Published online Oct 26, 2017. doi: 10.4330/wjc.v9.i10.787
Peer-review started: October 31, 2016
First decision: January 14, 2017
Revised: May 12, 2017
Accepted: May 22, 2017
Article in press: May 23, 2017
Published online: October 26, 2017
Processing time: 214 Days and 10.5 Hours
To determine whether the need for additional tricuspid valve repair is an independent risk factor when surgery is required for a left-sided heart disease.
One hundred and eighty patients (68 ± 12 years, 79 males) underwent tricuspid annuoplasty. Cox proportional-hazards regression model for multivariate analysis was performed for variables found significant in univariate analyses.
Tricuspid regurgitation etiology was functional in 154 cases (86%), organic in 16 cases (9%), and mixed in 10 cases (6%), respectively. Postoperative mortality at 30 days was 11.7%. Mean follow-up was 51.7 mo with survival at 5 years of 73.5%. Risk factors for mortality were acute endocarditis [hazard ratio (HR) = 9.22 (95%CI: 2.87-29.62), P < 0.001], ischemic heart disease requiring myocardial revascularization [HR = 2.79 (1.26-6.20), P = 0.012], and aortic valve stenosis [HR = 2.6 (1.15-5.85), P = 0.021]. Significant predictive factors from univariate analyses were double-valve replacement combined with tricuspid annuloplasty [HR = 2.21 (1.11-4.39), P = 0.003] and preoperatively impaired ejection fraction [HR = 1.98 (1.04-3.92), P = 0.044]. However, successful mitral valve repair showed a protective effect [HR = 0.32 (0.10-0.98), P = 0.046]. Additionally, in instances where tricuspid regurgitation required the need for concomitant tricuspid valve repair, mortality predictor scores such as Euroscore 2 could be shortened to a simple Euroscore-tricuspid comprised of only 7 inputs. The explanation may lie in the fact that significant tricuspid regurgitation following left-sided heart disease represents an independent risk factor encompassing several other factors such as pulmonary arterial hypertension and dyspnea.
Tricuspid annuloplasty should be used more often as a concomitant procedure in the presence of relevant tricuspid regurgitation, although it usually reveals an overly delayed correction of a left-sided heart disease.
Core tip: Tricuspid valve repair with flexible ring is easy to achieve in patients undergoing heart surgery. Predictor scores such as Euroscore 2 could be shortened to a simple Euroscore-tricuspid of only 7 inputs. A significant tricuspid regurgitation following a left-sided heart disease is an independent risk factor that encompasses several other factors such as pulmonary arterial hypertension and dyspnea. Patients with functional damage of the right side of the heart and significant functional tricuspid regurgitation have poor mid-term results with high mortality. A concomitant tricuspid regurgitation usually reveals a delayed correction of a left-sided heart disease.
- Citation: Azarnoush K, Nadeemy AS, Pereira B, Leesar MA, Lambert C, Azhari A, Eljezi V, Dauphin N, Geoffroy E, Camilleri L. Clinical outcomes of tricuspid valve repair accompanying left-sided heart disease. World J Cardiol 2017; 9(10): 787-793
- URL: https://www.wjgnet.com/1949-8462/full/v9/i10/787.htm
- DOI: https://dx.doi.org/10.4330/wjc.v9.i10.787
The concomitant correction of functional tricuspid regurgitation secondary to left heart disease requiring surgery remains underused[1] despite recent data showing late development of severe tricuspid regurgitation in patients with mild regurgitation at the time of cardiac procedures (e.g., mitral valve surgery)[2]. Several factors including pulmonary arterial hypertension, right ventricular dilatation, increased tricuspid annulus diameter and the occurrence of right-sided heart failure promote persistent or even deteriorating functional tricuspid insufficiency[3,4]. Associated tricuspid regurgitation is present in almost 50% of patients undergoing mitral-valve surgery[5]. Most patients presenting with significant tricuspid regurgitation suffer from functional regurgitation due to dilatation of the tricuspid annulus, caused by dilatation of the right ventricle[6].
Rare organic tricuspid insufficiencies may be secondary to iatrogenic injury (i.e., pacing leads), or of rheumatic, infectious, congenital or carcinoid origin[7].
Data on concomitant tricuspid valve annuloplasty are rare and usually focus on different techniques for repair. A recent review seemingly demonstrated evidence for tricuspid annuloplasty to be a low-risk procedure[8]. However, as highlighted in the present work, a concomitant tricuspid regurgitation reveals a delayed correction of left-sided heart disease. Our data demonstrate that standard Euroscore 2 mortality risk factors such as gender, pulmonary hypertension, renal impairment or weight of the intervention should no longer be taken into account when significant tricuspid regurgitation appears prior to surgery of left-sided heart disease and the need for tricuspid repair becomes an independent mortality risk factor.
The present study aimed to confirm that the need of concomitant tricuspid annuloplasty according to guidelines represents a far too late treatment. Patients should be addressed to heart surgery centers for an early correction of the left-side heart disease before the need of additional tricuspid valve repair procedure.
All patients undergoing concomitant tricuspid valve annuloplasty between January 2005 and December 2009 were included in this retrospective, single-center study. The study was approved by the local ethics committee and all patients gave their written informed consent for the procedure as well as for inclusion in this retrospective study[9].
All surgeries were performed using full median sternotomy and extracorporeal circulation with cardiac arrest using blood cardioplegia. Tricuspid annuloplasty was performed either with a De Vega tricuspid repair[10], a flexible Sovering® ring (Sorin Biomedica Cardio S.r.I., Saluggia, Italy) sized 26 to 36 mm[11], or a fexible Bex® linear reducer (Gamida, France)[12], respectively. If necessary, annuloplasty was combined with concomitant procedures to the tricuspid valve such as resection of vegetations in case of endocarditis, implantation of artificial chords or tricuspid pillar reinsertion in case of prolapse.
The patients’ health status was obtained through a questionnaire submitted to the cardiologist, to the attending physician or, in the absence of the latter, by interviewing the patient or his/her relatives by phone (if the patient was deceased).
Data are presented as the mean ± SD for continuous data and as the number of patients and associated percentages for categorical parameters. Cox proportional-hazards regression model was performed to evaluate the impact of several covariates on mortality in a multivariate context and define prognostic factors (using a stepwise backward and forward algorithm, from variables with a P < 0.10 in univariate analyses) according to the results of univariate analysis and clinical relevance.
All analyses were conducted using Stata v12® (Stata Corp, College Station, United States). The tests were two-sided, with a type I error set at α = 0.05 (except for multiple comparisons).
Between January 2005 and December 2009, a total 180 consecutive patients underwent tricuspid valve annuloplasty in our institution. During the same period, another 3 patients underwent isolated tricuspid valve replacement and were not included in the present study. Among the 180 included patients, there were 79 males (44%) and 101 females (56%). Age ranged from 12 to 89 years; mean age was 68.3 ± 12.4 years (Table 1).
| n (%) | |
| Age (yr) | 68.3 ± 12.4 |
| Gender | |
| Female | 101 (56) |
| Male | 79 (44) |
| Dyspnea (New York Heart Association) | |
| Class I | 7 (4) |
| Class II | 25 (14) |
| Class III | 125 (69) |
| Class IV | 23 (13) |
| Cardiac rhythm | |
| Sinus rhythm | 77 (43) |
| Atrial fibrillation | 87 (48) |
| Branch block | 40 (22) |
| Pacemaker | 16 (9) |
| Risk factors | |
| Arterial hypertension | 79 (44) |
| Hypercholesterolemia | 77 (43) |
| Tobacco | 44 (24) |
| Diabetes | 42 (23) |
| Lower limb or supra-aortic obstructive arteriopathy | 20 (11) |
| Pulmonary disease | 25 (14) |
| Cerebrovascular accident or transient ischemic attack | 19 (11) |
| Rheumatic valve disease | 40 (22) |
| Myocardial infarction | 12 (7) |
| Pacemaker implantation | 16 (9) |
| Reoperation | 26 (14) |
| Other heart disease | |
| Aortic regurgitation | 19 (11) |
| Aortic stenosis | 30 (17) |
| Combined aortic stenosis/regurgitation | 15 (8) |
| Mitral regurgitation | 99 (55) |
| Mitral stenosis | 21 (12) |
| Combined mitral stenosis/regurgitation | 21 (12) |
| Pulmonary valve regurgitation | 1 (0.6) |
| Coronary artery disease | 28 (16) |
| Acute endocarditis | 9 (5) |
| Interventricular or interatrial septal defect | 3 (1.7) |
Tricuspid valve regurgitation etiology was classified as functional in 154 cases (86%), organic in 16 cases (9%) and mixed in 10 cases (6%). In instances of functional tricuspid regurgitation, the main cause was degenerative mitral valve disease. In instances of organic tricuspid regurgitation, the predominant pathologies were rheumatism disease and infectious endocarditis, 9 of which required urgent surgery for acute endocarditis (Table 1).
Ninety-seven patients (45%) suffered from at least one heart failure episode, 22 with left-sided HF, 15 with right-sided HF, and 60 with global heart failure, respectively. Eighty-five patients (47%) suffered from persistent atrial fibrillation preoperatively. Further cardiovascular risk factors of the study patients are summarized in Table 1, along with preoperative echocardiographic findings in Table 2.
| mean ± SD | n (%) | |
| Preoperative parameter | ||
| Left ventricular ejection fraction (%) | 58.6 ± 12.5 | |
| Systolic pulmonary arterial pressure (mmHg) | 58.0 ± 16.7 | |
| Tricuspid regurgitation | ||
| I | 9 (5) | |
| II | 69 (38) | |
| III | 70 (39) | |
| IV | 32 (18) | |
| Left ventricular end-diastolic diameter (mm) | 52.7 ± 9.5 (29-74) | |
| Left ventricular end-systolic diameter (mm) | 34.4 ± 9.3 (17-63) | |
| Postoperative parameter | ||
| Left ventricular ejection fraction (%) | 54.4 ± 12.2 (10-82) | |
| Systolic pulmonary arterial pressure (mmHg) | 38.6 ± 10.6 (19-76) | |
| Tricuspid regurgitation | ||
| 0-I | 150 (83) | |
| II | 28 (15) | |
| III | 1 (0.6) | |
| IV | 1 (0.6) | |
| Left ventricular end-diastolic diameter (mm) | 50.4 ± 7.4 | |
| Left ventricular end-systolic diameter (mm) | 35.2 ± 8.3 |
Tricuspid annuloplasty with a prosthetic ring was performed in 176 patients; a Sovering® ring was used in 156 cases and a Bex® linear reducer in 20 cases. In 20 cases, annuloplasty was combined with concomitant procedures for tricuspid valve: Valve repair (leaflet slit or cleft closure), vegetation resection, implantation of a Gore-Tex® cord, and one tricuspid pillar reinsertion for iatrogenic tricuspid incompetence as a consequence of pacemaker lead removal. Four 4 De Vega tricuspid repairs were performed while the remaining procedures consisted of the following: Aortic valve replacement in 29 cases (16%), mitral valve replacement in 67 cases (37%), double mitro-aortic valvular replacement in 42 cases (23%), mitral valve repair in 38 cases (21%), pulmonary valve replacement in one case (0.6%), coronary artery bypass grafting in 26 cases (14%), and other procedures in 21 cases. Only 9 patients (5%) underwent surgery for isolated tricuspid regurgitation. These patients presented with preoperative grade III or IV tricuspid incompetence. Three of these patients had a previous history of mitral or aortic valvular surgery, with tricuspid insufficiency appearing within two years postoperatively. Two of these patients had a preoperative pulmonary artery hypertension with peak gradients over 60 mmHg at their first operation. The other five patients did not present any associated left-sided heart disease.
Mean hospital stay was 17.8 ± 19.3 d (range 2 to 165 d). Postoperative complications were reoperation for bleeding in 15 cases (8%) and one postoperative stroke. A total of 21 patients (11.7%) died within 30 d. The main causes of death were multi-organ failure in 20 cases, two of whom were from massive bleeding, and one unexplained sudden death.
All patients underwent early postoperative echocardiography, demonstrating marked reduction in both tricuspid insufficiency and in systolic pulmonary artery pressure (Table 2).
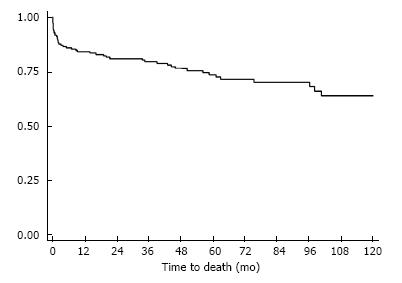
Among hospital survivors, two patients (1%) were lost at follow-up, the initial analysis thus resulting in a 99% follow-up. Data from these two patients were subsequently collected in 2014. One patient living in Kathmandu returned for a control cardiology visit in our university hospital and the second patient had a control consultation in the thoracic surgery department in Clermont-Ferrand, allowing us to complete the initial follow-up. Mean follow-up was 51.7 ± 39 mo with 5-year survival at 73.5% and 10-year survival at 63.8% (Figure 1). The main cause of death during the follow-up was heart failure. Only one tricuspid valve repair failed. Eight patients had a cerebrovascular event during the study period and seven patients presented with a late complete atrioventricular block requiring pacemaker implantation.
Univariate and multivariate analyses and parameters affecting global mortality (in-hospital and post-discharge) are detailed in Tables 3 and 4.
| Variable | n (%) | Univariate HR | P |
| Gender (male) | 79 (44) | 1.38 [0.77-2.44] | 0.27 |
| Age (≥ 75 yr) | 63 (35) | 1.75 [0.98-3.11] | 0.06 |
| Tobacco use | 44 (24) | 1.16 [0.60-2.24] | 0.66 |
| Pulmonary disease | 25 (14) | 1.58 [0.74-3.40] | 0.24 |
| Pacemaker | 16 (9) | 2.40 [1.12-5.13] | 0.02 |
| Branch block | 40 (22) | 1.88 [1.01-3.47] | 0.04 |
| Previous heart failure | 75 (42) | 1.67 [0.94-2.95] | 0.08 |
| Ejection fraction (> 50%) | 132 (73) | 0.49 [0.25-0.98] | 0.044 |
| Hypertension | 79 (44) | 1.81 [1.01-3.20] | 0.04 |
| Diabetes on insulin | 8 (4) | 2.33 [0.83-6.51] | 0.11 |
| Aortic regurgitation | 19 (11) | 0.52 [0.16-1.67] | 0.27 |
| Aortic disease | 15 (8) | 1.29 [0.51-3.26] | 0.59 |
| Mitral regurgitation | 99 (55) | 1.01 [0.56-1.79] | 0.98 |
| Mitral stenosis | 21 (12) | 0.96 [0.38-2.44] | 0.93 |
| Mitral disease | 21 (12) | 0.55 [0.19-1.54] | 0.26 |
| Dyslipidemia | 77 (43) | 0.88 [0.49-1.57] | 0.67 |
| NIDD | 34 (19) | 1.31 [0.64-2.64] | 0.45 |
| Cerebrovascular accident | 19 (11) | 1.44 [0.60-3.40] | 0.41 |
| Myocardial infarction | 12 (7) | 1.70 [0.67-4.30] | 0.26 |
| New York Heart Association (III/IV) | 148 (82) | 1.99 [0.78-5.04] | 0.14 |
| Redo vs Tridux | 26 (14) | 1.49 [0.74-3.00] | 0.26 |
| Double valve replacement associated with tricuspid repair | 43 (24) | 2.21 [1.11-4.39] | 0.024 |
| Sinus rhythm | 77 (43) | 0.74 [0.41-1.35] | 0.33 |
| Sovering ring | 156 (87) | 1.10 [0.49-2.46] | 0.81 |
| Bex device | 20 (11) | 1.16 [0.52-2.60] | 0.71 |
| Systolic pulmonary artery pressure (> 59 mmHg) | 98 (54) | 1.06 [0.60-1.88] | 0.83 |
| Systolic pulmonary artery pressure (> 49 mmHg) | 31 (17) | 1.16 [0.56-2.40] | 0.69 |
| Tricuspid annulus diameter (> 40 mm) | 20 (11) | 0.31 [0.07-1.27] | 0.10 |
| Postoperative ejection fraction (> 50%) | 98 (66) | 2.58 [1.31-5.08] | 0.006 |
| Variable | n (%) | Univariate | Multivariate | ||
| HR | P | HR | P | ||
| Aortic stenosis | 30 (17) | 2.69 [1.24-5.42] | 0.011 | 2.60 [1.15-5.85] | 0.021 |
| Coronary disease | 28 (16) | 4.12 [2.06-8.21] | < 0.001 | 2.79 [1.26-6.20] | 0.012 |
| Mitral-valve repair | 38 (21) | 0.27 [0.08-0.88] | 0.03 | 0.32 [0.10-0.98] | 0.046 |
| Infective endocarditis | 9 (5) | 5.06 [1.7-14.62] | 0.003 | 9.22 [2.87-29.62] | < 0.001 |
Of note, there was no significant correlation between death and several Euroscore factors such as gender, pulmonary hypertension, NYHA dyspnea level, chronic lung disease, renal impairment or weight of the intervention (Table 3). From multivariate analyses (Table 4), the adverse factors for mortality were the presence of acute endocarditis, ischemic heart disease that required myocardial revascularization, and aortic valve stenosis, respectively. In contrast, a successful mitral valve repair appeared to have a protective effect.
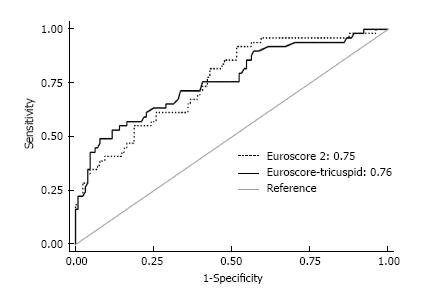
When taking into account a tricuspid valve regurgitation requiring an additional tricuspid valve repair accompanying a left-sided heart disease surgery, the 18 predictive risk factors of Euroscore 2 could be reduced to a Euroscore-tricuspid of only 7 factors with an at least equivalent statistical power (Figure 2). This new Euroscore-tricuspid would require only the following patient data: Age, ischemic heart disease, insulin-treated diabetes, previous cardiac surgery (redo intervention), active endocarditis, critical preoperative state and left ventricle function less than 50%.
Both the current American and European guidelines recommend correction of relevant functional tricuspid insufficiency if other cardiac diseases are corrected surgically[4,7] since functional tricuspid regurgitation, a frequent finding in patients undergoing cardiac surgery for other reasons[5], has proven to increase over time when not corrected during first surgery[2], mainly due to progressive annular dilatation[3]. However, although factors influencing the natural course of tricuspid regurgitation over time[4] and even during long-term follow-up of over 5 years[13] as well as its deleterious effect on mortality[14] are well known, its concomitant correction has yet to be performed to an adequate extent[1]. In the present study, the conducting of a successful mitral valve repair was found to be a protective factor when tricuspid annuloplasty was performed in patients with significant mitral regurgitation.
Acute endocarditis, associated ischemic heart disease and double valve replacement combined with tricuspid regurgitation were the main risk factors for hospital mortality in this study. Surprisingly, there was no correlation between elevated pulmonary arterial pressure, advanced age, preexisting arrhythmias and mid-term mortality results as conversely reported by others[15].
The 11.7% hospital mortality rate observed herein, mainly driven by multi-organ failure, is a reflection of the high rate of concomitant procedures. Other studies have reported hospital mortality rates of up to 35% in patients undergoing tricuspid valve repair as a concomitant procedure to other cardiac surgery[16,17].
The use of a flexible ring represented the technique of choice in the present series. Easy implantation, avoidance of a suture close to the conduction system, measured reduction of the tricuspid annulus and preservation of the valve’s normal physiological shape are among the related advantages of this approach[18]. For dilatation of the tricuspid annulus, annuloplasty alone provides excellent results in the absence of valvular or subvalvular disease[3]; however, it is no longer effective in correcting tricuspid regurgitation if there is also damage of the leaflets and of the subvalvular apparatus, and/or in instances where additional procedures are required[18,19]. Accordingly, less-than-moderate tricuspid regurgitation prior to discharge after tricuspid annuloplasty during redo valve surgery additionally proved to be an independent risk factor for better long-term outcome in terms of survival in a recent retrospective analysis[20]. Furthermore, concomitant tricuspid annuloplasty using flexible bands offered improved durability as compared to suture annuloplasty for preventing postoperative tricuspid regurgitation progression in two retrospective comparative analyses[21,22]. In the current series, 20 patients underwent a concomitant valvular or subvalvular procedure, without any added mortality or morbidity.
Recent clinical studies have demonstrated that moderate to severe residual tricuspid reurgitation still persists in 10% of patients who have undergone surgical repair[18]. Tricuspid regurgitation is related to the degree of limited leaflet motion and to the severity of the dilatation of the tricuspid annulus. The severity of preoperative tricuspid reurgitation, together with right ventricular dysfunction, contributes to postoperative residual insufficiency. Risk factors for recurrent tricuspid reurgitation are preoperative severe regurgitation, tricuspid repair without a prosthetic ring or with an oversized ring (large tricuspid valve), pacemaker catheters that pass through the tricuspid valve, mitral valve replacement rather than mitral repair, left ventricular dysfunction associated or not with advanced remodelling, cardiomegaly and atrial fibrillation[19].
Finally and surprisingly, we found that mortality predictor scores such as Euroscore 2 could be shortened to a simple Euroscore-tricuspid of only 7 inputs. From our standpoint, the explanation may reside in the fact that significant tricuspid regurgitation following a left-sided heart disease is an independent risk factor encompassing several other factors such as pulmonary arterial hypertension and dyspnea. Such finding has been reported in several studies of other diseases with regard to aortic and mitral valve diseases which also corroborate the present data embodying multiple diseases at once[23-25].
The present study demonstrates the efficacy and durability of tricuspid annuloplasty with an open flexible ring. This procedure may be performed in patients with severe left-sided valve disease. Patients with functional damage of the right side of the heart combined with significant functional tricuspid regurgitation have poor mid-term results along with high mortality. A concomitant tricuspid regurgitation typically reveals a delayed correction of left-sided heart disease.
The concomitant correction of functional tricuspid regurgitation secondary to left heart disease requiring surgery remains underused and an associated functional tricuspid regurgitation typically reveals a delayed correction of left-sided heart disease.
Functional tricuspid valve regurgitation concerns patients who are referred to heart surgery for left-sided heart disease too late. Results of this study contribute to clarify these patients’ clinical situation.
In this study, when a tricuspid regurgitation required the need for concomitant tricuspid valve repair, mortality predictor scores such as Euroscore 2 could be shortened to a simple Euroscore-tricuspid comprised of only 7 inputs. The explanation may lie in the fact that significant tricuspid regurgitation following left-sided heart disease represents an independent risk factor encompassing several other factors such as pulmonary arterial hypertension and dyspnea.
The present study demonstrates the efficacy and durability of tricuspid annuloplasty with an open flexible ring.
The study aimed the need of concomitant tricuspid annuloplasty for an early correction of the left-side heart disease. The author conducted retrospective multivariate analysis for significant variables in univariate analyses in 180 cases with tricuspid annuoplasty. The 5-10 years follow-up observation find out the risk factors for mortality were acute endocarditis, ischemic heart disease requiring myocardial revascularization, and aortic valve stenosis. Significant predictive factors from univariate analyses were double-vlave replacement combined with tricuspid annuloplasty and preoperatively impaired erection fraction. The author concluded that tricuspid annuloplasty should be used more often as concomitant procedure if relevant tricuspid regurgitation is present. The study suggests that the predictor scores could be shorten to a simple Euroscore-tricuspid of only 7 imputs. Functional tricuspid regurgitation may be frequently found in patients undergoing cardiac surgery from other reasons. It will become more severe over time if not corrected during first surgery. It is significant to have a investigation on the outcomes of tricuspid valve repair accompanying left-sided hart disease surgery. This manuscript retrospectively investigated this topic, discussed the advantages of the correction surgery at the same time, analyzed the risk factors and concluded to simplify using the predictive factors.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: France
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Ong HT, Zhang XQ S- Editor: Song XX L- Editor: A E- Editor: Lu YJ
| 1. | Rogers JH, Bolling SF. Valve repair for functional tricuspid valve regurgitation: anatomical and surgical considerations. Semin Thorac Cardiovasc Surg. 2010;22:84-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Chikwe J, Anyanwu AC. Surgical strategies for functional tricuspid regurgitation. Semin Thorac Cardiovasc Surg. 2010;22:90-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Dreyfus GD, Corbi PJ, Chan KM, Bahrami T. Secondary tricuspid regurgitation or dilatation: which should be the criteria for surgical repair? Ann Thorac Surg. 2005;79:127-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 580] [Cited by in RCA: 564] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 4. | Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Barón-Esquivias G, Baumgartner H, Borger MA, Carrel TP, De Bonis M, Evangelista A, Falk V, Lung B, Lancellotti P, Pierard L, Price S, Schäfers HJ, Schuler G, Stepinska J, Swedberg K, Takkenberg J, Von Oppell UO, Windecker S, Zamorano JL, Zembala M; ESC Committee for Practice Guidelines (CPG); Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC); European Association for Cardio-Thoracic Surgery (EACTS). Guidelines on the management of valvular heart disease (version 2012): the Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur J Cardiothorac Surg. 2012;42:S1-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 925] [Cited by in RCA: 1024] [Article Influence: 78.8] [Reference Citation Analysis (0)] |
| 5. | Freed LA, Levy D, Levine RA, Larson MG, Evans JC, Fuller DL, Lehman B, Benjamin EJ. Prevalence and clinical outcome of mitral-valve prolapse. N Engl J Med. 1999;341:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 733] [Cited by in RCA: 736] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 6. | Sagie A, Schwammenthal E, Padial LR, Vazquez de Prada JA, Weyman AE, Levine RA. Determinants of functional tricuspid regurgitation in incomplete tricuspid valve closure: Doppler color flow study of 109 patients. J Am Coll Cardiol. 1994;24:446-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 139] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Guyton RA, O’Gara PT, Ruiz CE, Skubas NJ, Sorajja P. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Thorac Cardiovasc Surg. 2014;148:e1-e132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 709] [Cited by in RCA: 712] [Article Influence: 64.7] [Reference Citation Analysis (0)] |
| 8. | Zhu TY, Wang JG, Meng X. Does concomitant tricuspid annuloplasty increase perioperative mortality and morbidity when correcting left-sided valve disease? Interact Cardiovasc Thorac Surg. 2015;20:114-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Claudot F, Alla F, Fresson J, Calvez T, Coudane H, Bonaïti-Pellié C. Ethics and observational studies in medical research: various rules in a common framework. Int J Epidemiol. 2009;38:1104-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 10. | De Vega NG, De Rábago G, Castillón L, Moreno T, Azpitarte J. A new tricuspid repair. Short-term clinical results in 23 cases. J Cardiovasc Surg (Torino). 1973;Spec No:384-386. [PubMed] |
| 11. | Della Barbera M, Laborde F, Thiene G, Arata V, Pettenazzo E, Pasquino E, Behr L, Valente M. Sovering annuloplasty rings: experimental pathology in the sheep model. Cardiovasc Pathol. 2005;14:96-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Camilleri LF, Miguel B, Bailly P, Legault BJ, D’Agrosa-Boiteux MC, Polvani GL, de Riberolles CM. Flexible posterior mitral annuloplasty: five-year clinical and Doppler echocardiographic results. Ann Thorac Surg. 1998;66:1692-1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Song H, Kim MJ, Chung CH, Choo SJ, Song MG, Song JM, Kang DH, Lee JW, Song JK. Factors associated with development of late significant tricuspid regurgitation after successful left-sided valve surgery. Heart. 2009;95:931-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 131] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 14. | Dreyfus GD, Chan KM. Functional tricuspid regurgitation: a more complex entity than it appears. Heart. 2009;95:868-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Kay GL, Morita S, Mendez M, Zubiate P, Kay JH. Tricuspid regurgitation associated with mitral valve disease: repair and replacement. Ann Thorac Surg. 1989;48:S93-S95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Bernal JM, Morales D, Revuelta C, Llorca J, Gutiérrez-Morlote J, Revuelta JM. Reoperations after tricuspid valve repair. J Thorac Cardiovasc Surg. 2005;130:498-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 125] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 17. | Scully HE, Armstrong CS. Tricuspid valve replacement. Fifteen years of experience with mechanical prostheses and bioprostheses. J Thorac Cardiovasc Surg. 1995;109:1035-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 80] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | McCarthy PM, Bhudia SK, Rajeswaran J, Hoercher KJ, Lytle BW, Cosgrove DM, Blackstone EH. Tricuspid valve repair: durability and risk factors for failure. J Thorac Cardiovasc Surg. 2004;127:674-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 502] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 19. | Navia JL, Nowicki ER, Blackstone EH, Brozzi NA, Nento DE, Atik FA, Rajeswaran J, Gillinov AM, Svensson LG, Lytle BW. Surgical management of secondary tricuspid valve regurgitation: annulus, commissure, or leaflet procedure? J Thorac Cardiovasc Surg. 2010;139:1473-1482.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 195] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 20. | Fukunaga N, Okada Y, Konishi Y, Murashita T, Koyama T. Persistent tricuspid regurgitation after tricuspid annuloplasty during redo valve surgery affects late survival and valve-related events. Circ J. 2014;78:2696-2703. [PubMed] |
| 21. | Murashita T, Okada Y, Kanemitsu H, Fukunaga N, Konishi Y, Nakamura K, Koyama T. Long-term outcomes of tricuspid annuloplasty for functional tricuspid regurgitation associated with degenerative mitral regurgitation: suture annuloplasty versus ring annuloplasty using a flexible band. Ann Thorac Cardiovasc Surg. 2014;20:1026-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Huang X, Gu C, Men X, Zhang J, You B, Zhang H, Wei H, Li J. Repair of functional tricuspid regurgitation: comparison between suture annuloplasty and rings annuloplasty. Ann Thorac Surg. 2014;97:1286-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Dahou A, Magne J, Clavel MA, Capoulade R, Bartko PE, Bergler-Klein J, Sénéchal M, Mundigler G, Burwash I, Ribeiro HB. Tricuspid Regurgitation Is Associated With Increased Risk of Mortality in Patients With Low-Flow Low-Gradient Aortic Stenosis and Reduced Ejection Fraction: Results of the Multicenter TOPAS Study (True or Pseudo-Severe Aortic Stenosis). JACC Cardiovasc Interv. 2015;8:588-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 24. | Barbanti M, Binder RK, Dvir D, Tan J, Freeman M, Thompson CR, Cheung A, Wood DA, Leipsic J, Webb JG. Prevalence and impact of preoperative moderate/severe tricuspid regurgitation on patients undergoing transcatheter aortic valve replacement. Catheter Cardiovasc Interv. 2015;85:677-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 25. | Briongos Figuero S, Moya Mur JL, García-Lledó A, Centella T, Salido L, Aceña Navarro Á, García Martín A, García-Andrade I, Oliva E, Zamorano JL. Predictors of persistent pulmonary hypertension after mitral valve replacement. Heart Vessels. 2016;31:1091-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |