Published online Sep 26, 2016. doi: 10.4330/wjc.v8.i9.534
Peer-review started: April 25, 2016
First decision: June 6, 2016
Revised: July 7, 2016
Accepted: July 20, 2016
Article in press: July 22, 2016
Published online: September 26, 2016
Processing time: 149 Days and 7.5 Hours
Cardiovascular implantable electronic device (CIED) infection and prosthetic valve endocarditis (PVE) remain a diagnostic challenge. Cardiac imaging plays an important role in the diagnosis and management of patients with CIED infection or PVE. Over the past few years, cardiac radionuclide imaging has gained a key role in the diagnosis of these patients, and in assessing the need for surgery, mainly in the most difficult cases. Both 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) and radiolabelled white blood cell single-photon emission computed tomography/computed tomography (WBC SPECT/CT) have been studied in these situations. In their 2015 guidelines for the management of infective endocarditis, the European Society of Cardiology incorporated cardiac nuclear imaging as part of their diagnostic algorithm for PVE, but not CIED infection since the data were judged insufficient at the moment. This article reviews the actual knowledge and recent studies on the use of 18F-FDG PET/CT and WBC SPECT/CT in the context of CIED infection and PVE, and describes the technical aspects of cardiac radionuclide imaging. It also discusses their accepted and potential indications for the diagnosis and management of CIED infection and PVE, the limitations of these tests, and potential areas of future research.
Core tip: Cardiovascular implantable electronic device infection and prosthetic valve endocarditis remain a diagnostic challenge. This review article describes the evolving role of cardiac radionuclide imaging in the diagnosis and management of cardiac infections. It focuses on recent published studies, indications and limitations of both 18F-fluorodeoxyglucose positron emission tomography/computed tomography and radiolabelled white blood cell single-photon emission computed tomography/computed tomography.
- Citation: Sarrazin JF, Philippon F, Trottier M, Tessier M. Role of radionuclide imaging for diagnosis of device and prosthetic valve infections. World J Cardiol 2016; 8(9): 534-546
- URL: https://www.wjgnet.com/1949-8462/full/v8/i9/534.htm
- DOI: https://dx.doi.org/10.4330/wjc.v8.i9.534
Cardiovascular implantable electronic device (CIED) infection and prosthetic valve endocarditis (PVE) carry significant morbidity and mortality as well as substantial financial burden to the society[1]. In some cases, establishing the diagnosis might be challenging since cultures are not always positive and they do not necessarily imply that the device/leads or heart valves are infected. Since device/lead extraction and repeat cardiac surgery are associated with significant risks, it is important to confirm the diagnosis and to plan the appropriate treatment. Cardiac imaging plays an important role in the pre-operative evaluation of patients with CIED infection and PVE. Radionuclide imaging has evolved over the past few years as an additional tool to confirm or exclude prosthetic infection and to guide the most appropriate clinical management, either complete removal or conservative treatment. In their 2015 guidelines for the management of infective endocarditis (IE), the European Society of Cardiology (ESC) addressed the use of nuclear medicine imaging for the diagnosis of IE[1]. The main objectives are to position 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) and white blood cell single-photon emission computed tomography/computed tomography (WBC SPECT/CT) imaging in clinical practice and to review the actual knowledge and recent studies as well as to address areas of future research.
CIED infection and PVE remain a diagnostic challenge. The clinical presentation can be highly variable because of multiple potential causative microorganisms, the presence of documented heart disease, cardiac devices or prosthetic valves, different modes of presentation, and sometimes non-specific symptoms at the time of initial presentation. The modified Duke criteria are considered the gold standard for the diagnosis of endocarditis[2,3]. However, the early diagnostic accuracy is often suboptimal with several patients being misclassified[4]. This is true mainly in patients with CIED infection and PVE. The early diagnosis of IE is imperative since postponement of antibiotic therapy and/or surgery can lead to a poor outcome[5,6].
A high level of expertise is required and it often includes cardiologists, nuclear medicine specialists, electrophysiologists, cardiac surgeons and infectious disease specialists. The concept of a “Heart Team approach” or “Endocarditis Team” has been proposed to improve the diagnosis and management of CIED infection and PVE. The use of a multidisciplinary task force with a well-defined protocol has been shown to decrease the 1-year mortality of patients with IE from 18.5% to 8.2%[7].
In addition, cardiac imaging plays an essential role in the diagnosis and management of IE. In recent guidelines, transthoracic echocardiography (TTE) and transesophageal echocardiography (TEE) remain the initial recommended imaging techniques for the diagnosis of IE (class I indication, level of evidence B)[1]. Some echocardiographic information is included as major criteria for the diagnosis of IE. The sensitivity for the identification of vegetations with TTE is 75% for native valves, but may be lower in patients with poor echogenicity or prosthetic valves or very small vegetations[8]. On the other hand, the sensitivity of TEE is superior at 85%-90%. However, a negative echocardiography does not rule out IE, and it has been recommended to repeat the TEE 3 to 5 d later or sooner when there is a high suspicion of IE or a change in the clinical status (class I, level of evidence B)[9]. In addition, non-infective vegetations such as strands or thrombi on valvular prosthesis or leads can lead to a false diagnosis of IE in up to 15% of cases[4]. These findings highlight the limitations of echocardiography and the potential benefits of other imaging techniques in such instances.
Investigation of patients with IE can also include other imaging techniques, such as multislice computed tomography for detection of abscesses or pseudoaneurysms, magnetic resonance imaging for detection of cerebral lesions, 18F-FDG PET/CT, and radiolabelled WBC hybrid SPECT/CT imaging.
PET imaging has been used for cancer diagnosis and staging and to detect infection in orthopaedic prostheses. In cardiology, it is used to evaluate myocardial viability, ischemia and to identify infection associated with vascular grafts, CIED and prosthetic valves.
With the combination of radionuclide imaging to CT scan (hybrid technology), nuclear imaging has provided significant supplementary information in patients with suspected IE. Two radionuclide imaging techniques are presently used in the diagnosis of CIED infection and PVE: (1) radiolabelled WBC SPECT/CT using either 111In-oxine or 99mTc-hexamethylpropyleneamine oxime (HMPAO); and (2) 18F-FDG PET/CT.
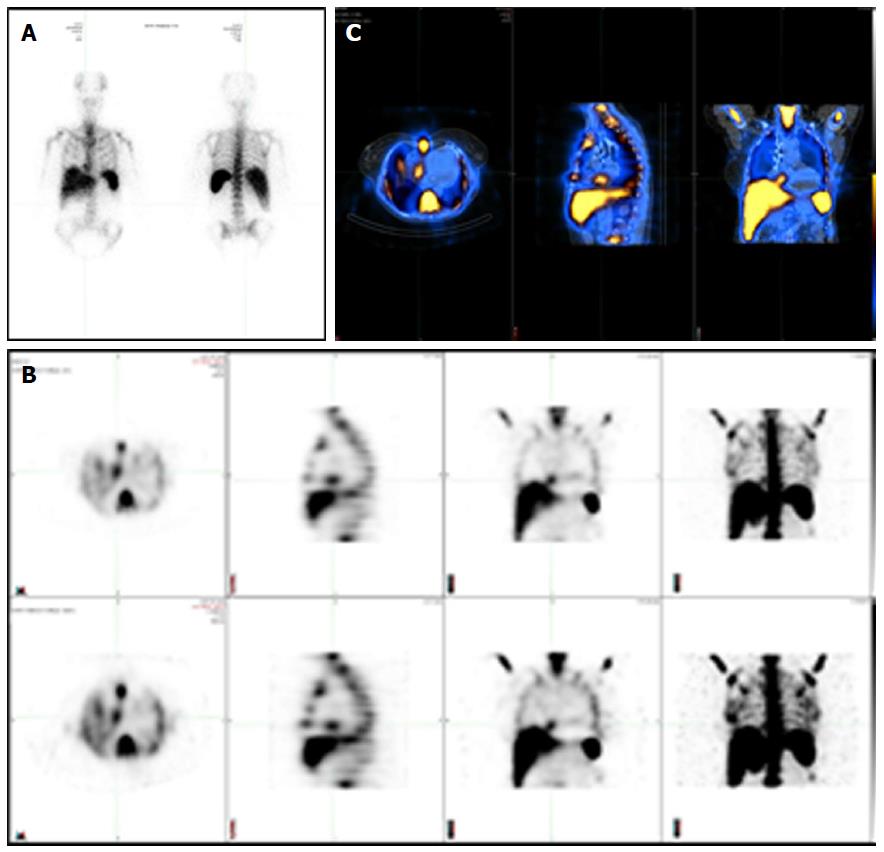
WBC SPECT/CT imaging uses autologous radiolabelled leukocytes (111In-oxine or 99mTc-HMPAO) that are injected intravenously back to the patient to look for infection in the body by imaging gamma rays. The accumulation of radiolabelled leukocytes is time-dependent between initial and late images. Planar images are obtained from different angulations with subsequent SPECT acquisition, 3D reconstruction and fusion with low-dose CT for further anatomical localization and attenuation correction. Figure 1 shows the differences between planar scintigraphy, conventional SPECT imaging and hybrid SPECT/CT. The sensitivity of this test depends on neutrophil granulocytes accumulation and is higher during acute infection. Studies have shown that cells participating in infection and inflammation, mainly neutrophils and macrophages, are able to express a great amount of glucose transporters, mainly GLUT1 or GLUT3 as well as hexokinase activity[10-14]. WBC SPECT/CT using 99mTc-HMPAO is performed 4 h following injection of radiolabelled leukocytes, although images at 24 h are possible but with loss of some image quality, whereas WBC SPECT/CT using 111In-oxine allows imaging up to 72 h with potentially better sensitivity (typically performed at 4, 24 and sometimes 48 h). This is based on the half-life of each radioactive isotope, being 6 h for 99mTc and 67 h for 111In. WBC SPECT/CT allows a higher specificity for the identification of active infection. However, leukocytes radiolabelling is more time-consuming. It also associated with manipulation of blood products.
18F-FDG PET/CT is a well-known non-invasive imaging technique that allows 3D calculation of metabolic activity within the body obtained from the emission of positrons subsequent to the disintegration of a radioactive compound. 18F-FDG is a glucose analogue, which is incorporated and retained within cells with a high metabolic activity, such as inflammatory cells. It is usually performed approximately 1 h after the injection of 18F-FDG. This tracer is actively incorporated by leukocytes, macrophages and CD4+ T-lymphocytes located at areas of infection via glucose transporters, primarily GLUT 1 and GLUT3, which are insulin sensitive and present in the myocardium[12-14]. Inside the cells, 18F-FDG is phosphorylated and remains intracellular without further transformation.
Each technique has advantages and weaknesses for the identification of active infection in cases of presumed PVE (Table 1). 18F-FDG PET/CT has the convenience of a shorter procedure time and a high sensitivity for the identification of hypermetabolic areas. It also has an excellent spatial resolution. However, it does not discriminate enough between infection and inflammation, mainly in the first few months postoperatively. Also, evaluation of 18F-FDG uptakes around cardiac valves can be more difficult if residual physiological myocardial uptake is present. For this reason, it is recommended to prepare the patient with the Atkins diet, which is a low-carbohydrate diet[15]. It is also suggested injecting a heparin bolus before administration of 18F-FDG. Unfractionated heparin increases plasma free fatty acids via activation of lipoprotein and hepatic lipases[16]. This can lead to a reduction in glucose consumption within the normal myocardium
| Advantages | Limitations |
| 18F-FDG PET/CT | |
| Excellent spatial resolution | Moderate radiation exposure (8-30 mSv depending on the study performed) |
| Short acquisition time | Not available in several centers |
| High sensitivity for the detection of hypermetabolic activity | Physiological uptake of 18F-FDG in the myocardium might prevent adequate detection of cardiac infection |
| Detection of peripheral events | Recent surgery may demonstrate residual inflammatory changes without evidence of infection |
| Detection of other sources of fever or bacteremia in patients with CIED | Possible uptakes can be found in active thrombi, cardiac tumours or metastasis, and foreign body reactions |
| Detection of CIED infection and PVE in cases of a negative TEE | Possible false-negative test in patients with small vegetations or prolonged antibiotic therapy |
| Less useful for infectious brain embolisms because of high glucose metabolism in the brain | |
| WBC SPECT/CT | |
| High specificity for the presence of active infection | Time-consuming |
| It involves blood products handling | |
| Cases of false-negative study seen with Candida and Enterococcus infection | |
In the literature, there are significant variations in the 18F-FDG PET/CT protocols used. Normally, 18F-FDG PET/CT is performed after a fasting period of 8 to 12 h. Eating foods rich in fat but very low in carbohydrates the evening prior to the exam is suggested in order to decrease the physiological uptake of 18F-FDG within the myocardium[17]. Patients should avoid bread, cereals, pasta, potatoes, rice, beans, fruit juice, chewing gum and drinking alcohol. Unfractionated heparin (50 IU/kg) can also be administered intravenously 15 min prior to 18F-FDG injection in an attempt to reduce more the physiological uptake. PET imaging is usually performed 1 h after the injection of 4-5 MBq/kg of 18F-FDG. Simultaneously, a whole-body low-dose CT without intravenous contrast is carried out for correction of attenuation and anatomic localization. The capillary glucose is measured, and patients receive an insulin injection if the fasting glucose is above 7.7 mmol/L or 140 mg/dL. The analysis is then performed using dedicated softwares. Both attenuation-corrected and non-attenuation-corrected images are reviewed in order to recognize potential artefacts that could be related to close proximity of objects of high density, such as device generator or prosthesis. A visual analysis is first performed to identify sites of hypermetabolic or abnormal 18F-FDG uptakes in close proximity to prosthetic valves and device generator/leads with further confirmation in the uncorrected images. In patients with CIED, focal uptake can be further classified based on the location (pocket infection, lead infection or both). Then, semi-quantitative analyses are done to measure the maximal standardized uptake value (SUVmax). However, it is important to recognize that these values have to be used with caution, since they can be falsely elevated due to the attenuation correction when measured in close proximity to a metallic object. For this reason, a semi-quantitative count ratio on non-attenuation-corrected images is likely superior to SUVmax (compared to an organ of reference, i.e., lung, mediastinum or liver parenchyma). In addition, whole-body acquisition allows for the detection of silent embolic events and extracardiac abnormal uptakes.
