Peer-review started: July 14, 2016
First decision: September 12, 2016
Revised: September 17, 2016
Accepted: November 16, 2016
Article in press: November 17, 2016
Published online: January 26, 2017
Processing time: 195 Days and 6.4 Hours
Many clinicians caring for patients with continuous flow left ventricular assist devices (CF-LVAD) use ramp right heart catheterization (RHC) studies to optimize pump speed and also to troubleshoot CF-LVAD malfunction. An investigational device, the ReliantHeart Heart Assist 5 (Houston, TX), provides the added benefit of an ultrasonic flow probe on the outflow graft that directly measures flow through the CF-LVAD. We performed a simultaneous ramp RHC and echocardiogram on a patient who received the above CF-LVAD to optimize pump parameters and investigate elevated flow through the CF-LVAD as measured by the flow probe. We found that the patient’s hemodynamics were optimized at their baseline pump speed, and that the measured cardiac output via the Fick principle was lower than that measured by the flow probe. Right heart catheterization may be useful to investigate discrepancies between flow measured by a CF-LVAD and a patient’s clinical presentation, particularly in investigational devices where little clinical experience exists. More data is needed to elucidate the correlation between the flow measured by an ultrasonic probe and cardiac output as measured by RHC.
Core tip: Commercially available left ventricular assist devices estimate flow through the device, but a new investigational device with an ultrasonic flow probe directly measures flow. Despite a reported accuracy in the flow probe’s measurement of flow, we found that this value was inaccurate in a patient whose flows were discrepant to the patient’s clinical status by performing echocardiography and right heart catheterization. Care should be taken to verify technical advances in mechanical circulatory support, and both imaging and hemodynamic evaluations can help clinicians make more informed decisions.
- Citation: Banerjee D, Dutt D, Duclos S, Sallam K, Wheeler M, Ha R. Simultaneous ramp right heart catheterization and echocardiography in a ReliantHeart left ventricular assist device. World J Cardiol 2017; 9(1): 55-59
- URL: https://www.wjgnet.com/1949-8462/full/v9/i1/55.htm
- DOI: https://dx.doi.org/10.4330/wjc.v9.i1.55
To optimize the function of continuous left ventricular assist devices (CF-LVAD) after implantation, we and others[1] routinely use ramp right heart catheterization (RHC) protocols. These studies may provide added data beyond ramp echocardiogram protocols[2], since a ramp RHC can simultaneously measure right and left sided filling pressures (central venous pressure and pulmonary capillary wedge pressure, respectively). While use of echocardiography has proven useful for optimization of HeartMate II (Pleasanton, CA) CF-LVAD pump speed, this has not proven helpful for other CF-LVADs[3]. As new CF-LVADs are developed and implanted, ramp RHC studies can be particularly helpful in defining optimal pump speed, since there is little clinical experience to turn to for guidance.
An investigational CF-LVAD, the ReliantHeart Heart Assist 5 (Houston, TX), incorporates an ultrasonic flow probe around the outflow graft that directly measures flow with a high reported accuracy[4]. There is no initial calibration with echo of RHC required, nor is there calibration based on blood viscosity (hematocrit) or patient’s heart rhythm.
Here we provide the initial report of a combined ramp RHC and ramp echocardiogram in a patient after ReliantHeart implantation, and provide a comparison of measured flow through the CF-LVAD to cardiac output measured by right heart catheterization.
A 50-year-old man underwent placement of a ReliantHeart LVAD as a bridge to cardiac transplantation at our hospital. In the intensive care unit his flow through the CF-LVAD as measured by the flow probe ranged between 5 and 6 L/min, with cardiac output measured via the Fick principle in the 7-8 L/min range, and cardiac output by thermodilution in the 7-8 L/min range as well. The flow through the CF-LVAD as measured by the flow probe increased to a range of 8-9 L/min on post-operative day 8, just prior to transfer out of the intensive care unit. Serum lactate dehydrogenase (LDH) levels were normal, and there were no signs of hemolysis in laboratory studies. The elevated measured flows with concomitant elevation in power consumption to 7-8 W raised a concern for pump malfunction, and we performed simultaneous ramp RHC and ramp echocardiography for further evaluation.
