Published online Dec 26, 2017. doi: 10.4330/wjc.v9.i12.822
Peer-review started: August 25, 2017
First decision: September 25, 2017
Revised: October 21, 2017
Accepted: November 10, 2017
Article in press: November 10, 2017
Published online: December 26, 2017
Processing time: 119 Days and 4.2 Hours
To determine endothelin-1 (ET-1) concentration before and after surgical coarctectomy and evaluate its association with left ventricular geometric change.
A prospective, cohort study of 24 patients aged 2 d to 10 years with coarctation of the aorta undergoing surgical repair. A sub-cohort of patients with age < 1 mo was classified as “neonates”. Echocardiograms were performed just prior to surgery and in the immediate post-op period to assess left ventricle mass index and relative wall thickness (RWT). Plasma ET-1 levels were assessed at both time points. Association between ET-1 levels and ventricular remodeling was assessed.
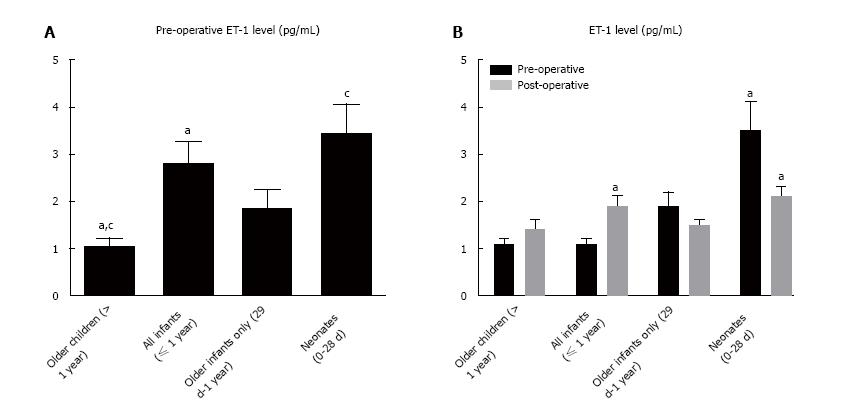
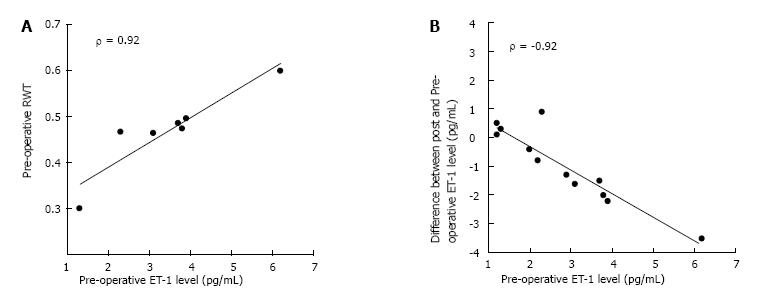
Patients < 1 year demonstrated higher pre-op ET-1 than post-op (2.8 pg/mL vs 1.9 pg/mL, P = 0.02). Conversely, patients > 1 year had no change in ET-1 concentration before and after surgery (1.1 vs 1.4, NS). Pre-op, patients < 1 year demonstrated significantly higher ET-1 than older children (2.8 vs 1.1, P = 0.001). Post-op there was no difference between the age groups (1.9 vs 1.4, NS). Neither RWT nor left ventricle mass index (LVMI) varied from pre-op to post-op. The subset of neonates showed a strong positive correlation between pre-op ET-1 and RWT (r = 0.92, P = 0.001). Patients with ET-1 > 2 pg/mL pre-op demonstrated higher LVMI (65.7 g/m2.7vs 38.5 g/m2.7, P = 0.004) and a trend towards higher RWT (45% vs 39%, P = 0.07) prior to repair than those with lower ET-1 concentration.
ET-1 concentration is significantly variable in the peri-operative period surrounding coarctectomy. Older children and infants have different responses to surgical repair suggesting different mechanisms of activation.
Core tip: Patients with coarctation of the aorta are at risk for a variety of short- and long-term complications after surgical repair. Endothelin-1 (ET-1) is a peptide hormone known to cause both cardiac myocyte hypertrophy and vasoconstriction that has been linked to late ventricular hypertrophy in this population. Peri-operative endothelin concentration in this population has not been previously defined. We demonstrate that neonates with coarctation of the aorta have high pre-operative ET-1 levels that decrease post-operatively. Older coarctation patients have more modest ET-1 activation that is unchanged post-operatively. These findings suggest two distinct patterns of ET-1 activation within this population.
- Citation: Frank BS, Urban TT, Tong S, Cassidy C, Mitchell MB, Nichols CS, Davidson JA. Endothelin-1 activation in pediatric patients undergoing surgical coarctation of the aorta repair. World J Cardiol 2017; 9(12): 822-829
- URL: https://www.wjgnet.com/1949-8462/full/v9/i12/822.htm
- DOI: https://dx.doi.org/10.4330/wjc.v9.i12.822
Isolated coarctation of the aorta (CoA) is found in 800 of every million live births, approximately 8% of all congenital heart disease[1]. Open surgical repair via thoracotomy or sternotomy is required for most patients presenting with severe obstruction. Although perioperative mortality is low in the modern era, the post-operative course is frequently complicated by either low cardiac output syndrome or recalcitrant hypertension[2,3]. Additionally, these children are at significant risk for long-term complications including persistent hypertension, a hypertensive response to exercise, altered cardiac mechanics, and left ventricular (LV) hypertrophy[4,5].
Vascular and cardiac changes are, in many cases, already present at the time of initial surgery. Pre-operatively, approximately 65% of all children with CoA will have LV hypertrophy and 33% of the subset who are diagnosed as neonates will have evidence of pulmonary hypertension[6]. Such echocardiographic evidence of physiologic derangements, present at diagnosis, suggests that there may be early activation of critical pathologic pathways. Better understanding of such pathways and their patterns of activation offers significant promise to understand the mechanisms of disease progression, improve prognostic accuracy, and guide future therapy.
Endothelin-1 (ET-1) is a 21-amino acid vasoactive peptide hormone with endocrine and paracrine effects. Previous mechanistic in vitro studies have demonstrated the capacity for ET-1 to cause vasoconstriction in both the pulmonary and systemic vasculature[7] as well as cardiac myocyte hypertrophy[8,9]. Additionally, a recent study showed increased ET-1 blood concentration was associated with left ventricular hypertrophy in a mouse model of coarctation of the aorta[10]. Clinical data in human subjects, however, are quite limited. While ET-1 concentration is increased compared to controls at late follow-up after coarctation repair[11], the clinical implications of this finding are yet to be evaluated. Further, no prior study has assessed either ET-1 concentration or its association with cardiovascular pathology at the time of initial surgery for CoA. While ET-1 activation is not thought to be causative of coarctation of the aorta, defining ET-1 activation in the perioperative period could offer significant insight as a marker of the variable short and long term physiologic responses to coarctation seen in this population.
Here we present a prospective, cohort study of ET-1 concentration and pathologic myocardial remodeling both prior to surgical correction of coarctation/aortic arch obstruction and in the immediate post-operative period. We sought to define pre-operative ET-1 activation, perioperative changes in concentrations with surgical relief, and the association with early myocardial change.
The Colorado Multiple Institution Review Board approved this study. Written informed consent was obtained from the study subjects’ parents in all cases. Written assent was obtained from all subjects aged between seven years and eighteen years.
We prospectively enrolled consecutive subjects (aged 0 to 18 years) undergoing surgical relief of coarctation of the aorta with or without associated transverse arch hypoplasia at Children’s Hospital Colorado from September 2015 through January 2017. Exclusion criteria included patients with significant co-morbid heart disease, those with a prior intervention (surgical or trans-catheter) on their aortic arch, and those weighing less than 2 kg, due to limitations in acceptable sample blood volumes for research.