Autologous radiolabelled WBC scintigraphy with 111In-oxine was introduced in the mid-1970s. Over the years, it has been mainly substituted by 99mTc-HMPAO, which has more advantageous physical characteristics, cost, availability, and lower radiation burden[18]. 99mTc has a shorter imaging time because of a half-life of 6 h compared to 67 h for Indium. However, Indium is often preferred for the detection of CIED infection and PVE since it allows acquisitions over a longer period of time (up to 72 h).
There are several methods for labelling WBC, but the main principles and technique are similar. Around 40-60 mL of venous blood is taken from the patient and then combined to 10 mL of acid-citrate-dextrose anticoagulant solution. This syringe is then put in an upright position for 1 to 2 h to facilitate erythrocyte sedimentation by gravity. After erythrocytes have been removed, blood centrifugation is then performed to separate leukocytes from platelets. HMPAO is labelled with 99mTc and incubated for 15 min with leukocytes. The routine dose of 111In labelled leukocytes is 10-20 MBq (0.3-0.5 mCi) while the quantity of 99mTc-HMPAO labelled leukocytes is 185-370 MBq (5-10 mCi). Radiolabelled leukocytes are separated from HMPAO by centrifugation. The majority of labelled leukocytes are neutrophils. For this reason, the procedure is mainly useful for identification of a neutrophil-mediated process, such as a bacterial infection. A labelling efficiency of at least 40% should be achieved. Radiolabelled leukocytes are tested by the trypan blue exclusion test for viability. The cells are then resuspended in plasma before reinjection into the patient. For 99mTc-HMPAO labelled leukocytes, the scintigraphy is performed 4 and 24 h (delayed images) after injection, and sometimes 48 h or rarely 72 h for 111In labelled leukocytes. Images are acquired using a SPECT/CT system. Scintigraphy is considered positive when an area of labelled WBC uptake superior to background activity is identified in the involved area and when the signal increases over time.
CIED infection is associated with significant morbidity and mortality. Device infection prevalence is increasing in parallel with broader indications for ICD implantation and cardiac resynchronization, the presence of more comorbidities, and the growing number of implants in the world[19]. It is known however that the infection burden increases more than the increase in device implantations. This is probably related to more comorbidities and change in pathogens[20]. Cardiac device infections can present as a superficial or deep generator pocket infection or cardiac-device-related IE with involvement of the leads and/or extension to cardiac valves. It should be initially suspected in patients with CIED who consult for unexplained fever. Deep pocket infection and/or lead infection require complete system extraction. However, superficial infection not in contact with the device can be treated with antibiotic therapy alone. The diagnosis is sometimes quite obvious in the presence of significant pocket redness or pus, bacteremia or lead vegetation on TEE. Unfortunately, several cases are more complicated to assess. Since device and lead extraction can be associated with significant morbidity (major complications = 1.5%-2%) and mortality (0.8%) even in an experienced center, a definite diagnosis is important[21]. On the other hand, CIED infection can be overestimated with echocardiography since non-infectious accretions can be found in up to 21% by TTE and 28% by TEE in CIED patients without infection[22]. These patients can have fever or bacteremia for another reason. Thus, another form of imaging is proposed before proceeding to extraction/surgery.
The first report of cardiac infection detected by 18F-FDG PET was published in 2006[23]. Afterwards, 2 small pilot studies were published on device infection and 18F-FDG PET/CT. Bensimhon et al[24] evaluated the diagnostic value of 18F-FDG PET/CT in 21 patients with presumed device infection, which were compared to 14 patients without infection. 18F-FDG PET/CT had a sensitivity and specificity of 80% and 100%, respectively for diagnosis of infection. Patients with false negative studies for lead infection had received antibiotics for a longer period of time prior to the 18F-FDG PET/CT (20 d vs 3.2 d; P < 0.01). The sensitivity was lower for the diagnosis of lead infection (60% compared to 100% for pocket infection). Ploux et al[25] investigated the role of 18F-FDG PET/CT in 10 patients with CIED and fever of unknown origin. These patients were compared to a control group of 40 patients. 18F-FDG PET/CT showed increased 18F-FDG uptakes along the leads in 6 out 10 patients who had initial comprehensive negative investigation. Subsequently, these patients had complete extraction of the implanted material and lead cultures were positive on all 6 patients. This showed the promising value of 18F-FDG PET/CT in difficult CIED cases.
In 2012, our group evaluated the usefulness of 18F-FDG PET/CT for the identification of CIED infection[26]. We compared 3 groups: 42 patients with suspected CIED infection, 12 patients with recent device implantation (between 4 and 8 wk postoperatively) but no clinical signs of infection, and 12 patients with devices implanted for more than 6 mo and also no device infection. We showed an excellent correlation between sites of 18F-FDG uptakes on 18F-FDG PET/CT and clinical findings on TEE or at the time of extraction. 18F-FDG PET/CT using a qualitative visual score had a sensitivity and specificity for diagnosis of CIED infection of 89% and 86%, respectively. We also demonstrated that 18F-FDG PET/CT could identify patients with superficial infection without direct involvement of the generator or leads that could be treated only with antibiotics. Negative 18F-FDG PET/CT identified a group of patients that had an excellent outcome without device extraction. Finally, we were able to identify a semi-quantitative ratio between the maximal uptake and normal lung parenchyma uptake, which was useful in differentiating between CIED infection and residual normal post-operative changes; a ratio of 1.5 had the best combination of sensitivity and specificity. Based on this information, we suggested an algorithm using 18F-FDG PET/CT for the evaluation of CIED infection (Figure 2). An important clinical aspect of 18F-FDG PET/CT is its high negative predictive value.