The patient was brought to the catheterization laboratory in the post-absorptive state. A Swan-Ganz catheter was placed via the right internal jugular vein via the Seldinger technique, and two pressure transducers were attached to the catheter to measure central venous pressure and pulmonary capillary wedge pressure simultaneously. We changed the speed by 400 revolutions per minute (rpm) and waited two minutes at each setting before measuring intracardiac pressures and cardiac output (CO) via an assumed Fick determination (CO = VO2 max/(oxygen concentration opf arterial blood - oxygen concentration of mixed venous blood). VO2max was assumed at 125 mL O2/BSA. At each setting, we also performed transthoracic echocardiography, measuring left ventricular end diastolic dimension, septal positioning, frequency of aortic valve opening, and the degree of mitral regurgitation.
Table 1 displays the measured changes in hemodynamic parameters with changes in RPM.
| RPM | RAP (mmHg) | RVP | PAP | PCWP | CO (L/min) | CI (L/min per meters square) | Flow (L/min) | Power (W) |
| 8300 | 11 | 44/20/28 | 15 | 6.3 | 2.2 | 7.3 | 6.4 | |
| 8700 | 10 | 40/18/25 | 14 | 6.4 | 2.5 | 7.9 | 7.1 | |
| 9100 baseline | 7 | 30/15 | 33/15/21 | 10 | 6.5 | 2.8 | 8.4 | 7.8 |
| 9500 | 6 | 35/15/22 | 10 | 6.7 | 2.9 | 8.7 | 8.6 |
The baseline speed was 9100 RPM. We changed speed by 400 RPM increments to determine the best hemodynamics (normal biventricular filling pressures with normal cardiac output), as well as optimal aortic valve opening (aortic valve opening frequency of at least 1:3 cardiac cycles).
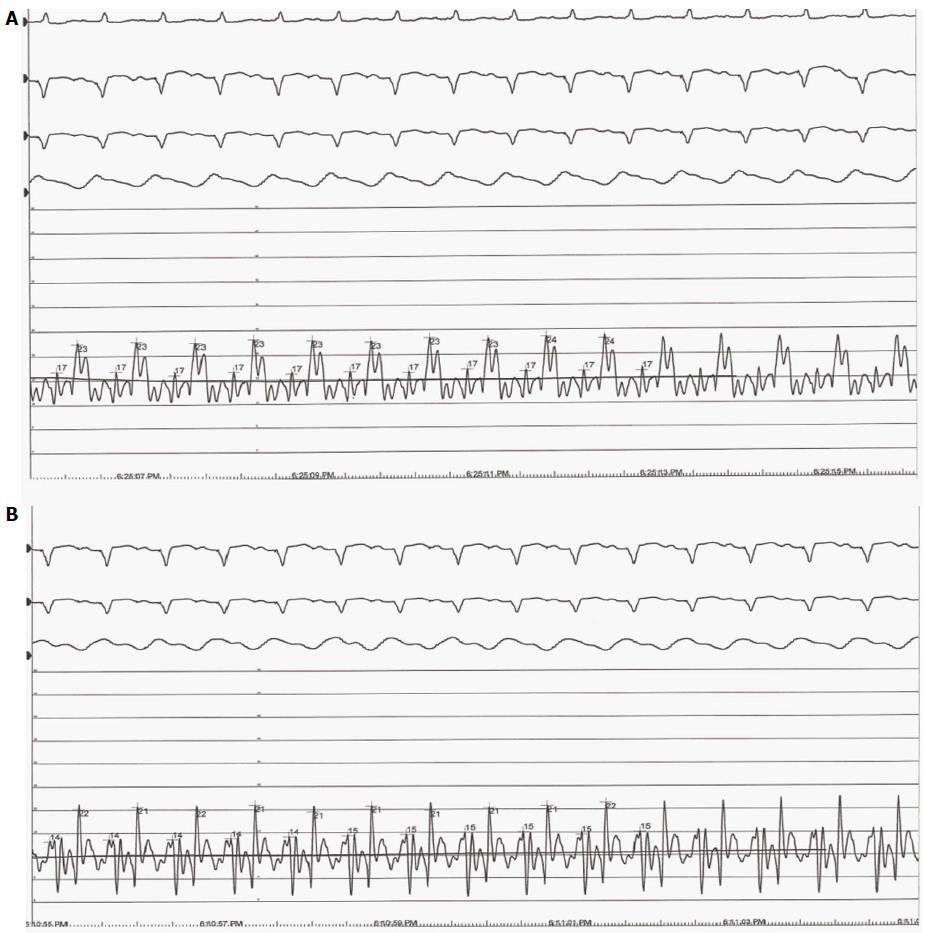
Biventricular filling pressures declined with an increase in pump speed, while cardiac output increased. The “v-wave” in the PCWP tracing was present at lower speeds (Figure 1A), but was reduced at 9100 RPM (Figure 1B). The flow measured through the CF-LVAD was consistently higher than the measured cardiac output by the Fick principle.
Echocardiography revealed that at 8300 RPM the aortic valve opened with every cardiac cycle, at 8700 RPM the valve opened 1:2 cardiac cycles, and the valve remained closed at 9100 RPM. At baseline RPM the left ventricular end diastolic dimension (LVIDd) was 8.0 cm and the interventricular septum (IVS) bowed mildly toward the LV. As pump speed increased, the degree of mitral regurgitation decreased, the LVIDd decreased further to 7.8 cm but the IVS shifted even more toward the LV. At lower speeds the IVS was midline, and at 8300 RPM the LVIDd was 8.3 cm. There was no evidence of aortic insufficiency or intracardiac shunting.
As a result, given acceptable hemodynamics at 8700 RPM and intermittent aortic valve opening with optimal position of the IVS, the speed was changed to 8700 RPM at the conclusion of the study.
We here report, to our knowledge, the first combined ramp RHC and ramp echocardiogram procedure in a patient receiving the ReliantHeart investigational CF-LVAD. This study reinforces the importance of incorporating simultaneous hemodynamic and echocardiographic data into defining the optimal pump speed for a patient with a CF-LVAD.
Our group commonly performs both ramp RHC and ramp echocardiography in patients for speed optimization post CF-LVAD placement, in the post-operative setting, as well as annually, and with clinical and laboratory data concerning for possible pump thrombosis. Published results corroborate the finding of our clinical practice. Ramp RHC in particular can demonstrate inadequate unloading of the left ventricle in patients who seem well compensated by clinical examination, or those with significant right ventricular dysfunction.
Ramp RHC can also be used to troubleshoot abnormal CF-LVAD parameters that are discrepant from clinical evaluation. In other CF-LVADs, high pump flows may signify pump thrombus or septic physiology. In this case, because an ultrasonic flow probe measured high flow, we worried the patient could indeed be in high flow state concerning for sepsis, intra-cardiac shunt or aortic regurgitation. On invasive hemodynamic assessment, we found the patient was adequately unloaded by the CF-LVAD (normal filling pressures and cardiac output) at the baseline RPM. After increasing the RPM, the loss of the V-wave on the PCWP tracing, coupled with aortic valve closure by echocardiogram, argued against the presence of a high cardiac output state, as these suggest further unloading by the CF-LVAD.
Interestingly, the flows measured by the flow probe at the time of the study were higher than the total cardiac output, suggesting a degree of inaccuracy in the flow probe measurement. We would have expected the measured flow through the CF-LVAD to be lower than the total cardiac output as measured by the Fick principle, as the total cardiac output should account for both flow through the native heart and flow through the CF-LVAD. That relationship was seen earlier in the patient’s hospital course, but was lost by the time the patient left the intensive care unit.
We do not have extensive data detailing the correlation between flow as measured by the ultrasonic flow probe of this investigational device and measured cardiac output via the Fick principle. We did note that the device’s power consumption also increased after implant to 8 W, although this was within the manufacturer specifications. This is an investigational device, and expected pump parameters still need to be described. In any case, early high pump powers are not necessarily indicative of future adverse events[5], and our patient had stable serum LDH values and no other evidence of pump thrombus.
One potential explanation for the unexpectedly high flows as measured by the flow probe is pressure drift, a slow change in the sensor that can shift its calibration and lead to inaccurate readings[6]. This drift has been seen in implantable left atrial pressure sensors over time. In addition, intraoperative placement of the flow probe is important. If the graft is not contacting the flow probe, then the measured flow may not be accurate. Shift of the flow probe over time could also lead to a shift in measured flow. Clinicians should use corroborative techniques (such as RHC) to confirm abnormal changes in CF-LVAD flows before acting on that data, both for CF-LVADs that estimate flows and those that directly measure flows.
One limitation of our study was the use of an assumed Fick calculation. We felt that was mitigated somewhat by the concomitant thermodilution data in the intensive care unit, which closely correlated with the Fick cardiac output. Simultaneous measurement of Fick and thermodilution cardiac outputs in the catheterization laboratory, as well as the use of metabolic cart to calculate peak oxygen consumption would more directly address this limitation.
In summary, we report here the utility of combined ramp RHC and ramp echocardiography to optimize speed in a patient receiving an investigational CF-LVAD, and troubleshoot abnormal parameters reported by that CF-LVAD. More data is needed to elucidate the correlation between the flow measured by the ultrasonic probe and cardiac output as measured by RHC.
A 59-year-old man with a severe nonischemic cardiomyopathy presented with elevated left ventricular assist device flows as measured by an ultrasonic flow probe despite normal clinical status.
Inaccurate flow estimation by ultrasonic flow probe.
Left ventricular assist devices (LVAD) pump thrombus, LVAD outflow graft malposition, infection.
All laboratory studies were within normal limits.
Echocardiogram revealed normal LVAD function with appropriate decompression of the left ventricle as speed increased.
Pressure drift has been noted in other pressure sensors, such as left atrial and pulmonary artery pressure monitors.
Ultrasonic.
Ultrasonic flow probes may provide inaccurate measures of flow through left ventricular assist devices, and more data is needed to elucidate the correlation between the flow measured by the ultrasonic probe and cardiac output as measured by right heart catheterization.
The paper is well written.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Ciccone MM, Peteiro J, Raina A, Shah N S- Editor: Qiu S L- Editor: A E- Editor: Wu HL
| 1. | Uriel N, Sayer G, Addetia K, Fedson S, Kim GH, Rodgers D, Kruse E, Collins K, Adatya S, Sarswat N. Hemodynamic Ramp Tests in Patients With Left Ventricular Assist Devices. JACC Heart Fail. 2016;4:208-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 164] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 2. | Estep JD, Vivo RP, Krim SR, Cordero-Reyes AM, Elias B, Loebe M, Bruckner BA, Bhimaraj A, Trachtenberg BH, Ashrith G. Echocardiographic Evaluation of Hemodynamics in Patients With Systolic Heart Failure Supported by a Continuous-Flow LVAD. J Am Coll Cardiol. 2014;64:1231-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 3. | Uriel N, Levin AP, Sayer GT, Mody KP, Thomas SS, Adatya S, Yuzefpolskaya M, Garan AR, Breskin A, Takayama H. Left Ventricular Decompression During Speed Optimization Ramps in Patients Supported by Continuous-Flow Left Ventricular Assist Devices: Device-Specific Performance Characteristics and Impact on Diagnostic Algorithms. J Card Fail. 2015;21:785-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 4. | Dean DA, Jia CX, Cabreriza SE, D’Alessandro DA, Dickstein ML, Sardo MJ, Chalik N, Spotnitz HM. Validation study of a new transit time ultrasonic flow probe for continuous great vessel measurements. ASAIO J. 1996;42:M671-M676. [PubMed] |
| 5. | Salerno CT, Sundareswaran KS, Schleeter TP, Moanie SL, Farrar DJ, Walsh MN. Early elevations in pump power with the HeartMate II left ventricular assist device do not predict late adverse events. J Heart Lung Transplant. 2014;33:809-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Yu L, Kim BJ, Meng E. Chronically implanted pressure sensors: challenges and state of the field. Sensors (Basel). 2014;14:20620-20644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |