Peer-review started: August 5, 2016
First decision: September 2, 2016
Revised: September 15, 2016
Accepted: November 16, 2016
Article in press: November 17, 2016
Published online: January 26, 2017
Processing time: 167 Days and 17.6 Hours
In the current case series we describe two cases of longitudinal stent deformation in ostial lesions treated with a new generation zotarolimus eluting stent and review current literature on longitudinal stent deformation. Historically not a common occurrence, longitudinal deformation occurred mainly in Promus Element everolimus eluting stents, which had only two rather than the commonly used 3 links between stent rings. Longitudinal deformation commonly occurs secondary to compression of the proximal edge of the stent by either the guide catheters, or intravascular balloons and imaging catheters. The degree of deformation however, depends on the longitudinal strength and design of the stent.
Core tip: In the current case series we describe two cases of longitudinal stent deformation in ostial lesions treated with a new generation zotarolimus eluting stent and review current literature on longitudinal stent deformation.
- Citation: Panoulas VF, Demir OM, Ruparelia N, Malik I. Longitudinal deformation of a third generation zotarolimus eluting stent: “The concertina returns!”. World J Cardiol 2017; 9(1): 60-64
- URL: https://www.wjgnet.com/1949-8462/full/v9/i1/60.htm
- DOI: https://dx.doi.org/10.4330/wjc.v9.i1.60
Over the last decade there has been remarkable progress in coronary stent design and materials. Early generation stents were stainless steel followed by the use of cobalt chromium and platinum chromium alloys, enabling stents to be thinner and more flexible, hence improving deliverability and conformability. In addition, thin stent struts have been associated with improved outcomes in drug eluting stents[1-3]. With the reduction in strut thickness, innovative designs have enabled maintenance of radial strength, however, longitudinal stent strength may have been compromised.
A recently observed complication includes longitudinal stent deformation, defined as the distortion or shortening of a stent in the longitudinal axis following successful stent deployment[4]. Longitudinal stent strength is dependent on the architectural composition - number, orientation, shape, thickness and material of the crest (or ring) and links. For example, the reduction in number of links between cells may enhance deliverability (by allowing more lateral bending) but as a consequence may compromise longitudinal strength. Longitudinal deformation can result in protrusion of struts into lumen. Extensive malapposition of struts may result in disruption of blood flow and increased risk of stent thrombosis[5,6]. In addition, longitudinal deformation of drug eluting stents may result in uneven drug delivery that can result in higher rates of instent restenosis[7].
In the current case series and review we describe two cases of longitudinal deformation with the new Resolute Onyx (Medtronic Inc., United States) zotarolimus eluting stents (ZES) and review the literature on longitudinal deformation. This new generation ZES has a novel design, manufactured from a single strand of core wire (platinum iridium) shaped into a continuous sinusoidal waveform. The strut thickness is 81 μm, rendering it extremely trackable and conformable. Every 4th crown is laser fused to provide uniform longitudinal strength across the length of the stent.
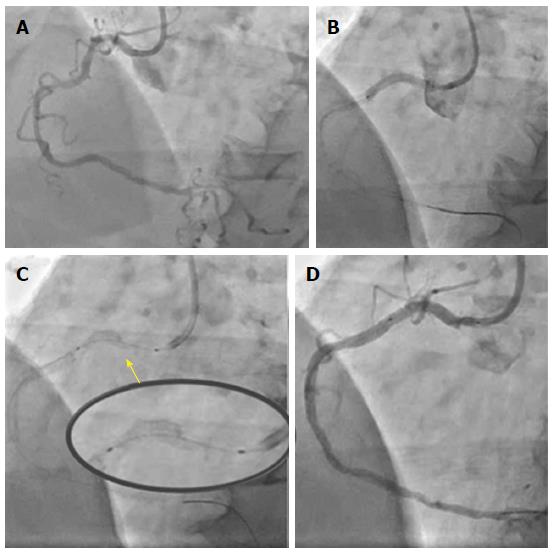
A 78-year-old male with diabetes mellitus, hypertension, hypercholesterolemia, and chronic renal failure presented with stable angina. Elective coronary angiography demonstrated significant lesions in the ostial and mid segments of a tortuous right coronary artery (RCA) (Figure 1A). The Judkins Right 4 catheter did not provide sufficient support therefore a 3 dimensional (3D) right coronary (Williams) guide catheter was used to intubate the RCA. This provided improved, yet suboptimal support, therefore a buddy wire was used to facilitate the implantation of a ZES (Resolute Onyx 2.75 mm × 38 mm, Medtronic) in the mid segment. Subsequently, the ostial-proximal lesion was pre-dilated, stented with a Resolute Onyx 3.0 mm × 22 mm ZES and post-dilated with a 3.0 mm non-compliant (NC) balloon (Figure 1B). Following stent deployment and removal of the buddy wire, significant longitudinal deformation was noted (Figure 1C), which was treated with non-compliant balloon dilatation and third ZES insertion (Resolute Onyx 3.0 mm × 18 mm) all the way to the ostium (Figure 1D).