Since, Cautela et al[27] demonstrated that 18F-FDG PET/CT had a high accuracy for the diagnosis of skin and pocket CIED infection (sensitivity 86.7% and specificity 100%), but a lower sensitivity of only 30.8% and a specificity of 62.5% for lead or cardiac involvement. Many patients with a false-negative test were already on antibiotics. The size of the vegetations might also have influenced the results. It cannot be excluded that some patients with lead extraction had a non-infectious cause for the vegetations seen on the lead. Finally, a possible limitation of this study is suboptimal patient preparation in order to partially explain the lower sensitivity observed for lead or cardiac involvement. It is of the utmost importance to make sure that physiologic myocardial uptake is suppressed to be able to realize an optimal evaluation. Ideally, every patient should be prepared with the Atkins diet and receive a heparin bolus before 18F-FDG injection. Ahmed et al[28] demonstrated that 18F-FDG PET/CT had a high diagnostic accuracy for the detection of patient with pocket infection that eventually required extraction. They find that the optimal semi-quantitative ratio cut-off value for the early identification of patients with pocket infection was > 2.0, giving a sensitivity of 97% and a specificity of 98%.
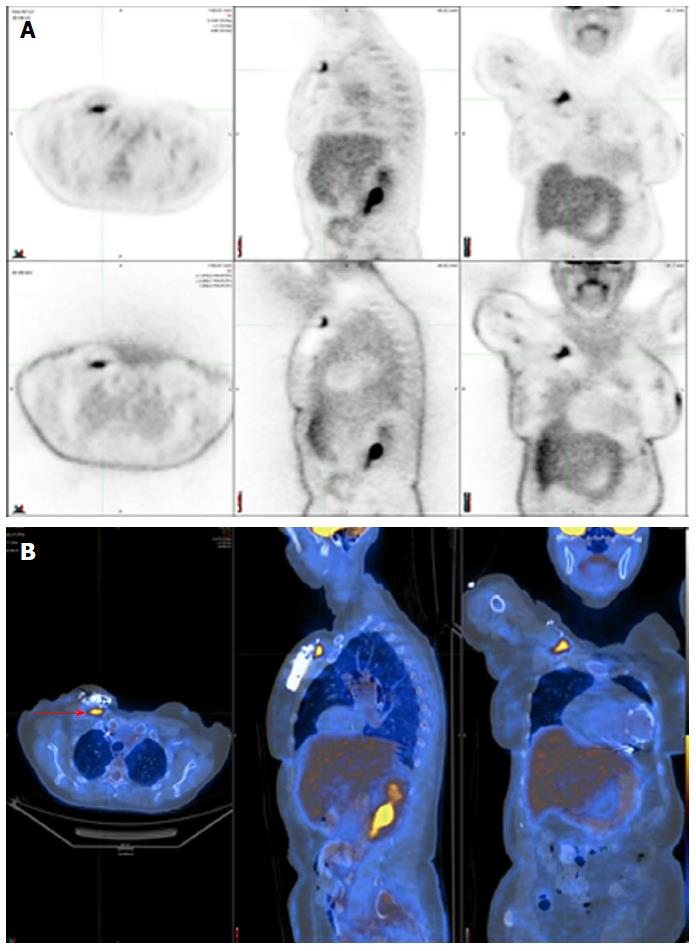
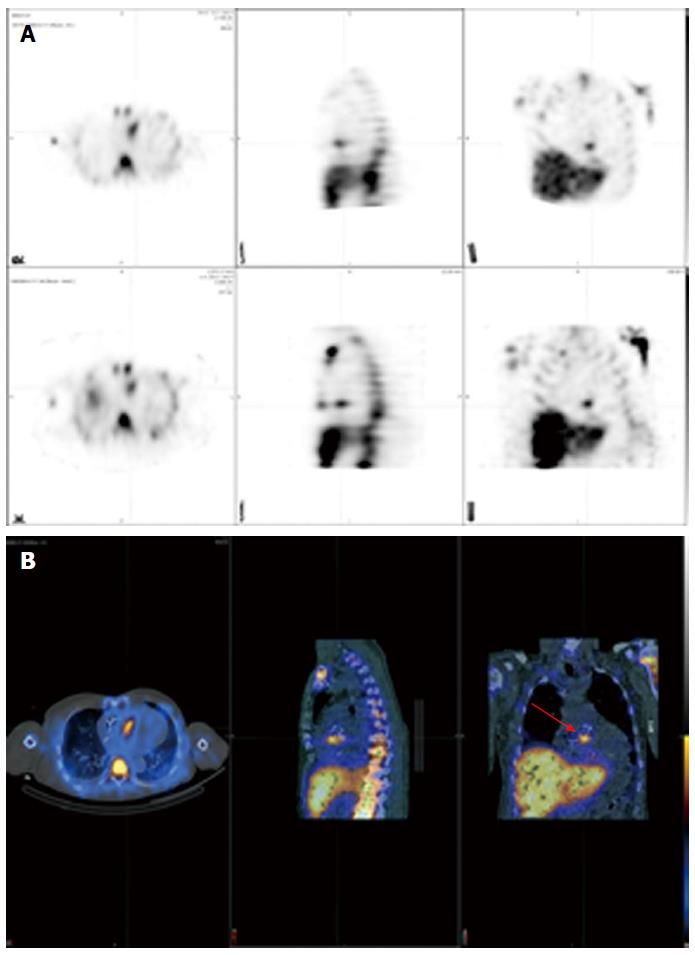
Figure 3 shows a positive 18F-FDG PET/CT in a patient with a deep pocket infection, while Figure 4 shows another positive 18F-FDG PET/CT but in a patient with a lead infection. Note how the physiologic myocardial uptake is well suppressed in this case.
The diagnostic accuracy of radiolabelled WBC scintigraphy was evaluated by Erba et al[29]. They obtained a sensitivity of 94% for both detection and localization of CIED infection. Two cases of false-negative scans were seen in patients with Candida and Enterococcus infection. No false-positive studies were seen, confirming the high specificity of this technique. They demonstrated the superiority of SPECT/CT over planar and SPECT alone imaging.
Based on these studies, 18F-FDG PET/CT and WBC SPECT/CT might play an additional role in the diagnosis of CIED infection, but data were judged not sufficient at the moment to be incorporated into the diagnostic criteria of IE involving pacemaker or defibrillator leads in the latest European guidelines[1]. Overall, 18F-FDG PET/CT seems to have an excellent sensitivity for the diagnosis of pocket infection, but a lower sensitivity in the context of lead infection.
Early diagnosis of PVE is also challenging. PVE is a severe form of IE and accounts for 10%-30% of all cases of IE. The diagnosis is often more difficult than in native valve endocarditis. Since the initial echocardiography is often normal or inconclusive in PVE, other imaging techniques are sometimes necessary. The use of 18F-FDG PET/CT in patients with PVE has evolved as a useful tool.
Case reports have demonstrated the possible benefits of 18F-FDG PET/CT in the diagnosis of prosthetic valves[30]. Saby et al[31] demonstrated the incremental benefit of using abnormal 18F-FDG uptake as a major criterion for the modified Duke criteria in the detection of PVE. They have shown that 18F-FDG PET/CT significantly increases the sensitivity of IE diagnosis from 70% to 97% (P = 0.008) on admission. They determined that 18F-FDG PET/CT had an adequate diagnostic value when abnormal 18F-FDG uptake is found near the prosthetic valve. They also showed that abnormal 18F-FDG uptake could be seen prior to detection of valvular damage by echocardiography in multiple patients, which emphasizes the benefit of 18F-FDG PET/CT to identify active infection before important damage has occurred.
Rouzet et al[32] evaluated the ability of 18F-FDG PET/CT and radiolabelled WBC imaging to diagnose PVE in 39 patients with presumed PVE but inconclusive echocardiography findings. 18F-FDG PET/CT had a higher sensitivity (93% vs 64%) but leukocyte scintigraphy had a higher specificity (100% vs 71%). Since it has a higher specificity for the detection of IE, it could be used in cases of equivocal 18F-FDG PET/CT or within the initial two months after heart valve surgery[32].
18F-FDG PET/CT can reduce the rate of misdiagnosed IE and help in the detection of peripheral events, including silent vascular phenomenon. 18F-FDG PET/CT can identify lesions of clinical importance not detected by conventional work-up in one out of seven IE patients[33]. It also improves the sensitivity of the modified Duke criteria in the most difficult situations. When endocarditis on a prosthetic valve is suspected, abnormal uptake around the site of insertion identified by 18F-FDG PET/CT (but more than 3 mo after prosthesis implantation) or radiolabelled WBC SPECT/CT could be considered a major diagnostic criterion. Results of 18F-FDG PET/CT should always be examined together with the other conventional diagnostic tools (clinical, microbiological and echocardiographic data). In addition, 18F-FDG PET/CT can be considered to monitor response to antibiotic therapy.
Erba et al[34] assessed in another study the value of 99mTc-HMPAO leukocyte scintigraphy in 131 patients with suspected endocarditis. In these patients, 51 had a confirmed diagnosis of IE and 35 had PVE (69%). Scintigraphy had a sensitivity of 90% and a specificity of 100%. No false-positive cases were seen, including patients evaluated for IE during the first two months after their surgery. However, false-negative studies were seen with Candida and Enterococcus endocarditis. It also identified cases of septic embolism. The test could be useful in patients with a high suspicion of IE but inconclusive TEE, in differentiating between infective and sterile vegetations identified with echocardiography, when other tests are contradictory, and to exclude valve involvement in patients with sepsis and prosthetic valve. In another study, Hyafil et al[35] looked at the role of radiolabelled leukocyte imaging in patients with presumed PVE and unconvincing echocardiography. They showed an excellent positive predictive value of intense signal with WBC scintigraphy for the presence of an abscess. Also, a negative scan predicted the absence of recurrent endocarditis in medically treated patients. Downsides of radiolabelled leukocyte scintigraphy are the necessity of blood handling, a longer procedure time, and a somewhat lower spatial resolution in contrast to 18F-FDG PET/CT.
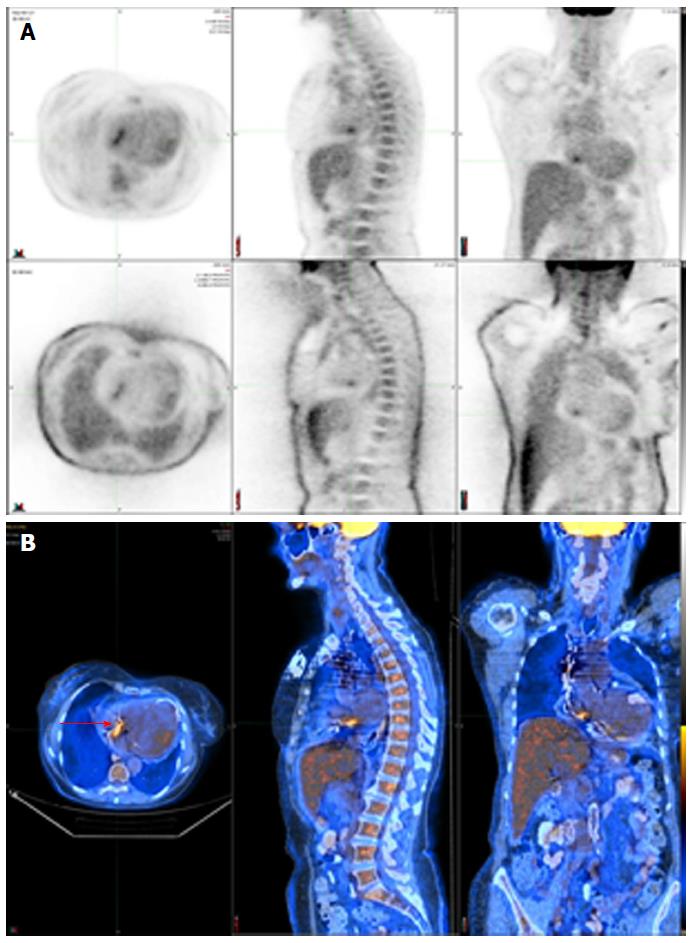
Figure 5 shows a positive 111In WBC SPECT/CT in a patient with endocarditis following an aortic valve replacement.
Table 2 shows the sensitivity and specificity of both 18F-FDG PET/CT and WBC SPECT/CT in the diagnosis of CIED infection and PVE.
| Test | Sensibility (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) | Accuracy (%) | |
| Prosthetic valve endocarditis | ||||||
| Saby et al[31] | PET/CT | 73 | 80 | 85 | 67 | 76 |
| Rouzet et al[32] | PET/CT | 93 | 71 | 68 | 94 | 80 |
| WBC | 64 | 100 | 100 | 81 | 86 | |
| Erba et al[34] | WBC | 90 | 100 | 100 | 94 | N/A |
| Cardiovascular implantable electronic device infection | ||||||
| Bensimhon et al[24] | PET/CT | 80 | 100 | 100 | 84.6 | N/A |
| 100 | 100 | 100 | 100 | N/A | ||
| Lead | 60 | 100 | 100 | 73 | N/A | |
| Ploux et al[25] | PET/CT | 100 | 93 | N/A | N/A | N/A |
| Sarrazin et al[26] | PET/CT | 88.6 | 85.7 | N/A | N/A | N/A |
| Cautela et al[27] | PET/CT | |||||
| 86.7 | 100 | N/A | N/A | N/A | ||
| Lead | 30.8 | 62.5 | N/A | N/A | N/A | |
| Ahmed et al[28] | PET/CT | |||||
| 97 | 98 | N/A | N/A | N/A | ||
| Erba et al[29] | WBC | 93.7 | 100 | 100 | 93.9 | 96.8 |
Despite its benefits, 18F-FDG PET/CT can have false-positive and false-negative results. Postoperative inflammatory changes can lead to non-specific 18F-FDG uptakes during the first several weeks after surgery, mainly following cardiac surgery or device implantation. Abnormal 18F-FDG uptake could also be caused by BioGlue surgical adhesive, a combination of bovine serum albumin and glutaraldehyde, used to seal the aortic root graft at time of surgery[36]. In addition, possible uptakes can be found in active thrombi, cardiac tumours or metastasis, post-surgical inflammation, and foreign body reactions like vascular grafts. At the other end of the spectrum, 18F-FDG PET/CT might be negative in patients with lower inflammation or when the test is performed after a long period of antibiotic therapy. The validity of 18F-FDG PET/CT in the context of slowly evolving infections is still unknown. Because of the high glucose metabolism in the brain, 18F-FDG PET/CT might not be the best test in order to detect infectious embolisms to the brain. However, an advantage of 18F-FDG PET/CT is the possibility to identify non-infectious causes of fever or underlying neoplasm. As opposed to echocardiography, cardiac nuclear imaging does not evaluate hemodynamic conditions associated with IE, such as valvular regurgitation, cardiac output, pulmonary arterial pressure and ventricular function. Another important issue remains that 18F-FDG PET/CT is less accessible than WBC SPECT/CT. The study quality could be improved by using respiratory and ECG gated techniques. This could minimize imaging artefacts, although it is technically more challenging and time-consuming. Cardiac nuclear imaging is a source of radiation. Administration of approximately 200 MBq of 18F-FDG for a PET study represents an effective dose between 3 and 4 mSv, which is similar to a low-dose CT. Then the total dose for a PET/CT would be approximately 7.5 mSv[37]. A follow-up study to monitor response to antibiotic therapy would increase radiation exposure. However, an initial PET scan combined to a low-dose CT and followed by a subsequent study would be equivalent to a percutaneous coronary intervention or an atrial fibrillation ablation procedure (approximately 15 mSv)[38].
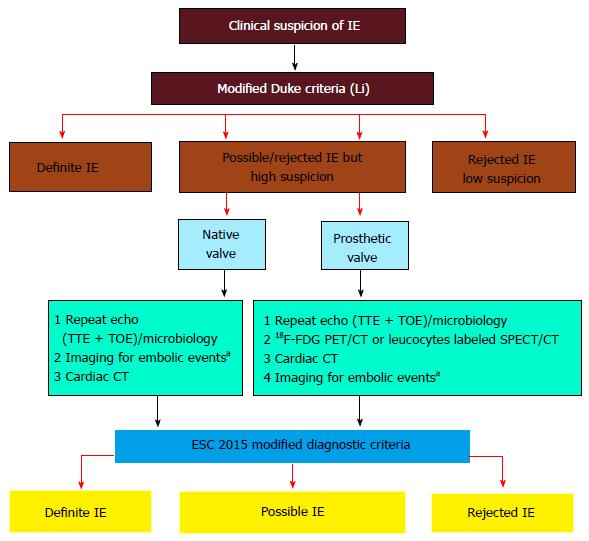
The ESC guidelines for the management of infectious endocarditis were updated in 2015[1]. The Task Force added 18F-FDG PET/CT or radiolabelled WBC SPECT/CT as a new major criterion if abnormal FDG uptakes are found around the area of prosthetic valve implantation in patients with a prosthesis implanted for more than 3 mo[1]. Nuclear imaging has also been incorporated in the new algorithm for the diagnosis of IE when the diagnosis is still possible or has been dismissed but when a high index of suspicion is still present (Figure 6). However, despite data for the key role of 18F-FDG PET/CT in the diagnosis of CIED infection, actual studies were judged insufficient to incorporate the results of 18F-FDG PET/CT at this time as a diagnostic criterion for device infection. For the moment, 18F-FDG PET/CT or radiolabelled leukocyte scintigraphy have a class IIb level of evidence C indication as an additional tool in patients with suspected CIED infection, positive blood cultures and negative echocardiography[1]. Also, the AHA scientific statement on IE judged that more clinical trials are still required to better clarify the utility of 18F-FDG PET/CT for the diagnosis and management of endocarditis[9]. Since most studies on cardiac radionuclide imaging have been published in the past 5 years, the use of 18F-FDG PET/CT in device infection was not discussed in the 2010 AHA scientific statement on CIED infections and their management[21].
Based on the recent ESC guidelines and previous studies, cardiac nuclear imaging could be considered in the following circumstances (Table 3): (1) accepted indication[1]: Possible or rejected IE diagnosis based on the modified Duke criteria, but persistent high clinical suspicion of infection in patients with a prosthetic valve; and (2) potential indications: Unclear diagnosis of CIED infection; Evaluation of the extent of infection when the results would affect the management of the patient, for example differentiation between superficial and deep pocket infection where device and lead extraction is recommended; Bacteremia with organisms not commonly a source of IE or fever of unknown origin in patients with CIED; High clinical suspicion of IE but negative TEE and/or negative blood cultures; Search for embolic events when it would affect the management of the patient; Monitoring the success of antibiotic therapy in medically treated patients.
| Accepted indication |
| Possible or rejected IE, but high suspicion of infection in patients with prosthetic valve |
| Potential indications |
| Unclear diagnosis of CIED infection |
| Evaluation of the extent of infection |
| Bacteremia or fever of unknown origin in patients with CIED |
| Cases with high clinical suspicion of IE but negative TEE and/or negative blood cultures |
| Search for embolic events |
| Monitoring the success of antibiotic therapy |
So far, available data on the diagnosis of CIED infection and PVE with either 18F-FDG PET/CT or WBC SPECT/CT come from small studies and limited number of patients. Larger studies would be useful to confirm the preliminary data suggesting the additional benefit of cardiac nuclear imaging. Despite encouraging results, some questions need to be answered. Is the use of 18F-FDG PET/CT cost-effective? Also, what is the consequence of prolonged antibiotic therapy prior to 18F-FDG PET/CT? There is also a need for standardization of the imaging techniques available since the imaging and data acquisition protocols are sometimes different from one center to another. There is still a need for further prospective studies in this field of research before 18F-FDG PET/CT should be systematically performed for the diagnosis of IE or used as a first line investigation. At the moment, it should be restricted to difficult cases of suspected CIED infection or PVE.
18F-FDG PET/CT appears to be a very promising imaging technique for the diagnosis of device infection and prosthetic valve endocarditis. Based on recent publications, there is growing evidence that cardiac nuclear imaging can play a key role in the diagnosis and management of patients with suspected CIED infections and PVE. This is now reflected in the most recent published guidelines. Although echocardiography remains an important initial test in the evaluation of these patients, 18F-FDG PET/CT and WBC SPECT/CT have clearly demonstrated their usefulness, mainly in difficult cases. Larger prospective studies will help to confirm the benefits of 18F-FDG PET/CT and clarify its role in the different algorithms of device and valve infections.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: Canada
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Falconi M, Peteiro J, Said SAM S- Editor: Gong XM L- Editor: A E- Editor: Wu HL
| 1. | Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del Zotti F, Dulgheru R, El Khoury G, Erba PA, Iung B. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36:3075-3128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2661] [Cited by in RCA: 3351] [Article Influence: 335.1] [Reference Citation Analysis (0)] |
| 2. | Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG, Ryan T, Bashore T, Corey GR. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30:633-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2699] [Cited by in RCA: 2825] [Article Influence: 113.0] [Reference Citation Analysis (0)] |
| 3. | Prendergast BD. Diagnostic criteria and problems in infective endocarditis. Heart. 2004;90:611-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 77] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | Habib G, Derumeaux G, Avierinos JF, Casalta JP, Jamal F, Volot F, Garcia M, Lefevre J, Biou F, Maximovitch-Rodaminoff A. Value and limitations of the Duke criteria for the diagnosis of infective endocarditis. J Am Coll Cardiol. 1999;33:2023-2029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 205] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 5. | Thuny F, Grisoli D, Cautela J, Riberi A, Raoult D, Habib G. Infective endocarditis: prevention, diagnosis, and management. Can J Cardiol. 2014;30:1046-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Vilacosta I, Graupner C, San Román JA, Sarriá C, Ronderos R, Fernández C, Mancini L, Sanz O, Sanmartín JV, Stoermann W. Risk of embolization after institution of antibiotic therapy for infective endocarditis. J Am Coll Cardiol. 2002;39:1489-1495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 285] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 7. | Botelho-Nevers E, Thuny F, Casalta JP, Richet H, Gouriet F, Collart F, Riberi A, Habib G, Raoult D. Dramatic reduction in infective endocarditis-related mortality with a management-based approach. Arch Intern Med. 2009;169:1290-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 220] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 8. | Habib G, Badano L, Tribouilloy C, Vilacosta I, Zamorano JL, Galderisi M, Voigt JU, Sicari R, Cosyns B, Fox K. Recommendations for the practice of echocardiography in infective endocarditis. Eur J Echocardiogr. 2010;11:202-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 357] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 9. | Baddour LM, Wilson WR, Bayer AS, Fowler VG, Tleyjeh IM, Rybak MJ, Barsic B, Lockhart PB, Gewitz MH, Levison ME. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation. 2015;132:1435-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1569] [Cited by in RCA: 2098] [Article Influence: 209.8] [Reference Citation Analysis (1)] |
| 10. | Gamelli RL, Liu H, He LK, Hofmann CA. Augmentations of glucose uptake and glucose transporter-1 in macrophages following thermal injury and sepsis in mice. J Leukoc Biol. 1996;59:639-647. [PubMed] |
| 11. | Fukuzumi M, Shinomiya H, Shimizu Y, Ohishi K, Utsumi S. Endotoxin-induced enhancement of glucose influx into murine peritoneal macrophages via GLUT1. Infect Immun. 1996;64:108-112. [PubMed] |
| 12. | Mochizuki T, Tsukamoto E, Kuge Y, Kanegae K, Zhao S, Hikosaka K, Hosokawa M, Kohanawa M, Tamaki N. FDG uptake and glucose transporter subtype expressions in experimental tumor and inflammation models. J Nucl Med. 2001;42:1551-1555. [PubMed] |
| 13. | Kubota R, Yamada S, Kubota K, Ishiwata K, Tamahashi N, Ido T. Intratumoral distribution of fluorine-18-fluorodeoxyglucose in vivo: high accumulation in macrophages and granulation tissues studied by microautoradiography. J Nucl Med. 1992;33:1972-1980. [PubMed] |
| 14. | Yamada S, Kubota K, Kubota R, Ido T, Tamahashi N. High accumulation of fluorine-18-fluorodeoxyglucose in turpentine-induced inflammatory tissue. J Nucl Med. 1995;36:1301-1306. [PubMed] |
