate assessment of LV volumes and remodelling. The evidence base suggests that in order to allow completion of the trio of IS, LVEF and LV volumetric changes, follow-up CMR should be performed at 12-mo post STEMI[5,7,18,20,21]. When correlating CMR and clinical outcomes, the longer timepoint of 12-mo also permits more reliable clinical follow-up.
Standardisation of LGE, AAR and IMH sequences and quantification methods is equally important in light of newer T1, T2 and T2*-mapping sequences and inherent image quality issues associated with T2w-TSE.
Contrast-enhanced CMR offers robust, validated and reproducible surrogate markers, providing an accurate representation of pathophysiology, assessment of myocardial function and injury, and predictive value for medium to long-term LV function, remodelling and prognosis following PPCI for STEMI. Tables 16 and 17 summarise the key prospective studies illustrating the independent predictive value of CMR markers for LV remodelling (studies where n > 100, follow-up CMR ≥ 3 mo post PPCI) and prognosis (studies where n > 100, ≥ 6 mo follow-up) respectively.
| CMR marker | Ref. | Year | n | CMR quantification | Main findings | Acute CMR time | Follow-up CMR time |
| IS | Husser et al[33] | 2012 | 304 | 2SD | IS extent IP for LVR in model with LVEF, IS, LV volumes, MVO | 6 d | 189 d |
| IS | Monmeneu et al[91] | 2012 | 118 | 2SD | Number of segments > 50% transmurality IP for LVR | 6 d | 6 mo |
| IS | Wu et al[94] | 2008 | 122 | Manual | IS extent at 2 d only IP for LVEF and LVR | 2 d | 4 mo |
| IS | Hombach et al[6] | 2005 | 110 | Manual | IS extent at 6 d was an IP for LVR in model with MVO, % transmurality | 6 d | 225 d |
| L-MVO | Weir et al[112] | 2010 | 100 | Manual | L-MVO extent was only IP of LVR in model with TIMI post PCI, E-MVO, IS | 4 d | 6 mo |
| L-MVO | Hombach et al[6] | 2005 | 110 | Manual | L-MVO extent IP of LVR in model with baseline IS, infarct transmurality | 6 d | 225 d |
| IMH | Carrick et al[74] | 2016 | 245 | T2* | IMH strongest IP of LVR in model with patient/angio characteristics, LVEDVI | 3 d | 7 mo |
| IMH | Husser et al[33] | 2012 | 304 | T2w-TSE | IMH strongest IP for LVR in model with LVEF, IS, LV volumes, L-MVO | 6 d | 189 d |
| MSI | Monmeneu et al[91] | 2012 | 118 | 2SD LGR/STIR | MSI univariate but not IP of LVR in model with IS, LVESVI, segments > 50% | 6 d | 6 mo |
| MSI | Masci et al[14] | 2011 | 260 | 2SD STIR, 5SD LGE | MSI univariate predictor of LVR and final LVEF. However not IP of either | 1 wk | 4 mo |
| MSI | Masci et al[26] | 2010 | 137 | > SD STIR, 5SD LGE | MSI strongest IP for LVR. However IS and MSI and IS and AAR correlated | 1 wk | 4 mo |
| T1 | Carrick et al[177] | 2016 | 300 | T1 map, 2SD STIR, 5SD LGE | Infarct core native T1 inverse relationship with LVR (OR 0.91 per -10 ms T1) | 2 d | 6 mo |
| CMR marker | Ref. | Year | n | CMR quantification | Main findings | Acute CMR time | Follow-up |
| IS | Husser et al[96] | 2013 | 250 | > 2SD | Extent of transmural infarction was only IP for MACE | 7 d | 163 wk |
| IS | Izquierdo et al[97] | 2013 | 440 | > 2SD | IS was IP for arrhythmic cardiac events in model including LVEF, hypertension | 7 d | 123 wk |
| IS | Eitel et al[34] | 2011 | 208 | > 5SD | IS was IP of MACE in model with MVO, LVEF, MSI, Killip, TIMI flow post-PPCI | 3 d | 18.5 mo |
| IS | Larose et al[67] | 2010 | 103 | FWHM | IS strongest IP for MACE in model with LVEF, CK. LGE > 23% for MACE | 4.5 h | 2 yr |
| IS | Bodi et al[38] | 2009 | 214 | > 2SD | Extent of transmural infarction (no. of segments > 50% transmurality) IP for MACE | 7 d | 553 d |
| IS | Wu et al[99] | 2008 | 122 | Manual | IS only IP of 2 yr MACE in model containing LVEF, LVESVI (HR 1.06) | 2 d | 538 d |
| L-MVO | Regenfus et al[117] | 2015 | 249 | Manual | MVO extent strongest IP for MACE in model with IS, LVEF, TIMI and no. vessels | 3.7 d | 72 mo |
| L-MVO | Eitel et al[119] | 2014 | 738 | > 5SD | L-MVO > 1.4% LVM IP of MACE in model with LVEDVI, LVEF, clinical markers | 7 d | 6 mo |
| L-MVO | de Waha et al[120] | 2012 | 438 | Manual | L-MVO extent IP for MACE in model with IS, LV volumes. L-MVO/IS strongest IP | 3 d | 19 mo |
| L-MVO | de Waha et al[36] | 2010 | 438 | Manual | L-MVO strongest IP of MACE/mortality in model with IS, LVEF, STR, TIMI post | 3 d | 19 mo |
| L-MVO | Cochet et al[37] | 2009 | 184 | Manual | L-MVO strongest IP for MACE in model with GRACE, IS, LVEF. E-MVO weaker IP | “3-7 d” | 12 mo |
| L-MVO | Bruder et al[116] | 2008 | 143 | Manual | L-MVO extent > 0.5% LV mass IP for MACE in model with IS, LVEF, age, DM, sex | 4.5 d | 12 mo |
| L-MVO | Hombach et al[6] | 2005 | 110 | Manual | L-MVO IP for MACE (P = 0.04) in model with LV end-diastolic volume and LVEF | 6 d | 268 d |
| IMH | Carrick et al[74] | 2016 | 245 | T2* | IMH strongest IP of CV death and HF. Multivariate model, L-MVO not predictor | 3 d | 830 d |
| IMH | Amabile et al[133] | 2012 | 114 | T2w-TSE | IMH presence was strongest predictor of MACE in model with MVO, LVEF, STR | 4 d | 12 mo |
| IMH | Husser et al[33] | 2012 | 304 | T2w-TSE | IMH IP for MACE in model with AAR, IS, L-MVO. T2w. No inc. value with LGE | 6 d | 140 wk |
| IMH | Eitel et al[125] | 2011 | 346 | T2w-TSE | IMH IP of MACE in model with L-MVO. T2w inc. value with LGE and cine | 3 d | 6 mo |
| MSI | Eitel et al[34] | 2011 | 208 | > 2SD/> 5SD | MSI only CMR IP of mortality in model with age, IS, MVO, LVEF, TIMI post, IS | 3 d | 19 mo |
| MSI | Eitel et al[161] | 2010 | 208 | > 2SD/> 5SD | MSI only IP for MACE/mortality in model with LVEF, MVO, IS, STR, TIMI post | 3 d | 6 mo |
| T1 | Carrick et al[177] | 2016 | 300 | T1 map, > 2SD STIR, > 5SD | Infarct core T1 inverse association with risk of mortality and heart failure hospitalisation, in model with LVEF, infarct T2, IMH. Similar prognostic as L-MVO | 2 d | 2.5 yr |
In the acute phase, CMR can be performed accurately for up to 7 d post PPCI. CMR delivers no radiation to the patient and this makes it ideal for serial studies. The multimodal nature of CMR allows a multiparametric study of cardiac function, structure and volumes within a single study, which can be undertaken within approximately 45 min in the majority of patients. It is likely that CMR will become the mainstay of cardiac imaging, providing an important role in risk stratification and treatment post STEMI. Focus needs to be continued in translating findings on the prognostic importance of surrogate markers to development of therapeutic targets post STEMI.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): A, A, A, A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Barison A, Cheng TH, Cosmi E, Kato M, Sato A S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Group BDW. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4510] [Cited by in RCA: 4130] [Article Influence: 172.1] [Reference Citation Analysis (0)] |
| 2. | Desch S, Eitel I, de Waha S, Fuernau G, Lurz P, Gutberlet M, Schuler G, Thiele H. Cardiac magnetic resonance imaging parameters as surrogate endpoints in clinical trials of acute myocardial infarction. Trials. 2011;12:204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Pitcher A, Ashby D, Elliott P, Petersen SE. Cardiovascular MRI in clinical trials: expanded applications through novel surrogate endpoints. Heart. 2011;97:1286-1292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res. 2011;46:399-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6382] [Cited by in RCA: 7506] [Article Influence: 536.1] [Reference Citation Analysis (0)] |
| 5. | Ripa RS, Nilsson JC, Wang Y, Søndergaard L, Jørgensen E, Kastrup J. Short- and long-term changes in myocardial function, morphology, edema, and infarct mass after ST-segment elevation myocardial infarction evaluated by serial magnetic resonance imaging. Am Heart J. 2007;154:929-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Hombach V, Grebe O, Merkle N, Waldenmaier S, Höher M, Kochs M, Wöhrle J, Kestler HA. Sequelae of acute myocardial infarction regarding cardiac structure and function and their prognostic significance as assessed by magnetic resonance imaging. Eur Heart J. 2005;26:549-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 367] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 7. | Engblom H, Hedström E, Heiberg E, Wagner GS, Pahlm O, Arheden H. Rapid initial reduction of hyperenhanced myocardium after reperfused first myocardial infarction suggests recovery of the peri-infarction zone: one-year follow-up by MRI. Circ Cardiovasc Imaging. 2009;2:47-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Ghugre NR, Ramanan V, Pop M, Yang Y, Barry J, Qiang B, Connelly KA, Dick AJ, Wright GA. Quantitative tracking of edema, hemorrhage, and microvascular obstruction in subacute myocardial infarction in a porcine model by MRI. Magn Reson Med. 2011;66:1129-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 9. | Ibrahim T, Hackl T, Nekolla SG, Breuer M, Feldmair M, Schömig A, Schwaiger M. Acute myocardial infarction: serial cardiac MR imaging shows a decrease in delayed enhancement of the myocardium during the 1st week after reperfusion. Radiology. 2010;254:88-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 10. | Lund GK, Stork A, Muellerleile K, Barmeyer AA, Bansmann MP, Knefel M, Schlichting U, Müller M, Verde PE, Adam G. Prediction of left ventricular remodeling and analysis of infarct resorption in patients with reperfused myocardial infarcts by using contrast-enhanced MR imaging. Radiology. 2007;245:95-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 11. | Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990;81:1161-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 1887] [Article Influence: 53.9] [Reference Citation Analysis (0)] |
| 12. | Bolognese L, Neskovic AN, Parodi G, Cerisano G, Buonamici P, Santoro GM, Antoniucci D. Left ventricular remodeling after primary coronary angioplasty: patterns of left ventricular dilation and long-term prognostic implications. Circulation. 2002;106:2351-2357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 446] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 13. | Ahn KT, Song YB, Choe YH, Yang JH, Hahn JY, Choi JH, Choi SH, Chang SA, Lee SC, Lee SH. Impact of transmural necrosis on left ventricular remodeling and clinical outcomes in patients undergoing primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. Int J Cardiovasc Imaging. 2013;29:835-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Masci PG, Ganame J, Francone M, Desmet W, Lorenzoni V, Iacucci I, Barison A, Carbone I, Lombardi M, Agati L. Relationship between location and size of myocardial infarction and their reciprocal influences on post-infarction left ventricular remodelling. Eur Heart J. 2011;32:1640-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 15. | Wagner A, Mahrholdt H, Holly TA, Elliott MD, Regenfus M, Parker M, Klocke FJ, Bonow RO, Kim RJ, Judd RM. Contrast-enhanced MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: an imaging study. Lancet. 2003;361:374-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 970] [Cited by in RCA: 892] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 16. | Bellenger NG, Grothues F, Smith GC, Pennell DJ. Quantification of right and left ventricular function by cardiovascular magnetic resonance. Herz. 2000;25:392-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 53] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4399] [Cited by in RCA: 4577] [Article Influence: 199.0] [Reference Citation Analysis (0)] |
| 18. | Mather AN, Fairbairn TA, Artis NJ, Greenwood JP, Plein S. Timing of cardiovascular MR imaging after acute myocardial infarction: effect on estimates of infarct characteristics and prediction of late ventricular remodeling. Radiology. 2011;261:116-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 19. | Beek AM, Kühl HP, Bondarenko O, Twisk JW, Hofman MB, van Dockum WG, Visser CA, van Rossum AC. Delayed contrast-enhanced magnetic resonance imaging for the prediction of regional functional improvement after acute myocardial infarction. J Am Coll Cardiol. 2003;42:895-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 236] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 20. | Ganame J, Messalli G, Masci PG, Dymarkowski S, Abbasi K, Van de Werf F, Janssens S, Bogaert J. Time course of infarct healing and left ventricular remodelling in patients with reperfused ST segment elevation myocardial infarction using comprehensive magnetic resonance imaging. Eur Radiol. 2011;21:693-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Dall'Armellina E, Karia N, Lindsay AC, Karamitsos TD, Ferreira V, Robson MD, Kellman P, Francis JM, Forfar C, Prendergast BD. Dynamic changes of edema and late gadolinium enhancement after acute myocardial infarction and their relationship to functional recovery and salvage index. Circ Cardiovasc Imaging. 2011;4:228-236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 209] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 22. | Orn S, Manhenke C, Anand IS, Squire I, Nagel E, Edvardsen T, Dickstein K. Effect of left ventricular scar size, location, and transmurality on left ventricular remodeling with healed myocardial infarction. Am J Cardiol. 2007;99:1109-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 123] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 23. | Husser O, Bodí V, Sanchis J, Núnez J, Mainar L, Rumiz E, López-Lereu MP, Monmeneu J, Chaustre F, Trapero I. The sum of ST-segment elevation is the best predictor of microvascular obstruction in patients treated successfully by primary percutaneous coronary intervention. Cardiovascular magnetic resonance study. Rev Esp Cardiol. 2010;63:1145-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Beek AM, Nijveldt R, van Rossum AC. Intramyocardial hemorrhage and microvascular obstruction after primary percutaneous coronary intervention. Int J Cardiovasc Imaging. 2010;26:49-55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 86] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 25. | Ganame J, Messalli G, Dymarkowski S, Rademakers FE, Desmet W, Van de Werf F, Bogaert J. Impact of myocardial haemorrhage on left ventricular function and remodelling in patients with reperfused acute myocardial infarction. Eur Heart J. 2009;30:1440-1449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 221] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 26. | Masci PG, Ganame J, Strata E, Desmet W, Aquaro GD, Dymarkowski S, Valenti V, Janssens S, Lombardi M, Van de Werf F. Myocardial salvage by CMR correlates with LV remodeling and early ST-segment resolution in acute myocardial infarction. JACC Cardiovasc Imaging. 2010;3:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 27. | Canali E, Masci P, Bogaert J, Bucciarelli Ducci C, Francone M, McAlindon E, Carbone I, Lombardi M, Desmet W, Janssens S. Impact of gender differences on myocardial salvage and post-ischaemic left ventricular remodelling after primary coronary angioplasty: new insights from cardiovascular magnetic resonance. Eur Heart J Cardiovasc Imaging. 2012;13:948-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | Nijveldt R, van der Vleuten PA, Hirsch A, Beek AM, Tio RA, Tijssen JG, Piek JJ, van Rossum AC, Zijlstra F. Early electrocardiographic findings and MR imaging-verified microvascular injury and myocardial infarct size. JACC Cardiovasc Imaging. 2009;2:1187-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Norris RM, Barnaby PF, Brandt PW, Geary GG, Whitlock RM, Wild CJ, Barratt-Boyes BG. Prognosis after recovery from first acute myocardial infarction: determinants of reinfarction and sudden death. Am J Cardiol. 1984;53:408-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 182] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 30. | White HD, Norris RM, Brown MA, Brandt PW, Whitlock RM, Wild CJ. Left ventricular end-systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation. 1987;76:44-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1690] [Cited by in RCA: 1618] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 31. | Burns RJ, Gibbons RJ, Yi Q, Roberts RS, Miller TD, Schaer GL, Anderson JL, Yusuf S. The relationships of left ventricular ejection fraction, end-systolic volume index and infarct size to six-month mortality after hospital discharge following myocardial infarction treated by thrombolysis. J Am Coll Cardiol. 2002;39:30-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 365] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 32. | El Aidi H, Adams A, Moons KG, Den Ruijter HM, Mali WP, Doevendans PA, Nagel E, Schalla S, Bots ML, Leiner T. Cardiac magnetic resonance imaging findings and the risk of cardiovascular events in patients with recent myocardial infarction or suspected or known coronary artery disease: a systematic review of prognostic studies. J Am Coll Cardiol. 2014;63:1031-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 111] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 33. | Husser O, Monmeneu JV, Sanchis J, Nunez J, Lopez-Lereu MP, Bonanad C, Chaustre F, Gomez C, Bosch MJ, Hinarejos R. Cardiovascular magnetic resonance-derived intramyocardial hemorrhage after STEMI: Influence on long-term prognosis, adverse left ventricular remodeling and relationship with microvascular obstruction. Int J Cardiol. 2013;167:2047-2054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 34. | Eitel I, Desch S, de Waha S, Fuernau G, Gutberlet M, Schuler G, Thiele H. Long-term prognostic value of myocardial salvage assessed by cardiovascular magnetic resonance in acute reperfused myocardial infarction. Heart. 2011;97:2038-2045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 35. | Amabile N, Jacquier A, Gaudart J, Sarran A, Shuaib A, Panuel M, Moulin G, Bartoli JM, Paganelli F. Value of a new multiparametric score for prediction of microvascular obstruction lesions in ST-segment elevation myocardial infarction revascularized by percutaneous coronary intervention. Arch Cardiovasc Dis. 2010;103:512-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 36. | de Waha S, Desch S, Eitel I, Fuernau G, Zachrau J, Leuschner A, Gutberlet M, Schuler G, Thiele H. Impact of early vs. late microvascular obstruction assessed by magnetic resonance imaging on long-term outcome after ST-elevation myocardial infarction: a comparison with traditional prognostic markers. Eur Heart J. 2010;31:2660-2668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 176] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 37. | Cochet AA, Lorgis L, Lalande A, Zeller M, Beer JC, Walker PM, Touzery C, Wolf JE, Brunotte F, Cottin Y. Major prognostic impact of persistent microvascular obstruction as assessed by contrast-enhanced cardiac magnetic resonance in reperfused acute myocardial infarction. Eur Radiol. 2009;19:2117-2126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 38. | Bodi V, Sanchis J, Nunez J, Mainar L, Lopez-Lereu MP, Monmeneu JV, Rumiz E, Chaustre F, Trapero I, Husser O. Prognostic value of a comprehensive cardiac magnetic resonance assessment soon after a first ST-segment elevation myocardial infarction. JACC Cardiovasc Imaging. 2009;2:835-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 39. | St John Sutton M, Lee D, Rouleau JL, Goldman S, Plappert T, Braunwald E, Pfeffer MA. Left ventricular remodeling and ventricular arrhythmias after myocardial infarction. Circulation. 2003;107:2577-2582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 174] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 40. | Otterstad JE, St John Sutton M, Frøland G, Skjaerpe T, Graving B, Holmes I. Are changes in left ventricular volume as measured with the biplane Simpson’s method predominantly related to changes in its area or long axis in the prognostic evaluation of remodelling following a myocardial infarction? Eur J Echocardiogr. 2001;2:118-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 41. | St John Sutton M, Pfeffer MA, Plappert T, Rouleau JL, Moyé LA, Dagenais GR, Lamas GA, Klein M, Sussex B, Goldman S. Quantitative two-dimensional echocardiographic measurements are major predictors of adverse cardiovascular events after acute myocardial infarction. The protective effects of captopril. Circulation. 1994;89:68-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 559] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 42. | Eitel I, Pöss J, Jobs A, Eitel C, de Waha S, Barkhausen J, Desch S, Thiele H. Left ventricular global function index assessed by cardiovascular magnetic resonance for the prediction of cardiovascular events in ST-elevation myocardial infarction. J Cardiovasc Magn Reson. 2015;17:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 43. | Ibrahim el-SH. Myocardial tagging by cardiovascular magnetic resonance: evolution of techniques--pulse sequences, analysis algorithms, and applications. J Cardiovasc Magn Reson. 2011;13:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 187] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 44. | Götte MJ, van Rossum AC, Twisk JWR JT, Visser CA. Quantification of regional contractile function after infarction: strain analysis superior to wall thickening analysis in discriminating infarct from remote myocardium. J Am Coll Cardiol. 2001;37:808-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 120] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 45. | Axel L, Dougherty L. MR imaging of motion with spatial modulation of magnetization. Radiology. 1989;171:841-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 941] [Cited by in RCA: 739] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 46. | Lima JA, Jeremy R, Guier W, Bouton S, Zerhouni EA, McVeigh E, Buchalter MB, Weisfeldt ML, Shapiro EP, Weiss JL. Accurate systolic wall thickening by nuclear magnetic resonance imaging with tissue tagging: correlation with sonomicrometers in normal and ischemic myocardium. J Am Coll Cardiol. 1993;21:1741-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 122] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 47. | Yeon SB, Reichek N, Tallant BA, Lima JA, Calhoun LP, Clark NR, Hoffman EA, Ho KK, Axel L. Validation of in vivo myocardial strain measurement by magnetic resonance tagging with sonomicrometry. J Am Coll Cardiol. 2001;38:555-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 85] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 48. | Shehata ML, Cheng S, Osman NF, Bluemke DA, Lima JA. Myocardial tissue tagging with cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2009;11:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 143] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 49. | Hor KN, Gottliebson WM, Carson C, Wash E, Cnota J, Fleck R, Wansapura J, Klimeczek P, Al-Khalidi HR, Chung ES. Comparison of magnetic resonance feature tracking for strain calculation with harmonic phase imaging analysis. JACC Cardiovasc Imaging. 2010;3:144-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 318] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 50. | Khan JN, Singh A, Nazir SA, Kanagala P, Gershlick AH, McCann GP. Comparison of cardiovascular magnetic resonance feature tracking and tagging for the assessment of left ventricular systolic strain in acute myocardial infarction. Eur J Radiol. 2015;84:840-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 110] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 51. | Götte MJ, van Rossum AC, Marcus JT, Kuijer JP, Axel L, Visser CA. Recognition of infarct localization by specific changes in intramural myocardial mechanics. Am Heart J. 1999;138:1038-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 52. | Neizel M, Lossnitzer D, Korosoglou G, Schäufele T, Peykarjou H, Steen H, Ocklenburg C, Giannitsis E, Katus HA, Osman NF. Strain-encoded MRI for evaluation of left ventricular function and transmurality in acute myocardial infarction. Circ Cardiovasc Imaging. 2009;2:116-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 53. | Gerber BL, Garot J, Bluemke DA, Wu KC, Lima JA. Accuracy of contrast-enhanced magnetic resonance imaging in predicting improvement of regional myocardial function in patients after acute myocardial infarction. Circulation. 2002;106:1083-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 283] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 54. | Wong DT, Leong DP, Weightman MJ, Richardson JD, Dundon BK, Psaltis PJ, Leung MC, Meredith IT, Worthley MI, Worthley SG. Magnetic resonance-derived circumferential strain provides a superior and incremental assessment of improvement in contractile function in patients early after ST-segment elevation myocardial infarction. Eur Radiol. 2014;24:1219-1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 55. | Buss SJ, Krautz B, Hofmann N, Sander Y, Rust L, Giusca S, Galuschky C, Seitz S, Giannitsis E, Pleger S. Prediction of functional recovery by cardiac magnetic resonance feature tracking imaging in first time ST-elevation myocardial infarction. Comparison to infarct size and transmurality by late gadolinium enhancement. Int J Cardiol. 2015;183:162-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 56. | Ersbøll M, Andersen MJ, Valeur N, Mogensen UM, Fakhri Y, Thune JJ, Møller JE, Hassager C, Søgaard P, Køber L. Early diastolic strain rate in relation to systolic and diastolic function and prognosis in acute myocardial infarction: a two-dimensional speckle-tracking study. Eur Heart J. 2014;35:648-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (1)] |
| 57. | Ersbøll M, Valeur N, Mogensen UM, Andersen MJ, Møller JE, Velazquez EJ, Hassager C, Søgaard P, Køber L. Prediction of all-cause mortality and heart failure admissions from global left ventricular longitudinal strain in patients with acute myocardial infarction and preserved left ventricular ejection fraction. J Am Coll Cardiol. 2013;61:2365-2373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 314] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 58. | Hung CL, Verma A, Uno H, Shin SH, Bourgoun M, Hassanein AH, McMurray JJ, Velazquez EJ, Kober L, Pfeffer MA. Longitudinal and circumferential strain rate, left ventricular remodeling, and prognosis after myocardial infarction. J Am Coll Cardiol. 2010;56:1812-1822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 242] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 59. | Antoni ML, Mollema SA, Delgado V, Atary JZ, Borleffs CJ, Boersma E, Holman ER, van der Wall EE, Schalij MJ, Bax JJ. Prognostic importance of strain and strain rate after acute myocardial infarction. Eur Heart J. 2010;31:1640-1647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 160] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 60. | Mahrholdt H, Wagner A, Holly TA, Elliott MD, Bonow RO, Kim RJ, Judd RM. Reproducibility of chronic infarct size measurement by contrast-enhanced magnetic resonance imaging. Circulation. 2002;106:2322-2327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 291] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 61. | Nesto RW, Kowalchuk GJ. The ischemic cascade: temporal sequence of hemodynamic, electrocardiographic and symptomatic expressions of ischemia. Am J Cardiol. 1987;59:23C-30C. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 348] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 62. | Petersen SE, Mohrs OK, Horstick G, Oberholzer K, Abegunewardene N, Ruetzel K, Selvanayagam JB, Robson MD, Neubauer S, Thelen M. Influence of contrast agent dose and image acquisition timing on the quantitative determination of nonviable myocardial tissue using delayed contrast-enhanced magnetic resonance imaging. J Cardiovasc Magn Reson. 2004;6:541-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 63. | Kim RJ, Chen EL, Lima JA, Judd RM. Myocardial Gd-DTPA kinetics determine MRI contrast enhancement and reflect the extent and severity of myocardial injury after acute reperfused infarction. Circulation. 1996;94:3318-3326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 437] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 64. | Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, Bundy J, Finn JP, Klocke FJ, Judd RM. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999;100:1992-2002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1774] [Cited by in RCA: 1744] [Article Influence: 67.1] [Reference Citation Analysis (0)] |
| 65. | Pennell DJ. Cardiovascular magnetic resonance. Circulation. 2010;121:692-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 66. | Amado LC, Gerber BL, Gupta SN, Rettmann DW, Szarf G, Schock R, Nasir K, Kraitchman DL, Lima JA. Accurate and objective infarct sizing by contrast-enhanced magnetic resonance imaging in a canine myocardial infarction model. J Am Coll Cardiol. 2004;44:2383-2389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 373] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 67. | Larose E, Rodés-Cabau J, Pibarot P, Rinfret S, Proulx G, Nguyen CM, Déry JP, Gleeton O, Roy L, Noël B. Predicting late myocardial recovery and outcomes in the early hours of ST-segment elevation myocardial infarction traditional measures compared with microvascular obstruction, salvaged myocardium, and necrosis characteristics by cardiovascular magnetic resonance. J Am Coll Cardiol. 2010;55:2459-2469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 186] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 68. | Zia MI, Ghugre NR, Roifman I, Strauss BH, Walcarius R, Mohammed M, Sparkes JD, Dick AJ, Wright GA, Connelly KA. Comparison of the frequencies of myocardial edema determined by cardiac magnetic resonance in diabetic versus nondiabetic patients having percutaneous coronary intervention for ST elevation myocardial infarction. Am J Cardiol. 2014;113:607-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 69. | Robbers LF, Eerenberg ES, Teunissen PF, Jansen MF, Hollander MR, Horrevoets AJ, Knaapen P, Nijveldt R, Heymans MW, Levi MM. Magnetic resonance imaging-defined areas of microvascular obstruction after acute myocardial infarction represent microvascular destruction and haemorrhage. Eur Heart J. 2013;34:2346-2353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 164] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 70. | Małek ŁA, Śpiewak M, Kłopotowski M, Miśko J, Rużyłło W, Witkowski A. The size does not matter - the presence of microvascular obstruction but not its extent corresponds to larger infarct size in reperfused STEMI. Eur J Radiol. 2012;81:2839-2843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 71. | Kim HW, Farzaneh-Far A, Kim RJ. Cardiovascular magnetic resonance in patients with myocardial infarction: current and emerging applications. J Am Coll Cardiol. 2009;55:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 257] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 72. | Khan JN, Nazir SA, Horsfield MA, Singh A, Kanagala P, Greenwood JP, Gershlick AH, McCann GP. Comparison of semi-automated methods to quantify infarct size and myocardial salvage by cardiac mri at 1.5t and 3.0t field strengths. Heart. 2014;100:A76-A78. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 73. | O'Regan DP, Ariff B, Baksi AJ, Gordon F, Durighel G, Cook SA. Salvage assessment with cardiac MRI following acute myocardial infarction underestimates potential for recovery of systolic strain. Eur Radiol. 2013;23:1210-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 74. | Carrick D, Haig C, Ahmed N, McEntegart M, Petrie MC, Eteiba H, Hood S, Watkins S, Lindsay MM, Davie A. Myocardial Hemorrhage After Acute Reperfused ST-Segment-Elevation Myocardial Infarction: Relation to Microvascular Obstruction and Prognostic Significance. Circ Cardiovasc Imaging. 2016;9:e004148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 165] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 75. | Judd RM, Lugo-Olivieri CH, Arai M, Kondo T, Croisille P, Lima JA, Mohan V, Becker LC, Zerhouni EA. Physiological basis of myocardial contrast enhancement in fast magnetic resonance images of 2-day-old reperfused canine infarcts. Circulation. 1995;92:1902-1910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 307] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 76. | Fieno DS, Hillenbrand HB, Rehwald WG, Harris KR, Decker RS, Parker MA, Klocke FJ, Kim RJ, Judd RM. Infarct resorption, compensatory hypertrophy, and differing patterns of ventricular remodeling following myocardial infarctions of varying size. J Am Coll Cardiol. 2004;43:2124-2131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 121] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 77. | Aletras AH, Tilak GS, Natanzon A, Hsu LY, Gonzalez FM, Hoyt RF, Arai AE. Retrospective determination of the area at risk for reperfused acute myocardial infarction with T2-weighted cardiac magnetic resonance imaging: histopathological and displacement encoding with stimulated echoes (DENSE) functional validations. Circulation. 2006;113:1865-1870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 419] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 78. | Lowe JE, Reimer KA, Jennings RB. Experimental infarct size as a function of the amount of myocardium at risk. Am J Pathol. 1978;90:363-379. [PubMed] |
| 79. | Reimer KA, Jennings RB, Cobb FR, Murdock RH, Greenfield JC, Becker LC, Bulkley BH, Hutchins GM, Schwartz RP, Bailey KR. Animal models for protecting ischemic myocardium: results of the NHLBI Cooperative Study. Comparison of unconscious and conscious dog models. Circ Res. 1985;56:651-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 268] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 80. | Christian TF, Schwartz RS, Gibbons RJ. Determinants of infarct size in reperfusion therapy for acute myocardial infarction. Circulation. 1992;86:81-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 170] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 81. | Hirsch A, Nijveldt R, Haeck JD, Beek AM, Koch KT, Henriques JP, van der Schaaf RJ, Vis MM, Baan J, de Winter RJ. Relation between the assessment of microvascular injury by cardiovascular magnetic resonance and coronary Doppler flow velocity measurements in patients with acute anterior wall myocardial infarction. J Am Coll Cardiol. 2008;51:2230-2238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 82. | Francone M, Bucciarelli-Ducci C, Carbone I, Canali E, Scardala R, Calabrese FA, Sardella G, Mancone M, Catalano C, Fedele F. Impact of primary coronary angioplasty delay on myocardial salvage, infarct size, and microvascular damage in patients with ST-segment elevation myocardial infarction: insight from cardiovascular magnetic resonance. J Am Coll Cardiol. 2009;54:2145-2153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 219] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 83. | Cochet A, Zeller M, Lalande A, L’huillier I, Walker PM, Touzery C, Verges B, Wolf JE, Brunotte F, Cottin Y. Utility of Cardiac Magnetic Resonance to assess association between admission hyperglycemia and myocardial damage in patients with reperfused ST-segment elevation myocardial infarction. J Cardiovasc Magn Reson. 2008;10:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 84. | Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, Klocke FJ, Bonow RO, Judd RM. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2376] [Cited by in RCA: 2212] [Article Influence: 88.5] [Reference Citation Analysis (0)] |
| 85. | Khan JN, Nazir SA, Singh A, Shetye A, Lai FY, Peebles C, Wong J, Greenwood JP, McCann GP. Relationship of Myocardial Strain and Markers of Myocardial Injury to Predict Segmental Recovery After Acute ST-Segment-Elevation Myocardial Infarction. Circ Cardiovasc Imaging. 2016;9:e003457. [PubMed] |
| 86. | Natale L, Napolitano C, Bernardini A, Meduri A, Marano R, Lombardo A, Crea F, Bonomo L. Role of first pass and delayed enhancement in assessment of segmental functional recovery after acute myocardial infarction. Radiol Med. 2012;117:1294-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 87. | Shapiro MD, Nieman K, Nasir K, Nomura CH, Sarwar A, Ferencik M, Abbara S, Hoffmann U, Gold HK, Jang IK. Utility of cardiovascular magnetic resonance to predict left ventricular recovery after primary percutaneous coronary intervention for patients presenting with acute ST-segment elevation myocardial infarction. Am J Cardiol. 2007;100:211-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 88. | Kitagawa K, Ichikawa Y, Hirano T, Makino K, Kobayashi S, Takeda K, Sakuma H. Diagnostic value of late gadolinium-enhanced MRI and first-pass dynamic MRI for predicting functional recovery in patients after acute myocardial infarction. Radiat Med. 2007;25:263-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 89. | Janssens S, Dubois C, Bogaert J, Theunissen K, Deroose C, Desmet W, Kalantzi M, Herbots L, Sinnaeve P, Dens J. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet. 2006;367:113-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 982] [Cited by in RCA: 905] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 90. | Motoyasu M, Sakuma H, Ichikawa Y, Ishida N, Uemura S, Okinaka T, Isaka N, Takeda K, Nakano T. Prediction of regional functional recovery after acute myocardial infarction with low dose dobutamine stress cine MR imaging and contrast enhanced MR imaging. J Cardiovasc Magn Reson. 2003;5:563-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 91. | Monmeneu JV, Bodí V, López-Lereu MP, Sanchis J, Núñez J, Chaustre F, Husser O, Merlos P, Bonanad C, Miñana G. Analysis of post-infarction salvaged myocardium by cardiac magnetic resonance. Predictors and influence on adverse ventricular remodeling. Rev Esp Cardiol (Engl Ed). 2012;65:634-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 92. | Ezekowitz JA, Armstrong PW, Granger CB, Theroux P, Stebbins A, Kim RJ, Patel MR. Predicting chronic left ventricular dysfunction 90 days after ST-segment elevation myocardial infarction: An Assessment of Pexelizumab in Acute Myocardial Infarction (APEX-AMI) Substudy. Am Heart J. 2010;160:272-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 93. | Bodí V, Husser O, Sanchis J, Núñez J, López-Lereu MP, Monmeneu JV, Mainar L, Chaustre F, Riegger GA, Bosch MJ. Contractile reserve and extent of transmural necrosis in the setting of myocardial stunning: comparison at cardiac MR imaging. Radiology. 2010;255:755-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 94. | Wu KC, Zerhouni EA, Judd RM, Lugo-Olivieri CH, Barouch LA, Schulman SP, Blumenthal RS, Lima JA. Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation. 1998;97:765-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 985] [Cited by in RCA: 924] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 95. | Steg PG, James SK, Atar D, Badano LP, Blömstrom-Lundqvist C, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fernandez-Aviles F. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569-2619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3540] [Cited by in RCA: 3705] [Article Influence: 285.0] [Reference Citation Analysis (0)] |
| 96. | Husser O, Monmeneu JV, Bonanad C, Gomez C, Chaustre F, Nunez J, Lopez-Lereu MP, Minana G, Sanchis J, Mainar L. Head-to-head comparison of 1 week versus 6 months CMR-derived infarct size for prediction of late events after STEMI. Int J Cardiovasc Imaging. 2013;29:1499-1509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 97. | Izquierdo M, Ruiz-Granell R, Bonanad C, Chaustre F, Gomez C, Ferrero A, Lopez-Lereu P, Monmeneu JV, Nuñez J, Chorro FJ. Value of early cardiovascular magnetic resonance for the prediction of adverse arrhythmic cardiac events after a first noncomplicated ST-segment-elevation myocardial infarction. Circ Cardiovasc Imaging. 2013;6:755-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 98. | Miszalski-Jamka T, Klimeczek P, Tomala M, Krupiński M, Zawadowski G, Noelting J, Lada M, Sip K, Banyś R, Mazur W. Extent of RV dysfunction and myocardial infarction assessed by CMR are independent outcome predictors early after STEMI treated with primary angioplasty. JACC Cardiovasc Imaging. 2010;3:1237-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 99. | Wu E, Ortiz JT, Tejedor P, Lee DC, Bucciarelli-Ducci C, Kansal P, Carr JC, Holly TA, Lloyd-Jones D, Klocke FJ. Infarct size by contrast enhanced cardiac magnetic resonance is a stronger predictor of outcomes than left ventricular ejection fraction or end-systolic volume index: prospective cohort study. Heart. 2008;94:730-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 330] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 100. | Reimer KA, Jennings RB. The “wavefront phenomenon” of myocardial ischemic cell death. II. Transmural progression of necrosis within the framework of ischemic bed size (myocardium at risk) and collateral flow. Lab Invest. 1979;40:633-644. [PubMed] |
| 101. | Kloner RA, Ganote CE, Jennings RB. The “no-reflow” phenomenon after temporary coronary occlusion in the dog. J Clin Invest. 1974;54:1496-1508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1400] [Cited by in RCA: 1355] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 102. | Rochitte CE, Lima JA, Bluemke DA, Reeder SB, McVeigh ER, Furuta T, Becker LC, Melin JA. Magnitude and time course of microvascular obstruction and tissue injury after acute myocardial infarction. Circulation. 1998;98:1006-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 327] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 103. | Wong DT, Leung MC, Richardson JD, Puri R, Bertaso AG, Williams K, Meredith IT, Teo KS, Worthley MI, Worthley SG. Cardiac magnetic resonance derived late microvascular obstruction assessment post ST-segment elevation myocardial infarction is the best predictor of left ventricular function: a comparison of angiographic and cardiac magnetic resonance derived measurements. Int J Cardiovasc Imaging. 2012;28:1971-1981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 104. | Klug G, Mayr A, Schenk S, Esterhammer R, Schocke M, Nocker M, Jaschke W, Pachinger O, Metzler B. Prognostic value at 5 years of microvascular obstruction after acute myocardial infarction assessed by cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2012;14:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 105. | Wu KC, Kim RJ, Bluemke DA, Rochitte CE, Zerhouni EA, Becker LC, Lima JA. Quantification and time course of microvascular obstruction by contrast-enhanced echocardiography and magnetic resonance imaging following acute myocardial infarction and reperfusion. J Am Coll Cardiol. 1998;32:1756-1764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 229] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 106. | Gerber BL, Rochitte CE, Melin JA, McVeigh ER, Bluemke DA, Wu KC, Becker LC, Lima JA. Microvascular obstruction and left ventricular remodeling early after acute myocardial infarction. Circulation. 2000;101:2734-2741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 187] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 107. | Bekkers SC, Backes WH, Kim RJ, Snoep G, Gorgels AP, Passos VL, Waltenberger J, Crijns HJ, Schalla S. Detection and characteristics of microvascular obstruction in reperfused acute myocardial infarction using an optimized protocol for contrast-enhanced cardiovascular magnetic resonance imaging. Eur Radiol. 2009;19:2904-2912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 108. | Ørn S, Manhenke C, Greve OJ, Larsen AI, Bonarjee VV, Edvardsen T, Dickstein K. Microvascular obstruction is a major determinant of infarct healing and subsequent left ventricular remodelling following primary percutaneous coronary intervention. Eur Heart J. 2009;30:1978-1985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 109. | Bogaert J, Kalantzi M, Rademakers FE, Dymarkowski S, Janssens S. Determinants and impact of microvascular obstruction in successfully reperfused ST-segment elevation myocardial infarction. Assessment by magnetic resonance imaging. Eur Radiol. 2007;17:2572-2580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 110. | Khan JN, Razvi N, Nazir SA, Singh A, Masca NG, Gershlick AH, Squire I, McCann GP. Prevalence and extent of infarct and microvascular obstruction following different reperfusion therapies in ST-elevation myocardial infarction. J Cardiovasc Magn Reson. 2014;16:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 111. | Nijveldt R, Beek AM, Hirsch A, Stoel MG, Hofman MB, Umans VA, Algra PR, Twisk JW, van Rossum AC. Functional recovery after acute myocardial infarction: comparison between angiography, electrocardiography, and cardiovascular magnetic resonance measures of microvascular injury. J Am Coll Cardiol. 2008;52:181-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 272] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 112. | Weir RA, Murphy CA, Petrie CJ, Martin TN, Balmain S, Clements S, Steedman T, Wagner GS, Dargie HJ, McMurray JJ. Microvascular obstruction remains a portent of adverse remodeling in optimally treated patients with left ventricular systolic dysfunction after acute myocardial infarction. Circ Cardiovasc Imaging. 2010;3:360-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 113. | Wong DT, Weightman MJ, Baumert M, Tayeb H, Richardson JD, Puri R, Bertaso AG, Roberts-Thomson KC, Sanders P, Worthley MI. Electro-mechanical characteristics of myocardial infarction border zones and ventricular arrhythmic risk: novel insights from grid-tagged cardiac magnetic resonance imaging. Eur Radiol. 2012;22:1651-1658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 114. | Ye YX, Basse-Lüsebrink TC, Arias-Loza PA, Kocoski V, Kampf T, Gan Q, Bauer E, Sparka S, Helluy X, Hu K. Monitoring of monocyte recruitment in reperfused myocardial infarction with intramyocardial hemorrhage and microvascular obstruction by combined fluorine 19 and proton cardiac magnetic resonance imaging. Circulation. 2013;128:1878-1888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 115. | Kidambi A, Mather AN, Motwani M, Swoboda P, Uddin A, Greenwood JP, Plein S. The effect of microvascular obstruction and intramyocardial hemorrhage on contractile recovery in reperfused myocardial infarction: insights from cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2013;15:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 116. | Bruder O, Breuckmann F, Jensen C, Jochims M, Naber CK, Barkhausen J, Erbel R, Sabin GV. Prognostic impact of contrast-enhanced CMR early after acute ST segment elevation myocardial infarction (STEMI) in a regional STEMI network: results of the “Herzinfarktverbund Essen”. Herz. 2008;33:136-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 117. | Regenfus M, Schlundt C, Krähner R, Schönegger C, Adler W, Ludwig J, Daniel WG, Schmid M. Six-Year Prognostic Value of Microvascular Obstruction After Reperfused ST-Elevation Myocardial Infarction as Assessed by Contrast-Enhanced Cardiovascular Magnetic Resonance. Am J Cardiol. 2015;116:1022-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 118. | van Kranenburg M, Magro M, Thiele H, de Waha S, Eitel I, Cochet A, Cottin Y, Atar D, Buser P, Wu E. Prognostic value of microvascular obstruction and infarct size, as measured by CMR in STEMI patients. JACC Cardiovasc Imaging. 2014;7:930-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 274] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 119. | Eitel I, de Waha S, Wöhrle J, Fuernau G, Lurz P, Pauschinger M, Desch S, Schuler G, Thiele H. Comprehensive prognosis assessment by CMR imaging after ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2014;64:1217-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 308] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 120. | de Waha S, Desch S, Eitel I, Fuernau G, Lurz P, Leuschner A, Grothoff M, Gutberlet M, Schuler G, Thiele H. Relationship and prognostic value of microvascular obstruction and infarct size in ST-elevation myocardial infarction as visualized by magnetic resonance imaging. Clin Res Cardiol. 2012;101:487-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 121. | Bekkers SC, Smulders MW, Passos VL, Leiner T, Waltenberger J, Gorgels AP, Schalla S. Clinical implications of microvascular obstruction and intramyocardial haemorrhage in acute myocardial infarction using cardiovascular magnetic resonance imaging. Eur Radiol. 2010;20:2572-2578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 122. | Russo JJ, Wells GA, Chong AY, So DY, Glover CA, Froeschl MP, Hibbert B, Marquis JF, Dick A, Blondeau M. Safety and Efficacy of Staged Percutaneous Coronary Intervention During Index Admission for ST-Elevation Myocardial Infarction With Multivessel Coronary Disease (Insights from the University of Ottawa Heart Institute STEMI Registry). Am J Cardiol. 2015;116:1157-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 123. | Lotan CS, Bouchard A, Cranney GB, Bishop SP, Pohost GM. Assessment of postreperfusion myocardial hemorrhage using proton NMR imaging at 1.5 T. Circulation. 1992;86:1018-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 71] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 124. | Basso C, Corbetti F, Silva C, Abudureheman A, Lacognata C, Cacciavillani L, Tarantini G, Marra MP, Ramondo A, Thiene G. Morphologic validation of reperfused hemorrhagic myocardial infarction by cardiovascular magnetic resonance. Am J Cardiol. 2007;100:1322-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 125. | Eitel I, Kubusch K, Strohm O, Desch S, Mikami Y, de Waha S, Gutberlet M, Schuler G, Friedrich MG, Thiele H. Prognostic value and determinants of a hypointense infarct core in T2-weighted cardiac magnetic resonance in acute reperfused ST-elevation-myocardial infarction. Circ Cardiovasc Imaging. 2011;4:354-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 163] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 126. | O'Regan DP, Ariff B, Neuwirth C, Tan Y, Durighel G, Cook SA. Assessment of severe reperfusion injury with T2* cardiac MRI in patients with acute myocardial infarction. Heart. 2010;96:1885-1891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 127. | Kali A, Kumar A, Cokic I, Tang R, Tsaftaris S, Friedrich M, Dharmakumar R. Chronic manifestation of post-reperfusion intramyocardial hemorrhage as regional iron deposition – a cardiovascular mr study with ex-vivo validation. Circulation: Cardiovascular Imaging. 2013;6:218-228. |
| 128. | Kali A, Tang RL, Kumar A, Min JK, Dharmakumar R. Detection of acute reperfusion myocardial hemorrhage with cardiac MR imaging: T2 versus T2. Radiology. 2013;269:387-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 129. | Zia MI, Ghugre NR, Connelly KA, Strauss BH, Sparkes JD, Dick AJ, Wright GA. Characterizing myocardial edema and hemorrhage using quantitative T2 and T2* mapping at multiple time intervals post ST-segment elevation myocardial infarction. Circ Cardiovasc Imaging. 2012;5:566-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 130. | O'Regan DP, Ahmed R, Karunanithy N, Neuwirth C, Tan Y, Durighel G, Hajnal JV, Nadra I, Corbett SJ, Cook SA. Reperfusion hemorrhage following acute myocardial infarction: assessment with T2* mapping and effect on measuring the area at risk. Radiology. 2009;250:916-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 131. | Mather AN, Fairbairn TA, Ball SG, Greenwood JP, Plein S. Reperfusion haemorrhage as determined by cardiovascular MRI is a predictor of adverse left ventricular remodelling and markers of late arrhythmic risk. Heart. 2011;97:453-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 123] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 132. | Kali A, Kumar A, Cokic I, Tang RL, Tsaftaris SA, Friedrich MG, Dharmakumar R. Chronic manifestation of postreperfusion intramyocardial hemorrhage as regional iron deposition: a cardiovascular magnetic resonance study with ex vivo validation. Circ Cardiovasc Imaging. 2013;6:218-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 133. | Amabile N, Jacquier A, Shuhab A, Gaudart J, Bartoli JM, Paganelli F, Moulin G. Incidence, predictors, and prognostic value of intramyocardial hemorrhage lesions in ST elevation myocardial infarction. Catheter Cardiovasc Interv. 2012;79:1101-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 134. | Abdel-Aty H, Simonetti O, Friedrich MG. T2-weighted cardiovascular magnetic resonance imaging. J Magn Reson Imaging. 2007;26:452-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 164] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 135. | Higgins CB, Herfkens R, Lipton MJ, Sievers R, Sheldon P, Kaufman L, Crooks LE. Nuclear magnetic resonance imaging of acute myocardial infarction in dogs: alterations in magnetic relaxation times. Am J Cardiol. 1983;52:184-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 286] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 136. | Arai AE. Magnetic resonance imaging for area at risk, myocardial infarction, and myocardial salvage. J Cardiovasc Pharmacol Ther. 2012;16:313-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 137. | García-Dorado D, Oliveras J, Gili J, Sanz E, Pérez-Villa F, Barrabés J, Carreras MJ, Solares J, Soler-Soler J. Analysis of myocardial oedema by magnetic resonance imaging early after coronary artery occlusion with or without reperfusion. Cardiovasc Res. 1993;27:1462-1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 183] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 138. | Carlsson M, Ubachs JF, Hedström E, Heiberg E, Jovinge S, Arheden H. Myocardium at risk after acute infarction in humans on cardiac magnetic resonance: quantitative assessment during follow-up and validation with single-photon emission computed tomography. JACC Cardiovasc Imaging. 2009;2:569-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 160] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 139. | Hedström E, Engblom H, Frogner F, Aström-Olsson K, Ohlin H, Jovinge S, Arheden H. Infarct evolution in man studied in patients with first-time coronary occlusion in comparison to different species - implications for assessment of myocardial salvage. J Cardiovasc Magn Reson. 2009;11:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 140. | Hadamitzky M, Langhans B, Hausleiter J, Sonne C, Kastrati A, Martinoff S, Schömig A, Ibrahim T. The assessment of area at risk and myocardial salvage after coronary revascularization in acute myocardial infarction: comparison between CMR and SPECT. JACC Cardiovasc Imaging. 2013;6:358-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 141. | Viallon M, Mewton N, Thuny F, Guehring J, O’Donnell T, Stemmer A, Bi X, Rapacchi S, Zuehlsdorff S, Revel D. T2-weighted cardiac MR assessment of the myocardial area-at-risk and salvage area in acute reperfused myocardial infarction: comparison of state-of-the-art dark blood and bright blood T2-weighted sequences. J Magn Reson Imaging. 2012;35:328-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 142. | Berry C, Kellman P, Mancini C, Chen MY, Bandettini WP, Lowrey T, Hsu LY, Aletras AH, Arai AE. Magnetic resonance imaging delineates the ischemic area at risk and myocardial salvage in patients with acute myocardial infarction. Circ Cardiovasc Imaging. 2010;3:527-535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 143. | Srichai MB, Lim RP, Lath N, Babb J, Axel L, Kim D. Diagnostic performance of dark-blood T2-weighted CMR for evaluation of acute myocardial injury. Invest Radiol. 2013;48:24-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 144. | Kellman P, Aletras AH, Mancini C, McVeigh ER, Arai AE. T2-prepared SSFP improves diagnostic confidence in edema imaging in acute myocardial infarction compared to turbo spin echo. Magn Reson Med. 2007;57:891-897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 211] [Cited by in RCA: 197] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 145. | Goldfarb JW, Arnold S, Han J. Recent myocardial infarction: assessment with unenhanced T1-weighted MR imaging. Radiology. 2007;245:245-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 146. | Croisille P, Kim HW, Kim RJ. Controversies in cardiovascular MR imaging: T2-weighted imaging should not be used to delineate the area at risk in ischemic myocardial injury. Radiology. 2012;265:12-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 147. | Simonetti OP, Kim RJ, Fieno DS, Hillenbrand HB, Wu E, Bundy JM, Finn JP, Judd RM. An improved MR imaging technique for the visualization of myocardial infarction. Radiology. 2001;218:215-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 148. | Payne AR, Casey M, McClure J, McGeoch R, Murphy A, Woodward R, Saul A, Bi X, Zuehlsdorff S, Oldroyd KG. Bright-blood T2-weighted MRI has higher diagnostic accuracy than dark-blood short tau inversion recovery MRI for detection of acute myocardial infarction and for assessment of the ischemic area at risk and myocardial salvage. Circ Cardiovasc Imaging. 2011;4:210-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 149. | Sörensson P, Heiberg E, Saleh N, Bouvier F, Caidahl K, Tornvall P, Rydén L, Pernow J, Arheden H. Assessment of myocardium at risk with contrast enhanced steady-state free precession cine cardiovascular magnetic resonance compared to single-photon emission computed tomography. J Cardiovasc Magn Reson. 2010;12:25. [PubMed] |
| 150. | Ubachs JF, Sörensson P, Engblom H, Carlsson M, Jovinge S, Pernow J, Arheden H. Myocardium at risk by magnetic resonance imaging: head-to-head comparison of T2-weighted imaging and contrast-enhanced steady-state free precession. Eur Heart J Cardiovasc Imaging. 2012;13:1008-1015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 151. | Verhaert D, Thavendiranathan P, Giri S, Mihai G, Rajagopalan S, Simonetti OP, Raman SV. Direct T2 quantification of myocardial edema in acute ischemic injury. JACC Cardiovasc Imaging. 2011;4:269-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 288] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 152. | Naßenstein K, Nensa F, Schlosser T, Bruder O, Umutlu L, Lauenstein T, Maderwald S, Ladd ME. Cardiac MRI: T2-Mapping Versus T2-Weighted Dark-Blood TSE Imaging for Myocardial Edema Visualization in Acute Myocardial Infarction. Rofo. 2014;186:166-172. [PubMed] |
| 153. | Lønborg J, Engstrøm T, Mathiasen AB, Vejlstrup N. Myocardial area at risk after ST-elevation myocardial infarction measured with the late gadolinium enhancement after scar remodeling and T2-weighted cardiac magnetic resonance imaging. Int J Cardiovasc Imaging. 2012;28:1455-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 154. | Ubachs JF, Engblom H, Erlinge D, Jovinge S, Hedström E, Carlsson M, Arheden H. Cardiovascular magnetic resonance of the myocardium at risk in acute reperfused myocardial infarction: comparison of T2-weighted imaging versus the circumferential endocardial extent of late gadolinium enhancement with transmural projection. J Cardiovasc Magn Reson. 2010;12:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 155. | Kociemba A, Pyda M, Katulska K, Łanocha M, Siniawski A, Janus M, Grajek S. Comparison of diffusion-weighted with T2-weighted imaging for detection of edema in acute myocardial infarction. J Cardiovasc Magn Reson. 2013;15:90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 156. | O'Gara PT, Kushner FG, Ascheim DD, Casey DE, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:e78-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1953] [Cited by in RCA: 2275] [Article Influence: 175.0] [Reference Citation Analysis (0)] |
| 157. | Masci PG, Andreini D, Francone M, Bertella E, De Luca L, Coceani M, Mushtaq S, Mariani M, Carbone I, Pontone G. Prodromal angina is associated with myocardial salvage in acute ST-segment elevation myocardial infarction. Eur Heart J Cardiovasc Imaging. 2013;14:1041-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 158. | Desch S, Engelhardt H, Meissner J, Eitel I, Sareban M, Fuernau G, de Waha S, Grothoff M, Gutberlet M, Schuler G. Reliability of myocardial salvage assessment by cardiac magnetic resonance imaging in acute reperfused myocardial infarction. Int J Cardiovasc Imaging. 2012;28:263-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 159. | Milavetz JJ, Giebel DW, Christian TF, Schwartz RS, Holmes DR, Gibbons RJ. Time to therapy and salvage in myocardial infarction. J Am Coll Cardiol. 1998;31:1246-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 101] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 160. | Newby LK, Rutsch WR, Califf RM, Simoons ML, Aylward PE, Armstrong PW, Woodlief LH, Lee KL, Topol EJ, Van de Werf F. Time from symptom onset to treatment and outcomes after thrombolytic therapy. GUSTO-1 Investigators. J Am Coll Cardiol. 1996;27:1646-1655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 161. | Eitel I, Desch S, Fuernau G, Hildebrand L, Gutberlet M, Schuler G, Thiele H. Prognostic significance and determinants of myocardial salvage assessed by cardiovascular magnetic resonance in acute reperfused myocardial infarction. J Am Coll Cardiol. 2010;55:2470-2479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 363] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 162. | Friedrich MG, Abdel-Aty H, Taylor A, Schulz-Menger J, Messroghli D, Dietz R. The salvaged area at risk in reperfused acute myocardial infarction as visualized by cardiovascular magnetic resonance. J Am Coll Cardiol. 2008;51:1581-1587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 351] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 163. | Desch S, Eitel I, Schmitt J, Sareban M, Fuernau G, Schuler G, Thiele H. Effect of coronary collaterals on microvascular obstruction as assessed by magnetic resonance imaging in patients with acute ST-elevation myocardial infarction treated by primary coronary intervention. Am J Cardiol. 2009;104:1204-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 164. | Abdel-Aty H, Cocker M, Meek C, Tyberg JV, Friedrich MG. Edema as a very early marker for acute myocardial ischemia: a cardiovascular magnetic resonance study. J Am Coll Cardiol. 2009;53:1194-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 175] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 165. | Fernández-Jiménez R, Sánchez-González J, Agüero J, García-Prieto J, López-Martín GJ, García-Ruiz JM, Molina-Iracheta A, Rosselló X, Fernández-Friera L, Pizarro G. Myocardial edema after ischemia/reperfusion is not stable and follows a bimodal pattern: imaging and histological tissue characterization. J Am Coll Cardiol. 2015;65:315-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 180] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 166. | Phrommintikul A, Abdel-Aty H, Schulz-Menger J, Friedrich MG, Taylor AJ. Acute oedema in the evaluation of microvascular reperfusion and myocardial salvage in reperfused myocardial infarction with cardiac magnetic resonance imaging. Eur J Radiol. 2010;74:e12-e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 167. | Larose E, Tizon-Marcos H, Rodés-Cabau J, Rinfret S, Déry JP, Nguyen CM, Gleeton O, Boudreault JR, Roy L, Noël B. Improving myocardial salvage in late presentation acute ST-elevation myocardial infarction with proximal embolic protection. Catheter Cardiovasc Interv. 2010;76:461-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 168. | Ndrepepa G, Mehilli J, Schwaiger M, Schühlen H, Nekolla S, Martinoff S, Schmitt C, Dirschinger J, Schömig A, Kastrati A. Prognostic value of myocardial salvage achieved by reperfusion therapy in patients with acute myocardial infarction. J Nucl Med. 2004;45:725-729. [PubMed] |
| 169. | Messroghli DR, Niendorf T, Schulz-Menger J, Dietz R, Friedrich MG. T1 mapping in patients with acute myocardial infarction. J Cardiovasc Magn Reson. 2003;5:353-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 123] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 170. | Mewton N, Liu CY, Croisille P, Bluemke D, Lima JA. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J Am Coll Cardiol. 2011;57:891-903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 813] [Cited by in RCA: 749] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 171. | Salerno M, Kramer CM. Advances in parametric mapping with CMR imaging. JACC Cardiovasc Imaging. 2013;6:806-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 154] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 172. | Roujol S, Weingärtner S, Foppa M, Chow K, Kawaji K, Ngo LH, Kellman P, Manning WJ, Thompson RB, Nezafat R. Accuracy, precision, and reproducibility of four T1 mapping sequences: a head-to-head comparison of MOLLI, ShMOLLI, SASHA, and SAPPHIRE. Radiology. 2014;272:683-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 252] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 173. | Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU, Ridgway JP. Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn Reson Med. 2004;52:141-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 902] [Cited by in RCA: 1057] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 174. | h-Ici DO, Jeuthe S, Al-Wakeel N, Berger F, Kuehne T, Kozerke S, Messroghli DR. T1 mapping in ischaemic heart disease. Eur Heart J Cardiovasc Imaging. 2014;15:597-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 175. | Messroghli DR, Plein S, Higgins DM, Walters K, Jones TR, Ridgway JP, Sivananthan MU. Human myocardium: single-breath-hold MR T1 mapping with high spatial resolution--reproducibility study. Radiology. 2006;238:1004-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 195] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 176. | Messroghli DR, Walters K, Plein S, Sparrow P, Friedrich MG, Ridgway JP, Sivananthan MU. Myocardial T1 mapping: application to patients with acute and chronic myocardial infarction. Magn Reson Med. 2007;58:34-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 277] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 177. | Carrick D, Haig C, Rauhalammi S, Ahmed N, Mordi I, McEntegart M, Petrie MC, Eteiba H, Hood S, Watkins S. Prognostic significance of infarct core pathology revealed by quantitative non-contrast in comparison with contrast cardiac magnetic resonance imaging in reperfused ST-elevation myocardial infarction survivors. Eur Heart J. 2016;37:1044-1059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 103] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 178. | Giri S, Chung YC, Merchant A, Mihai G, Rajagopalan S, Raman SV, Simonetti OP. T2 quantification for improved detection of myocardial edema. J Cardiovasc Magn Reson. 2009;11:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 518] [Cited by in RCA: 526] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 179. | Ugander M, Bagi PS, Oki AJ, Chen B, Hsu LY, Aletras AH, Shah S, Greiser A, Kellman P, Arai AE. Myocardial edema as detected by pre-contrast T1 and T2 CMR delineates area at risk associated with acute myocardial infarction. JACC Cardiovasc Imaging. 2012;5:596-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 263] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 180. | Anderson LJ, Holden S, Davis B, Prescott E, Charrier CC, Bunce NH, Firmin DN, Wonke B, Porter J, Walker JM. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J. 2001;22:2171-2179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1169] [Cited by in RCA: 1212] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 181. | Masci PG, Francone M, Desmet W, Ganame J, Todiere G, Donato R, Siciliano V, Carbone I, Mangia M, Strata E. Right ventricular ischemic injury in patients with acute ST-segment elevation myocardial infarction: characterization with cardiovascular magnetic resonance. Circulation. 2010;122:1405-1412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 182. | Puchalski MD, Williams RV, Askovich B, Minich LL, Mart C, Tani LY. Assessment of right ventricular size and function: echo versus magnetic resonance imaging. Congenit Heart Dis. 2007;2:27-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 183. | Galea N, Francone M, Carbone I, Cannata D, Vullo F, Galea R, Agati L, Fedele F, Catalano C. Utility of cardiac magnetic resonance (CMR) in the evaluation of right ventricular (RV) involvement in patients with myocardial infarction (MI). Radiol Med. 2014;119:309-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 184. | Jensen CJ, Jochims M, Hunold P, Sabin GV, Schlosser T, Bruder O. Right ventricular involvement in acute left ventricular myocardial infarction: prognostic implications of MRI findings. AJR Am J Roentgenol. 2010;194:592-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 185. | Bonanad C, Ruiz-Sauri A, Forteza MJ, Chaustre F, Minana G, Gomez C, Diaz A, Noguera I, de Dios E, Nunez J. Microvascular obstruction in the right ventricle in reperfused anterior myocardial infarction. Macroscopic and pathologic evidence in a swine model. Thromb Res. 2013;132:592-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 186. | Andreini D, Pontone G, Mushtaq S, Pepi M, Bogaert J, Masci PG. Microvascular obstruction complicating acute right ventricular myocardial infarction. J Cardiovasc Med (Hagerstown). 2015;16 Suppl 1:S12-S14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 187. | Grothoff M, Elpert C, Hoffmann J, Zachrau J, Lehmkuhl L, de Waha S, Desch S, Eitel I, Mende M, Thiele H. Right ventricular injury in st-elevation myocardial infarction: Risk stratification by visualization of wall motion, edema, and delayed-enhancement cardiac magnetic resonance. Circulation: Cardiovascular Imaging. 2012;5:60-68. [DOI] [Full Text] |
| 188. | Bodi V, Sanchis J, Mainar L, Chorro FJ, Nunez J, Monmeneu JV, Chaustre F, Forteza MJ, Ruiz-Sauri A, Lopez-Lereu MP. Right ventricular involvement in anterior myocardial infarction: a translational approach. Cardiovasc Res. 2010;87:601-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 189. | Larose E, Ganz P, Reynolds HG, Dorbala S, Di Carli MF, Brown KA, Kwong RY. Right ventricular dysfunction assessed by cardiovascular magnetic resonance imaging predicts poor prognosis late after myocardial infarction. J Am Coll Cardiol. 2007;49:855-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 148] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 190. | Hamon M, Agostini D, Le Page O, Riddell JW, Hamon M. Prognostic impact of right ventricular involvement in patients with acute myocardial infarction: meta-analysis. Crit Care Med. 2008;36:2023-2033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 191. | Shah PK, Maddahi J, Staniloff HM, Ellrodt AG, Pichler M, Swan HJ, Berman DS. Variable spectrum and prognostic implications of left and right ventricular ejection fractions in patients with and without clinical heart failure after acute myocardial infarction. Am J Cardiol. 1986;58:387-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 95] [Article Influence: 2.4] [Reference Citation Analysis (0)] |