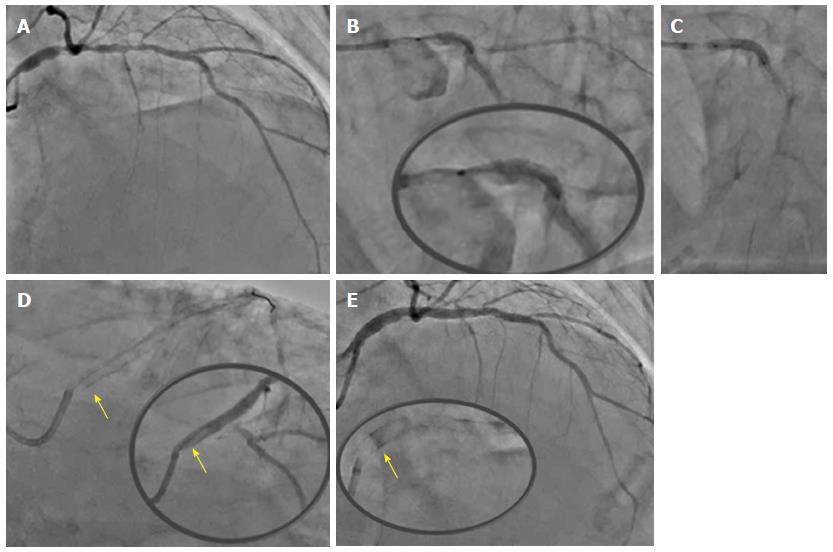
A 85-year-old male with end stage renal failure and severely impaired left ventricular systolic function presented with rapidly conducted atrial fibrillation and raised troponin (6000, upper normal limits 30) with inferolateral ST depression on electrocardiogram. On coronary angiography, he had severe three-vessel and left main disease. After being turned down for surgery, his proximal tight RCA lesion was stented with a Resolute Onyx 4.5 mm × 12 mm and post-dilated with a 5 mm non-compliant balloon. In view of the heavily calcified ostial/distal left main stem (LMS) and proximal left anterior descending (LAD) disease (Figure 2A) and bursting non-compliant balloons, decision was made to rotablate the lesions first with 1.5 mm burr. Subsequently a 3.0 mm non-compliant balloon was used to pre-dilate all lesions successfully. The proximal LAD was stented with a Resolute Onyx 3.0 mm × 26 mm stent. The whole length of the LMS into the proximal LAD was stented with a 3.5 mm × 34 mm Resolute Onyx covering the LMS ostium (Figure 2B and C). The LMS segment of the stent was post-dilated with a 4.5 mm NC balloon. Immediately after and despite taking care in removing the trapped LCx wire, there was longitudinal deformation of the stent, which no longer covered the LMS ostium (Figure 2D). After ballooning the deformed stent with a 4.5 mm non-compliant balloon the ostium was covered with another 4.0 mm × 8 mm stent and post-dilated with 4.5 mm NC balloon with an excellent final result (Figure 2E).
To our knowledge this case series demonstrates the first reported cases of longitudinal deformation in patients treated with the new Resolute Onyx stent platform.
Traditionally, longitudinal strength was not considered standard characteristics for stent performance. However, recent evidence has highlighted possible complications since longitudinal deformation was first reported by Hanratty et al[8], describing 3 cases where longitudinal compression of a previously deployed stent resulted in stent deformation. Two were identified angiographically and one with the aid of intravascular imagining. It was first documented with the Promus Element (Boston Scientific) stent which was related to guide catheter compression of stents deployed in an ostial location[8]. However, Hanratty et al[8] have observed this phenomenon in other drug eluting stents. A retrospective analysis of 4455 interventional cases over a four-year period showed stent deformation occurred in 0.2% of patients affecting 0.097% of stents deployed[7]. In 6 cases, Promus Element was involved, and there was 1 case each involving Endeavor (Medtronic), Biomatrix (Biosensors Interventional Technologies), and TAXUS Liberté (Boston Scientific) stents. The rate of stent deformation varied from 0% in several other stent types to 0.86% in the case of the Promus Element stent. In the same series, there was one case of late stent thrombosis attributable to longitudinal stent deformation[7]. In the DUTCH-PEERS study 906 patients were assigned to receive third generation zotarolimus-eluting stents (Resolute Integrity, Medtronic) and 905 to receive everolimus-eluting stents (Promus Element, Boston Scientific)[9]. Longitudinal stent deformation was seen only in the everolimus-eluting stent group [nine (1.0%) of 905 vs 0 of 906, P = 0.002; nine of 1591 (0.6%) everolimus-eluting stents implanted became deformed], but was not associated with any adverse events.
Despite drug eluting stents having improved remarkably the safety and efficacy of revascularization procedures, stent design is a continuously developing field that aims to balance numerous performance attributes such as stent flexibility, shortening on expansion, trackability, scaffolding, radiopacity, longitudinal strength, radial strength and recoil. An experimental evaluation of longitudinal strength of four commercially available stent design families demonstrated that a 50 g force resulted in longitudinal compression of 1.25-5.30 mm (4.46%-18.93%, compared with the nominal expanded stent length). The Promus Element stent platform had an average longitudinal compression of 13.20 mm (47.07%), demonstrating marked lower resistance to longitudinal compression (Table 1)[10]. Newer stent platforms with ultrathin struts (down to 60 μm) have shown non-inferiority to established everolimus platforms[11] but their longitudinal strength has yet to be assessed on the bench.
| Stents | Xience V | Xience PRIME/ Xience Xpedition | Promus Element | Promus Premier | SYNERGY | Resolute Onyx |
| Stent platform | Vision: CoCr | Multilink-9: CoCr | PtCr | PtCr | PtCr | PtIr core Co alloy outer |
| Strut thickness | 81 μm | 81 μm | 81 μm | 81 μm | 74 μm | 81 μm (up to 4.0 mm) 91 μm (4.5 and 5.0 mm) |
| Connectors | 3 links | 3 links | 2 links | 2 links (4 between the 3 proximal hoops) | 2 links | Every 4th crown laser fused (in the 2.75, 3.0 mm platforms every 5th crown fused) |
| Drug eluting | Everolimus | Everolimus | Everolimus | Everolimus | Everolimus | Zotarolimus |
| Polymer | Primer layer PBMA Drug matrix layer A semicrystalline random copolymer: PvDF-HFP | Primer layer PBMA Drug matrix layer A semicrystalline random copolymer: PvDF-HFP | Primer layer PBMA Drug matrix layer A semicrystalline random copolymer: PvDF-HFP | Primer layer PBMA Drug matrix layer A semicrystalline random copolymer: PvDF-HFP | Bioabsorbable PLGA | Biocompatible BioLinx polymer |
| Manufacturer | Abbott vascular, Santa Clara, CA, United States | Abbott vascular, Santa Clara, CA, United States | Boston Scientific, Natick, MA, United States | Boston Scientific, Natick, MA, United States | Boston Scientific, Natick, MA, United States | Medtronic CardioVascular Ltd, MN, United States |
In our series both cases of longitudinal deformation occurred in the hands of very experienced operators implanting the scaffold at an ostial location. Potential reasons behind the deformation could include guide catheter compression of the proximal edge of the ostial stent post removal of jailed wires (case 1 or 2) or aggressive post-dilatation of the stent with a significantly larger NC balloon causing longitudinal shortening (case 2). It is unclear whether laser fusion provides less support compared to traditional links and further evidence is required prior to drawing any conclusions.
In conclusion, longitudinal stent deformation can occur even with new generation ZES and identification is important as, if left untreated, it may associate with a risk of future stent thrombosis, restenosis, and challenges in rewiring and retreating these lesions in the future.
The authors presented two cases, one with stable angina and one presenting with a non ST elevation myocardial infarction, requiring treatment of ostial lesions with new generation drug eluting stents.
Both cases were treated with the third generation zotarolimus eluting stent Resolut Onyx, and in both cases longitudinal deformation of the stents was observed. This was managed with further ballooning and stenting.
Longitudinal stent deformation can occur even with new generation ZES and identification is important.
This manuscript reports two cases of longitudinal deformation of a 22-mm and 34-mm third generation zotarolimus eluting stent. The issue brought up by the authors is interesting and the cases well documented.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: De Ponti R, Lee TM, Tang JM S- Editor: Song XX L- Editor: A E- Editor: Wu HL
| 1. | Moreno R, Jimenez-Valero S, Sanchez-Recalde A, Galeote G, Calvo L, Martin-Reyes R, Sabate M, Plaza I, Macaya C, Lopez-Sendon JL. Periprocedural (30-day) risk of myocardial infarction after drug-eluting coronary stent implantation: a meta-analysis comparing cobalt-chromium and stainless steel drug-eluting coronary stents. EuroIntervention. 2011;6:1003-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Sen H, Lam MK, Löwik MM, Danse PW, Jessurun GA, van Houwelingen KG, Anthonio RL, Tjon Joe Gin RM, Hautvast RW, Louwerenburg JH. Clinical Events and Patient-Reported Chest Pain in All-Comers Treated With Resolute Integrity and Promus Element Stents: 2-Year Follow-Up of the DUTCH PEERS (DUrable Polymer-Based STent CHallenge of Promus ElemEnt Versus ReSolute Integrity) Randomized Trial (TWENTE II). JACC Cardiovasc Interv. 2015;8:889-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 3. | Maeng M, Tilsted HH, Jensen LO, Krusell LR, Kaltoft A, Kelbæk H, Villadsen AB, Ravkilde J, Hansen KN, Christiansen EH. Differential clinical outcomes after 1 year versus 5 years in a randomised comparison of zotarolimus-eluting and sirolimus-eluting coronary stents (the SORT OUT III study): a multicentre, open-label, randomised superiority trial. Lancet. 2014;383:2047-2056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 4. | Kwok OH. Stent “concertina: “ stent design does matter. J Invasive Cardiol. 2013;25:E114-E119. [PubMed] |
| 5. | Ormiston JA, Webber B, Ubod B, White J, Webster MW. Stent longitudinal strength assessed using point compression: insights from a second-generation, clinically related bench test. Circ Cardiovasc Interv. 2014;7:62-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Ormiston JA, Webber B, Webster MW. Stent longitudinal integrity bench insights into a clinical problem. JACC Cardiovasc Interv. 2011;4:1310-1317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 142] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 7. | Williams PD, Mamas MA, Morgan KP, El-Omar M, Clarke B, Bainbridge A, Fath-Ordoubadi F, Fraser DG. Longitudinal stent deformation: a retrospective analysis of frequency and mechanisms. EuroIntervention. 2012;8:267-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 8. | Hanratty CG, Walsh SJ. Longitudinal compression: a “new” complication with modern coronary stent platforms--time to think beyond deliverability? EuroIntervention. 2011;7:872-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 9. | von Birgelen C, Sen H, Lam MK, Danse PW, Jessurun GA, Hautvast RW, van Houwelingen GK, Schramm AR, Gin RM, Louwerenburg JW. Third-generation zotarolimus-eluting and everolimus-eluting stents in all-comer patients requiring a percutaneous coronary intervention (DUTCH PEERS): a randomised, single-blind, multicentre, non-inferiority trial. Lancet. 2014;383:413-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 155] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 10. | Prabhu S, Schikorr T, Mahmoud T, Jacobs J, Potgieter A, Simonton C. Engineering assessment of the longitudinal compression behaviour of contemporary coronary stents. EuroIntervention. 2012;8:275-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Pilgrim T, Heg D, Roffi M, Tüller D, Muller O, Vuilliomenet A, Cook S, Weilenmann D, Kaiser C, Jamshidi P. Ultrathin strut biodegradable polymer sirolimus-eluting stent versus durable polymer everolimus-eluting stent for percutaneous coronary revascularisation (BIOSCIENCE): a randomised, single-blind, non-inferiority trial. Lancet. 2014;384:2111-2122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 225] [Article Influence: 20.5] [Reference Citation Analysis (0)] |