| 15. | Coulden R, Chung P, Sonnex E, Ibrahim Q, Maguire C, Abele J. Suppression of myocardial 18F-FDG uptake with a preparatory “Atkins-style” low-carbohydrate diet. Eur Radiol. 2012;22:2221-2228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Persson E. Lipoprotein lipase, hepatic lipase and plasma lipolytic activity. Effects of heparin and a low molecular weight heparin fragment (Fragmin). Acta Med Scand Suppl. 1988;724:1-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 38] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Ahmed FZ, James J, Memmott MJ, Arumugam P. Radionuclide Imaging of Cardiovascular Infection. Cardiol Clin. 2016;34:149-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | de Vries EF, Roca M, Jamar F, Israel O, Signore A. Guidelines for the labelling of leucocytes with (99m)Tc-HMPAO. Inflammation/Infection Taskgroup of the European Association of Nuclear Medicine. Eur J Nucl Med Mol Imaging. 2010;37:842-848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 175] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 19. | Voigt A, Shalaby A, Saba S. Rising rates of cardiac rhythm management device infections in the United States: 1996 through 2003. J Am Coll Cardiol. 2006;48:590-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 280] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 20. | Greenspon AJ, Patel JD, Lau E, Ochoa JA, Frisch DR, Ho RT, Pavri BB, Kurtz SM. 16-year trends in the infection burden for pacemakers and implantable cardioverter-defibrillators in the United States 1993 to 2008. J Am Coll Cardiol. 2011;58:1001-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 493] [Cited by in RCA: 577] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 21. | Baddour LM, Epstein AE, Erickson CC, Knight BP, Levison ME, Lockhart PB, Masoudi FA, Okum EJ, Wilson WR, Beerman LB. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation. 2010;121:458-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 743] [Cited by in RCA: 760] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 22. | Dundar C, Tigen K, Tanalp C, Izgi A, Karaahmet T, Cevik C, Erkol A, Oduncu V, Kirma C. The prevalence of echocardiographic accretions on the leads of patients with permanent pacemakers. J Am Soc Echocardiogr. 2011;24:803-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Vos FJ, Bleeker-Rovers CP, van Dijk AP, Oyen WJ. Detection of pacemaker and lead infection with FDG-PET. Eur J Nucl Med Mol Imaging. 2006;33:1245. [PubMed] |
| 24. | Bensimhon L, Lavergne T, Hugonnet F, Mainardi JL, Latremouille C, Maunoury C, Lepillier A, Le Heuzey JY, Faraggi M. Whole body [(18) F]fluorodeoxyglucose positron emission tomography imaging for the diagnosis of pacemaker or implantable cardioverter defibrillator infection: a preliminary prospective study. Clin Microbiol Infect. 2011;17:836-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 25. | Ploux S, Riviere A, Amraoui S, Whinnett Z, Barandon L, Lafitte S, Ritter P, Papaioannou G, Clementy J, Jais P. Positron emission tomography in patients with suspected pacing system infections may play a critical role in difficult cases. Heart Rhythm. 2011;8:1478-1481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 26. | Sarrazin JF, Philippon F, Tessier M, Guimond J, Molin F, Champagne J, Nault I, Blier L, Nadeau M, Charbonneau L. Usefulness of fluorine-18 positron emission tomography/computed tomography for identification of cardiovascular implantable electronic device infections. J Am Coll Cardiol. 2012;59:1616-1625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 204] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 27. | Cautela J, Alessandrini S, Cammilleri S, Giorgi R, Richet H, Casalta JP, Habib G, Raoult D, Mundler O, Deharo JC. Diagnostic yield of FDG positron-emission tomography/computed tomography in patients with CEID infection: a pilot study. Europace. 2013;15:252-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 28. | Ahmed FZ, James J, Cunnington C, Motwani M, Fullwood C, Hooper J, Burns P, Qamruddin A, Al-Bahrani G, Armstrong I. Early diagnosis of cardiac implantable electronic device generator pocket infection using 18F-FDG-PET/CT. Eur Heart J Cardiovasc Imaging. 2015;16:521-530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 29. | Erba PA, Sollini M, Conti U, Bandera F, Tascini C, De Tommasi SM, Zucchelli G, Doria R, Menichetti F, Bongiorni MG. Radiolabeled WBC scintigraphy in the diagnostic workup of patients with suspected device-related infections. JACC Cardiovasc Imaging. 2013;6:1075-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 30. | Plank F, Mueller S, Uprimny C, Hangler H, Feuchtner G. Detection of bioprosthetic valve infection by image fusion of (18)fluorodeoxyglucose-positron emission tomography and computed tomography. Interact Cardiovasc Thorac Surg. 2012;14:364-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 31. | Saby L, Laas O, Habib G, Cammilleri S, Mancini J, Tessonnier L, Casalta JP, Gouriet F, Riberi A, Avierinos JF. Positron emission tomography/computed tomography for diagnosis of prosthetic valve endocarditis: increased valvular 18F-fluorodeoxyglucose uptake as a novel major criterion. J Am Coll Cardiol. 2013;61:2374-2382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 361] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 32. | Rouzet F, Chequer R, Benali K, Lepage L, Ghodbane W, Duval X, Iung B, Vahanian A, Le Guludec D, Hyafil F. Respective performance of 18F-FDG PET and radiolabeled leukocyte scintigraphy for the diagnosis of prosthetic valve endocarditis. J Nucl Med. 2014;55:1980-1985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 154] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 33. | Asmar A, Ozcan C, Diederichsen AC, Thomassen A, Gill S. Clinical impact of 18F-FDG-PET/CT in the extra cardiac work-up of patients with infective endocarditis. Eur Heart J Cardiovasc Imaging. 2014;15:1013-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 34. | Erba PA, Conti U, Lazzeri E, Sollini M, Doria R, De Tommasi SM, Bandera F, Tascini C, Menichetti F, Dierckx RA. Added value of 99mTc-HMPAO-labeled leukocyte SPECT/CT in the characterization and management of patients with infectious endocarditis. J Nucl Med. 2012;53:1235-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 157] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 35. | Hyafil F, Rouzet F, Lepage L, Benali K, Raffoul R, Duval X, Hvass U, Iung B, Nataf P, Lebtahi R. Role of radiolabelled leucocyte scintigraphy in patients with a suspicion of prosthetic valve endocarditis and inconclusive echocardiography. Eur Heart J Cardiovasc Imaging. 2013;14:586-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 36. | Schouten LR, Verberne HJ, Bouma BJ, van Eck-Smit BL, Mulder BJ. Surgical glue for repair of the aortic root as a possible explanation for increased F-18 FDG uptake. J Nucl Cardiol. 2008;15:146-147. [PubMed] |
| 37. | Xia T, Alessio AM, De Man B, Manjeshwar R, Asma E, Kinahan PE. Ultra-low dose CT attenuation correction for PET/CT. Phys Med Biol. 2012;57:309-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 38. | Picano E, Vañó E, Rehani MM, Cuocolo A, Mont L, Bodi V, Bar O, Maccia C, Pierard L, Sicari R. The appropriate and justified use of medical radiation in cardiovascular imaging: a position document of the ESC Associations of Cardiovascular Imaging, Percutaneous Cardiovascular Interventions and Electrophysiology. Eur Heart J. 2014;35:665-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 251] [Article Influence: 22.8] [Reference Citation Analysis (0)] |