Clinical information was extracted from the electronic medical record (Epic Systems, Verona, WI). Demographic variables, peri-operative details, and key clinical variables were recorded. Study data were collected and managed using REDCap electronic data capture tools hosted at University of Colorado[12].
Blood samples were obtained immediately prior to surgery and between 12-48 h post-operatively. Extracted plasma aliquots were then stored at -80 °C for batch analysis. ET-1 and B-type natriuretic peptide (BNP) analysis were performed by enzyme-linked immunosorbent assay (ELISA) per manufacturer’s recommendations (R and D Systems, Inc. Minneapolis, MN).
Echocardiograms were obtained immediately prior to surgical repair and between 24 and 72 h post-operatively. All images were obtained with a GE Vivid E9 or E95 machine (General Electric, Chicago, Ill). Relative wall thickness (RWT) was measured at end-diastole from the parasternal short axis view as the ratio of the sum of the posterior and septal mural thickness to the left ventricular internal end-diastolic diameter; a value of 41% is conventionally taken as the upper limit of normal for RWT[4]. LV mass was calculated by the area-length (AL) method, indexed to height2.7, and compared to previously published normal values[13,14].
Demographics were summarized using descriptive statistics as indicated by the distribution of the data. Changes in echocardiographic indices were compared using the Signed-Rank test. Pearson’s correlation test, two-sample T-Test, and general linear modeling compared ET-1 levels among groups and correlation with echocardiographic indices. All the statistical analyses were performed with SAS V9.4. The primary outcome for analysis was change in ET-1 concentration from the pre-operative to the post-operative sample. Other associations were tested as secondary outcomes. Statistician S Tong from University of Colorado, Denver reviewed the statistical methods of this study.
Twenty-four patients were enrolled in the study. Their demographics and baseline/surgical characteristics are presented in Table 1, represented as median (range) or n (%). Five patients, all < 1 mo in age, underwent aortic arch reconstruction on cardio-pulmonary bypass, while the other nineteen underwent coarctectomy by lateral thoracotomy without bypass. Six patients (all in the neonatal cohort) had evidence of a patent ductus arteriosus on echocardiogram and were on prostaglandin infusion at the time of repair. In each of those six patients, ductal flow was right-to-left in systole, indicating that pressure in the pulmonary artery was equal to or greater than pressure in the aorta. One patient was on continuous milrinone. No patient received an endothelin receptor antagonist during the study period.
| Age (d) | 356 (24039) |
| Age class | |
| ≤ 1 yr | 12 (50) |
| > 1 yr | 12 (50) |
| Age class | |
| ≤ 28 d | 7 (29.2) |
| 28 d-1 yr | 5 (20.8) |
| > 1 yr | 12 (50) |
| Weight preop | 7.9 (3.1, 81.8) |
| Weight postop | 8.0 (2.9, 81.8) |
| Race | |
| African American | 1 (4.2) |
| White | 20 (83.3) |
| Hispanic | 3 (12.5) |
| Gender | |
| Male | 17 (70.8) |
| Female | 7 (29.2) |
Clinical presentation varied by age at diagnosis. Five of the neonates were diagnosed prenatally, started on prostaglandin within the first hours of life, and remained stable until repair. Two neonates presented within the first week of life with clinical evidence of decreased systemic perfusion and were medically stabilized before proceeding to operative repair. The patients between one month and one year of life had the greatest variability in clinical presentation, ranging from asymptomatic murmur to symptomatic left ventricular failure with decreased tissue oxygen delivery. Children older than one year were all clinically stable at presentation, referred for right upper extremity hypertension, decreased femoral pulses, or an asymptomatic murmur.
Pre-op ET-1 level in our entire cohort was 1.9 pg/mL (Figure 1). Patients < 1-year-old showed significantly higher concentrations when compared to the older cohort. This effect was most pronounced among neonates, who demonstrated the highest levels.
Analysis within age cohorts demonstrated two distinct patterns. Patients < 1-year-old showed an immediate decline in ET-1 level post-op (Figure 1). Conversely, older children demonstrated no significant change between pre-op and post-op. Overall, patients with the highest levels of ET-1 pre-op demonstrated the greatest post-op decline while those with more modest ET-1 concentration pre-op tended to have unchanged levels after repair (Figure 2).
For the entire population taken together, BNP concentration followed a right skewed distribution. Median pre-op BNP concentration was 73 pg/mL (upper limit of normal 99 pg/mL, range 21-4915). Neonatal subjects demonstrated significantly higher BNP levels than older children [1752 (30-4915) pg/mL vs 35 (21-74) pg/mL, P < 0.0001, represented as median (range)]. Pre-operative BNP was moderately correlated with pre-operative ET-1 (r = 0.65; P = 0.0002). Post-operatively, neonatal patients demonstrated a significant decrease in BNP level [1752 (30-4915) pg/mL vs 977 (192-2732) pg/mL, P = 0.02] while the concentration was unchanged among older children [35 (21-74) pg/mL vs 161 (124-451) pg/mL, NS]. Post-operative BNP was moderately correlated with post-operative ET-1 (r = 0.73; P = 0.0001).
Mean RWT (%) and left ventricle mass index (LVMI) (g/m2.7) were increased compared to normal values for all time points (Table 2). As expected, there was no significant change in RWT or LVMI between the pre-op and post-operative echocardiograms. Overall, infants showed higher LVMI compared to older children but no significant difference in RWT.
| Subjects | Preop | Posop | P value | |
| Relative wall thickness | ||||
| All subjects | 24 | 0.41 (0.09) | 0.42 (0.10) | NS |
| Neonates (0-28 d) | 7 | 0.44 (0.09) | 0.47 (0.09) | NS |
| Infants (29 d-1 yr) | 5 | 0.43 (0.08) | 0.40 (0.14) | NS |
| Children (> 1 yr) | 12 | 0.39 (0.09) | 0.39 (0.09) | NS |
| LV mass index | ||||
| All subjects | 24 | 49.4 (24.6) | 51.6 (17.5) | NS |
| Neonates (0-28 d) | 7 | 50.2 (22.3) | 57.6 (12.9) | NS |
| Infants (29 d-1 yr) | 5 | 79.7 (31.1) | 72.0 (19.9) | NS |
| Children (> 1 yr) | 12 | 36.4 (6.3) | 39.6 (7.1) | NS |
Including the entire cohort, RWT and LVMI were compared between patients with lower and higher levels of ET-1. Patients with ET-1 > 2 pg/mL pre-op demonstrated higher LVMI (65.7 g/m2.7vs 38.5 g/m2.7, P = 0.004) and a trend towards higher RWT (45% vs 39%, P = 0.07) prior to repair. Additionally, pre-op ET-1 > 2 pg/mL was associated with higher post-op LVMI and RWT. Among the neonatal cohort, pre-operative ET-1 showed a strong positive correlation with post-op RWT (Figure 2).
This study is the first to assess early ET-1 activation and its association with LV remodeling at the time of surgery for coarctation of the aorta. Key findings include increased concentration among neonates preoperatively with a decline after anatomic correction. This pattern is contrasted with more modest ET-1 level before surgery in older children and no significant post-operative change. Taking all patients together, our population also shows higher levels of ET-1 concentration than previously reported normal controls[11]. Similar changes in ET-1 level surrounding anatomic correction of a congenital heart lesion have not previously been described. The variable pattern of ET-1 concentration seen among patients with similar anatomic lesions but different physiologies is also a novel finding.
Increasing evidence suggests that ET-1 activation is regulated in part by pulmonary vascular stress. While data conflict on whether pulmonary artery pressure or pulmonary blood flow is the primary effector, abnormalities in pulmonary vascular physiology are known to associate with alterations in serum ET-1 concentration[15-18]. Subgroup analysis of our data supports this relationship. The youngest subset of patients demonstrated the highest pre-operative ET-1 levels; these patients, in addition to manifesting high LV afterload, all demonstrated elevated pressure in the pulmonary arteries due either to ductal dependent systemic blood flow or as an upstream consequence of left atrial hypertension. Those same patients also had a post-operative decline in ET-1 level associated with the acute physiologic change (rapid normalization of pulmonary hemodynamics). The older children, with isolated high LV afterload physiology that is slower to resolve, experienced no change in ET-1 concentration from pre-op to post-op. The variable activation pattern between neonatal and older patients supports a role for the different pulmonary artery mechanics between the two subgroups affecting the variable ET-1 levels seen.
One potential confounder in the different pre-op to post-op ET-1 patterns described is the role of cardio-pulmonary bypass in post-op ET-1 concentration (a majority of neonates were repaired on bypass while all older children were repaired off pump). However, prior studies of pediatric patients undergoing bypass for the Fontan operation[19] and adult patients undergoing bypass for coronary artery bypass grafting[20] have demonstrated, on average, higher plasma ET-1 concentrations after bypass. And, the two neonates who underwent coarctectomy without bypass showed the same pattern of ET-1 concentration as the rest of the neonatal cohort. The observation that patients with different pre- and post-op physiologies undergoing bypass have widely variable peri-op ET-1 concentration patterns further supports the conclusion that the peri-op physiology likely plays the dominant role in ET-1 level, rather than bypass alone.
Our data also provide preliminary evidence for a separate pathway of ET-1 regulation associated with LV afterload. All patients in our cohort, including older coarctation subjects with isolated high LV afterload and otherwise normal physiology, have higher ET-1 concentration than previously reported normal controls[11]. Previous studies have suggested such a correlation in a similar population, showing increased ET-1 levels compared to controls in hypertensive adults with high afterload physiology[21]. One possible driver of ET-1 activation in this group is sympathetic nervous system activation. Norepinephrine, an endogenous catecholamine and effector of the sympathetic nervous system, is known both to be over-expressed in hypertensive patients[22] and to stimulate ET-1 production by the vascular endothelium[23]. Given the normal pulmonary hemodynamics in our older cohort and the mechanistic explanation offered by previous work, we posit that the ET-1 levels seen in this sub-group could reflect sympathetic activation due to the abnormality in LV and systemic vascular physiology. Future studies will be needed to confirm this hypothesis.
Individual patients who deviated from the typical clinical presentation for their age cohort provide further evidence for the two distinct mechanisms of ET-1 regulation. A neonate with a severe coarctation, acutely decreased LV function, and normal pulmonary artery pressure developed post-operative low cardiac output syndrome and his function was slow to recover. His ET-1 concentration increased slightly from the pre-operative to post-operative sample, consistent with the pattern typical of older children whose ET-1 level is likely driven by LV stress. An eight month old with severe coarctation, a dilated LV with poor systolic function, and near-systemic pulmonary artery pressure secondary to longstanding LV failure demonstrated rapidly improved pulmonary hypertension and LV function post-operatively. As would be expected, ET-1 activation in this patient more closely mirrored those in the neonatal cohort: Pre-operative ET-1 concentration was quite high and post-operative level was well below the pre-operative value.
Measured BNP concentration follows a similar trend to ET-1 concentration in this patient population. Neonatal patients have higher pre-operative BNP levels than their older counterparts and show a significant post-operative decline. Older children have lower BNP levels (normal in many cases) pre-operatively with no statistical change after surgical correction. These data suggest that neonates and some older infants experience a broad neuro-hormonal activation prior to coarctation repair in response to ventricular and potentially pulmonary artery stress and that surgical repair can result in early reversal of this neuro-hormonal activation in many patients. The specific role of BNP in this pathophysiology is not fully defined, and future studies will be needed to clarify the relationship with ET-1 and other markers of neuro-hormonal activation.
The echocardiographic data provide another layer of evidence for ET-1’s potential role in the physiologic response to coarctation. Average LVMI and RWT in our patient population were significantly higher than previously published normal values and did not vary significantly through the perioperative period. This finding supports prior work demonstrating concentric LV remodeling and hypertrophy in the face of chronically high afterload with no immediate resolution following afterload reduction[6,24,25]. Mechanistically, animal studies have demonstrated that chronic ET-1 exposure induces cardiomyocyte hypertrophy via increased production of Extracellular Signal-Regulated Kinases 1 and 2 (ERK 1/2)[8,9,26]. Our data aligns with this finding, as patients with higher levels of ET-1 had more abnormal cardiac geometry. This novel finding combined with a biologically plausible link between ET-1 and myocyte hypertrophy raises the possibility that ET-1 could be not only a useful marker of LV remodeling but potentially an effector as well. Further, longitudinal studies will be needed to evaluate ET-1’s role both in myocyte hypertrophy prior to repair and reverse remodeling after surgical correction.
In summary, we conclude that ET-1 concentration is significantly altered in patients with coarctation of the aorta undergoing surgical repair. We find preliminary evidence supporting two potentially distinct stimuli for ET-1: One mechanism, associated with high LV afterload and sympathetic nervous system activation, persists through the immediate post-operative period and the other, likely driven by altered pulmonary artery physiology, is rapidly reversible. We further find preliminary evidence supporting an association between ET-1 activity and early pathologic LV remodeling.
This study is prospective, single center, and targets a relatively rare patient population. As such, statistical power was limited, particularly in sub-cohort analysis. Biological heterogeneity, particularly among patients between 1 mo and 1 year of age, also limited statistical analysis. Therefore, validation of these findings in similar cohorts at other centers will be of great importance. Our study design does not allow for conclusions regarding causality in the relationship between ET-1 level and LV remodeling. Due to the young age of the subject population, control serum samples for ET-1 analysis were not obtained. While historical controls from previous studies were noted for discussion, an ideal comparison including unaffected, age-matched controls will be a goal of future studies. Additionally, small patient size and safety concerns preclude routine direct clinical monitoring of pulmonary artery and left atrial pressure in the peri-operative period. While hemodynamic inferences can be drawn from available clinical data, future animal studies will be helpful to directly measure these pressures and more precisely elucidate their role in the physiology described.
Patients with coarctation of the aorta are at risk for a variety of short- and long-term complications after surgical repair. Endothelin-1 (ET-1) is a peptide hormone known to cause both cardiac myocyte hypertrophy and vasoconstriction that has been linked to late ventricular hypertrophy in this population. Peri-operative endothelin concentration in this population has not been previously defined.
Defining ET-1 activation in the perioperative period could offer significant insight into the variable short and long term physiologic responses to coarctation seen in this population.
The authors sought to define pre-operative ET-1 activation, perioperative changes in concentrations with surgical relief, and the association with early myocardial change.
Here authors present a prospective, cohort study of ET-1 concentration and pathologic myocardial remodeling both prior to surgical correction of coarctation/aortic arch obstruction and in the immediate post-operative period. ET-1 analysis was performed by ELISA. Echocardiograms were obtained immediately prior to surgical repair and between 24 and 72 h post-operatively. All images were obtained with a GE Vivid E9 or E95 machine (General Electric, Chicago, Ill). Relative wall thickness (RWT) was measured at end-diastole from the parasternal short axis view as the ratio of the sum of the posterior and septal mural thickness to the left ventricular internal end-diastolic diameter; a value of 41% is conventionally taken as the upper limit of normal for RWT. LV mass was calculated by the area-length (AL) method, indexed to height2.7, and compared to previously published normal values.
The authors demonstrate that neonates with coarctation of the aorta have high pre-operative ET-1 levels that decrease post-operatively. Older coarctation patients have more modest ET-1 activation that is unchanged post-operatively.
This study is the first to assess early ET-1 activation and its association with LV remodeling at the time of surgery for coarctation of the aorta. Key findings include increased concentration among neonates preoperatively with a decline after anatomic correction. This pattern is contrasted with more modest ET-1 level before surgery in older children and no significant post-operative change. The authors find preliminary evidence supporting two potentially distinct stimuli for ET-1: One mechanism, associated with high LV afterload and sympathetic nervous system activation, persists through the immediate post-operative period and the other, likely driven by altered pulmonary artery physiology, is rapidly reversible. The authors further find preliminary evidence supporting an association between ET-1 activity and early pathologic LV remodeling.
Longitudinal studies will be needed to evaluate ET-1’s role both in myocyte hypertrophy prior to repair and reverse remodeling after surgical correction. Given the normal pulmonary hemodynamics in the author’s older cohort and the mechanistic explanation offered by previous work, the authors posit that the ET-1 levels seen in this sub-group could reflect sympathetic activation due to the abnormality in LV and systemic vascular physiology. Future studies will be needed to confirm this hypothesis. The variable activation pattern between neonatal and older patients supports a role for the different pulmonary artery mechanics between the two subgroups affecting the variable ET-1 levels seen. Further studies will be needed to clarify the role of pulmonary artery pressure and pulmonary blood flow on ET-1 concentration.
The authors would like to acknowledge the Children’s Hospital Colorado Core Laboratory and specifically Mary Harrington, MTASCP who performed the ET-1 ELISAs for all study samples. The authors would also like to acknowledge the University of Colorado Hospital Core Laboratory and specifically Karen Morgenthaler, MTASCP who performed the BNP ELISAs for all study samples.
Manuscript source: Unsolicited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D, D
Grade E (Poor): 0
P- Reviewer: Anan R, Altarabsheh SE, Bertini M, Ueda H S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890-1900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3680] [Cited by in RCA: 3720] [Article Influence: 161.7] [Reference Citation Analysis (1)] |
| 2. | Rocchini AP, Rosenthal A, Barger AC, Castaneda AR, Nadas AS. Pathogenesis of paradoxical hypertension after coarctation resection. Circulation. 1976;54:382-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 88] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Lim C, Kim WH, Kim SC, Rhyu JW, Baek MJ, Oh SS, Na CY, Kim CW. Aortic arch reconstruction using regional perfusion without circulatory arrest. Eur J Cardiothorac Surg. 2003;23:149-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Bocelli A, Favilli S, Pollini I, Bini RM, Ballo P, Chiappa E, Zuppiroli A. Prevalence and long-term predictors of left ventricular hypertrophy, late hypertension, and hypertensive response to exercise after successful aortic coarctation repair. Pediatr Cardiol. 2013;34:620-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Krieger EV, Clair M, Opotowsky AR, Landzberg MJ, Rhodes J, Powell AJ, Colan SD, Valente AM. Correlation of exercise response in repaired coarctation of the aorta to left ventricular mass and geometry. Am J Cardiol. 2013;111:406-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Klitsie LM, Roest AA, Kuipers IM, Van der Hulst AE, Hazekamp MG, Blom NA, Ten Harkel AD. Enhanced characterization of ventricular performance after coarctation repair in neonates and young children. Ann Thorac Surg. 2013;96:629-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Vignon-Zellweger N, Heiden S, Miyauchi T, Emoto N. Endothelin and endothelin receptors in the renal and cardiovascular systems. Life Sci. 2012;91:490-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 8. | Shimojo N, Jesmin S, Zaedi S, Otsuki T, Maeda S, Yamaguchi N, Aonuma K, Hattori Y, Miyauchi T. Contributory role of VEGF overexpression in endothelin-1-induced cardiomyocyte hypertrophy. Am J Physiol Heart Circ Physiol. 2007;293:H474-H481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Shimojo N, Jesmin S, Zaedi S, Maeda S, Soma M, Aonuma K, Yamaguchi I, Miyauchi T. Eicosapentaenoic acid prevents endothelin-1-induced cardiomyocyte hypertrophy in vitro through the suppression of TGF-beta 1 and phosphorylated JNK. Am J Physiol Heart Circ Physiol. 2006;291:H835-H845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Tsai SH, Lu G, Xu X, Ren Y, Hein TW, Kuo L. Enhanced endothelin-1/Rho-kinase signalling and coronary microvascular dysfunction in hypertensive myocardial hypertrophy. Cardiovasc Res. 2017;113:1329-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 11. | Tavli V, Saritas T, Guven B, Okur F, Saylan BC, Tavli T, Uyanik BS, Ari Z, Isbilen B. Myocardial performance after successful intervention for native aortic coarctation. Cardiol Young. 2010;20:33-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38562] [Cited by in RCA: 36912] [Article Influence: 2307.0] [Reference Citation Analysis (0)] |
| 13. | de Simone G, Daniels SR, Devereux RB, Meyer RA, Roman MJ, de Divitiis O, Alderman MH. Left ventricular mass and body size in normotensive children and adults: assessment of allometric relations and impact of overweight. J Am Coll Cardiol. 1992;20:1251-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1269] [Cited by in RCA: 1331] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 14. | Daniels SR, Kimball TR, Morrison JA, Khoury P, Meyer RA. Indexing left ventricular mass to account for differences in body size in children and adolescents without cardiovascular disease. Am J Cardiol. 1995;76:699-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 220] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 15. | Gorenflo M, Gross P, Bodey A, Schmitz L, Brockmeier K, Berger F, Bein G, Lange PE. Plasma endothelin-1 in patients with left-to-right shunt. Am Heart J. 1995;130:537-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Vincent JA, Ross RD, Kassab J, Hsu JM, Pinsky WW. Relation of elevated plasma endothelin in congenital heart disease to increased pulmonary blood flow. Am J Cardiol. 1993;71:1204-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 63] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Skiendzielewski J, Werner B. Importance of plasma endothelin-1 level in the evaluation of heart failure severity in infants with ventricular septal defect. Kardiol Pol. 2014;72:459-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 18. | Rosenberg AA, Kennaugh J, Koppenhafer SL, Loomis M, Chatfield BA, Abman SH. Elevated immunoreactive endothelin-1 levels in newborn infants with persistent pulmonary hypertension. J Pediatr. 1993;123:109-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 133] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Hiramatsu T, Imai Y, Takanashi Y, Seo K, Terada M, Aoki M, Nakazawa M. Time course of endothelin-1 and adrenomedullin after the Fontan procedure. Ann Thorac Surg. 1999;68:169-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Danová K, Pechán I, Olejárová I, Líska B. Natriuretic peptides and endothelin-1 in patients undergoing coronary artery bypass grafting. Gen Physiol Biophys. 2007;26:194-199. [PubMed] |
| 21. | Ergul A, Jupin D, Johnson MH, Prisant LM. Elevated endothelin-1 levels are associated with decreased arterial elasticity in hypertensive patients. J Clin Hypertens (Greenwich). 2006;8:549-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Strand AH, Gudmundsdottir H, Os I, Smith G, Westheim AS, Bjørnerheim R, Kjeldsen SE. Arterial plasma noradrenaline predicts left ventricular mass independently of blood pressure and body build in men who develop hypertension over 20 years. J Hypertens. 2006;24:905-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Cullingford TE, Markou T, Fuller SJ, Giraldo A, Pikkarainen S, Zoumpoulidou G, Alsafi A, Ekere C, Kemp TJ, Dennis JL. Temporal regulation of expression of immediate early and second phase transcripts by endothelin-1 in cardiomyocytes. Genome Biol. 2008;9:R32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Richey PA, Disessa TG, Somes GW, Alpert BS, Jones DP. Left ventricular geometry in children and adolescents with primary hypertension. Am J Hypertens. 2010;23:24-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 25. | Rassi AN, Pibarot P, Elmariah S. Left ventricular remodelling in aortic stenosis. Can J Cardiol. 2014;30:1004-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 26. | Marshall AK, Barrett OP, Cullingford TE, Shanmugasundram A, Sugden PH, Clerk A. ERK1/2 signaling dominates over RhoA signaling in regulating early changes in RNA expression induced by endothelin-1 in neonatal rat cardiomyocytes. PLoS One. 2010;5:e10027. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |