Published online Aug 26, 2016. doi: 10.4330/wjc.v8.i8.488
Peer-review started: February 16, 2016
First decision: April 15, 2016
Revised: May 5, 2016
Accepted: June 27, 2016
Article in press: June 29, 2016
Published online: August 26, 2016
Processing time: 190 Days and 21 Hours
To delineate the features and current therapeutic option of congenital and acquired aortocameral fistulas (ACF) secondary to iatrogenic or infectious disorders.
From a PubMed search using the term "aortocameral fistula", 30 suitable papers for the current review were retrieved. Reviews, case series and case reports published in English were considered. Abstracts and reports from scientific meetings were not included. A total of 38 reviewed subjects were collected and analyzed. In addition, another case - an adult male who presented with ACF between commissures of the right and non-coronary sinuses and right atrium as a late complication of Staphylococcus aureus infective endocarditis of the AV - is added, the world literature is briefly reviewed.
A total of thirty-eight subjects producing 39 fistulas were reviewed, analyzed and stratified into either congenital (47%) or acquired (53%) according to their etiology. Of all subjects, 11% were asymptomatic and 89% were symptomatic with dyspnea (21 ×) as the most common presentation. Diagnosis was established by a multidiagnostic approach in 23 (60%), single method in 14 (37%) (echocardiography in 12 and catheterization in 2), and at autopsy in 2 (3%) of the subjects. Treatment options included percutaneous transcatheter closure in 12 (30%) with the deployment of the Amplatzer duct or septal occluder and Gianturco coil and surgical correction in 24 (63%).
Acquired ACF is an infrequent entity which may occur late after an episode of endocarditis of the native AV. The management of ACF is generally by surgical correction but non-surgical device intervention has recently been introduced as a safe alternative.
Core tip: Aortocameral fistula is an uncommon complication of native aortic valve (AV) endocarditis, which is associated with high morbidity and mortality. Acquired aortocameral fistulas (ACF) may originate from any of the three sinuses of Valsalva. Audible continuous murmur may raise suspicion for the presence of ACF. Congenital fistulas are less commonly reported than the acquired types. Acquired ACF may occur late after an episode of endocarditis of the native AV. The management of ACF is generally by surgical correction but non-surgical device intervention has recently been introduced as a safe alternative. Another case is added and the world literature is briefly reviewed.
- Citation: Said SAM, Mariani MA. Acquired aortocameral fistula occurring late after infective endocarditis: An emblematic case and review of 38 reported cases. World J Cardiol 2016; 8(8): 488-495
- URL: https://www.wjgnet.com/1949-8462/full/v8/i8/488.htm
- DOI: https://dx.doi.org/10.4330/wjc.v8.i8.488
Aortocameral fistulas (ACF) may be congenital[1] or acquired complicating acute aortic dissection[2] following an intimal tear in the vicinity and proximity of the aortic root or after aortic valve (AV) replacement[3]. ACF is an uncommon complication of native AV endocarditis, which is associated with high morbidity and mortality. ACF may originate from any of the three sinuses of Valsalva. Audible continuous murmur may raise suspicion for the presence of ACF[4]. The clinical manifestations of ACF may include exertional dyspnea[2,5], chest pain[6,7], palpitation[6,8], congestive heart failure[9,10] and recurrent respiratory tract infection[11,12]. ACF may incidentally be found during routine preoperative examination[13]. Untreated ACF may cause significant morbidity and early mortality. The surgical correction of ACF is the treatment of choice but percutaneous transcatheter device intervention has recently been successfully introduced for the closure of ACF[5,6,8,9]. Acquired ACF is an infrequent entity which may occur late after an episode of endocarditis of the native AV. Another case of our own is added and the world literature is briefly reviewed.
From the PubMed search using the term "aortocameral fistula", 30 suitable papers for the current review were retrieved (Table 1). Reviews, case series and case reports published in English were considered. Abstracts and reports from scientific meetings were not included. From 30 publications, 38 reviewed subjects were collected and analyzed. Data were analyzed using descriptive statistics.
| Ref. | Age gender | ACF | Diagnostic modality | Clinical presentation/etiology | Management |
| Jung et al[19], 2011 | 49 M | NCS-right atrium | TTE | Dyspnea and high fever Ruptured sinus of Valsalva Infective endocarditis (Enterococcus gallinarum) | Patch repair AVR/TVR |
| Raufi et al[21], 2002 | 50 M | RCS-right atrium | TEE aortography cardiac cath | Dyspnea and chest pain Post-repair of aneurysm of right sinus of Valsalva | Repair of the ruptured sinus of Valsalva Closure of the fistula |
| Hsu et al[2], 2000 | 67 M | False lumen-Right atrium | TTE cardiac cath ioTEE | Dyspnea Acute aortic dissection | Fistula repair Bentall procedure |
| Chung et al[20], 2000 | 52 M | AAR-right atrium | TTE cardiac cath angiography MRI | Dyspnea and hemoptysis Post-repair (ARR) of acute aortic dissection | Closure of the fistula New composite aortic root graft |
| Ananthasubramaniam et al[24], 2005 | 66 M | LCS-left atrium | TTE TEE ioTEE | Dyspnea post-AVR | Surgical closure of the fistula/repair |
| Haddad et al[22], 2008 | 66 M | NCS-right atrium | TTE | Dyspnea post-repair of acute aortic dissection | Fistula repair Bentall procedure |
| Estévez-Loureiro et al[5], 2012 | 44 M | NCS-left atrium | TTE TEE MDCT CAG 3-D TEE | Dyspnea Infective endocarditis post-AVR (Streptococcus viridans) | Percutaneous Amplatzer vascular plug III occluder |
| Bouchez et al[13], 2012 | 61 M | LCS-left atrium | io TEE 3-D TEE | Asymptomatic | Conservative |
| Mundo-Sagardía et al[14], 2006 | 22 M | NCS-right atrium | TTE TEE | Infective endocarditis (Streptococcus mitis). Complex congenital heart disease. Perforation of sinus Valsalva aneurysm (NCS) | Closure/repair |
| Ladowski et al[4], 1984 | 56 F | NCS-right atrium | angiography | Iatrogenic dissection | Surgical closure/repair |
| Vydt et al[36], 2002 | 43 M | NCS-right atrium | TTE TEE cardiac cath angiography aortography | Chest pain, DOE, ruptured sinus Valsalva aneurysm | Surgical closure |
| Moiduddin et al[12], 2009 | 5 F | NCS-left atrium | TEE angiography cardiac cath | Amplatzer atrial septal occluder ASD PDA | Surgical closure/repair |
| Chandra et al[1], 2011 | 12 F | RCS-right atrium | TTE CTA aortography angiography cardiac cath | Dyspnea, palpitation | Percutaneous Amplatzer duct occluder |
| Noureddine et al[16], 2001 | 21 F | NCS-right atrium | TTE TEE | Dyspnea | Sudden death |
| Przybojewski et al[39], 1983 | 27 M | RCS/right atrium and right ventricle | Phonocardiography TTE cardiac cath angiography aortography | Dyspnea; biventricular heart failure, ruptured sinus Valsalva aneurysm (RCS) | Surgical closure/repair |
| Mujanovic et al[35], 2010 | 41 F | NCS-right atrium | TTE angiography | Heart failure; ruptured sinus Valsalva aneurysm (NCS) | Surgical closure/repair |
| Mello et al[33], 2005 | 16 F | NCS-left atrium | TTE TEE aortography | Asymptomatic, post-placement of Amplatzer atrial septal occluder (ASO) for ASDII | Surgical closure/repair |
| Grayburn et al[7], 2005 | 41 F | Aorta-right atrium | TTE TEE | Chest pain, post-placement of Amplatzer atrial septal occluder (ASO) for ASD | Surgical closure/repair |
| Chun et al[30], 2003 | 10 M | NCS-right atrium | TTE TEE | Asymptomatic, post-placement of Amplatzer atrial septal occluder (ASO) for ASD | Surgical closure/repair |
| Knirsch et al[32], 2005 | 3 M | NCS-left atrium | TTE | Asymptomatic, post-placement of Amplatzer atrial septal occluder (ASO) for ASD | Surgical closure/repair |
| Jang et al[31], 2005 | 54 F | NCS-right atrium | TTE | Dyspnea, palpitation, hematuria, post-placement of Amplatzer atrial septal occluder (ASO) for ASDII | Surgical closure/repair |
| Ozay et al[45], 2007 | 22 F | NCS-right atrium | TTE TEE aortography cardiac catheter | Palpitation post-surgical repair of VSD and ASDII | Surgical closure/repair/ correction |
| Elwatidy et al[46], 2003 | 3 F | Aortic isthmus-RA | TTE cardiac catheter | Presented as a case of PDA | Surgical closure/repair/ correction |
| Akowuah et al[10], 2002 | 52 F | NCS-right atrium | TTE cardiac catheter | CHF, IE, TV, Staphylococcus aureus MRSA | Surgical TVR correction |
| Darwzah et al[15], 2006 | 23 M | NCS/LCS-right atrium | TTE | PVE of AVR Staphylococcus epidermidis | Surgical Re-re AVR correction |
| Russo et al[47], 2001 | 70 F | NCS-right atrium | TTE TEE | Chest pain, dyspnea, CHF complication of AAD type 1 | Surgical closure/repair/ correction |
| Onorato et al[9], 2005 | 48 F | NCS-right atrium | TTE TEE ICE aortography cardiac catheter | Dyspnea, CHF, ruptured sinus Valsalva aneurysm | ADO catheter closure |
| Chang et al[8], 2006 | 47, 22 F (2 ×) and 22,18M (2 ×) | NCS-RA (1 ×) RCS-RA (1 ×) and RCS-RV (2 ×) | 4 × TEE 4 × aortography | Closure of VSD and AVR, IE (1 ×) Dyspnea and palpitation (3 ×) | ADO catheter closure (3 ×) and Gianturco coil (1 ×) |
| Szkutnik et al[6], 2009 congenital (4 ×) and acquired (1 ×) | 51, 23, 41 M (3 ×) and 18, 28 F (2 ×) | RCS-RVOT (3 ×), RCS-RA (1 ×) LCS-PA (1 ×) and NCS-RA (1 ×) | TTE TEE MDCT | Dyspnea, chest pain, palpitation and syncope | ADO (5 ×) and ASO (1 ×) catheter closure |
| Oram et al[17], 1955 | 36, 67 M (2 ×) | NCS-RA RCS-RA, RV | Catheterization, autopsy | Chest pain, palpitation, dyspnea Chest pain, dyspnea | Post-mortem |
| Said and Mariani 2016 | 44 M | NCS-RCS-RA | TTE, aortography cardiac cath, angiography | Easy fatigability | Surgical closure |
An adult male presented with ACF between the junction of RCS-NCS and RA as a late complication of Staphylococcus aureus infective endocarditis (IE) of the native AV, is added.
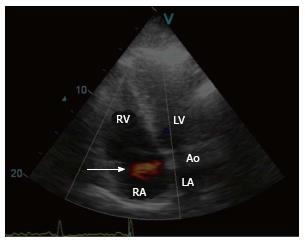
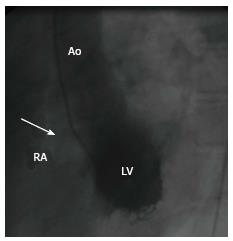
A 44-year-old male survivor of a prior episode of Staphylococcus aureus IE of the native AV (1998) presented with a recent history of rapid fatigability (2008) during sporting activities. He was afebrile and a continuous murmur was heard. Laboratory results and chest X-ray were normal. Resting ECG depicted sinus rhythm with signs of left ventricular hypertrophy (LVH). Two-dimensional transthoracic Doppler echocardiography revealed mild LVH, the right ventricle (RV) was dilated and normokinetic, and the tricuspid AV had no vegetation. Color flow mapping revealed evidence of a high velocity shunt between the commissures of the right coronary sinus (RCS) and non-coronary sinuses (NCS) terminating into the right atrium (RA) (Figure 1, Supplementary material online, Video 1). Cardiac catheterization demonstrated a shunt between the aorta and the RA and normal left ventricular kinetics (Figure 2, Supplementary material online, Video 2). Hemodynamic evaluation revealed a significant left-to-right shunt (Qp:Qs = 2.0:1.0) with normal pulmonary vascular resistance, normal intracardiac pressures and high resting cardiac output of 10 L/min. Computed tomography and cardiovascular magnetic resonance were not available at that time. The fistula was surgically closed (2008). The fistula was surgically closed (2008). After establishing median sternotomy, extracorporeal circulation was performed through standard cannulation of the aorta and right atrium. The heart was arrested with antegrade and selective blood cardioplegia. On inspection, no infectious masses or evidence of abscess or vegetations were visible. Further inspection revealed that the ascending aorta was not dilated or calcified and the LV showed moderate hypertrophy. After aortotomy, the AV could be inspected, which was tricuspid with mild thickening and the fistula was clearly visible between the RCS and NCS terminating into RA. The fistula was closed with 4.0 prolene suture and pledgets. The patient could easily be weaned off after an uneventful procedure. Postoperative transesophageal echocardiography revealed no rest shunt flow. The patient had an uneventful postoperative course. The patient had uneventful postoperative course and regained his non-professional sporting activities without any limitations. After 8 years of follow-up, he remains free of symptoms. The fistula was closed by 4.0 prolene suture and pledgets. The patient had uneventful postoperative course and regained his non-professional sporting activities without any limitations. After 7 years of follow-up, he remains free of symptoms.
In contrast to classic meta-analysis, the outcome is defined here as the percentages of an event (without comparison) in observed patients.
A total of 38 subjects were reviewed [21 males (55%) and 17 females (45%)], with a mean age of 36.8 years (range 3-70 years). The etiology was congenital in 18 (47%) and acquired in 20 (53%). They all had 39 fistulas. Of those, 37 had a single and 1 had dual origin, with a similar outflow distribution of 37 single and one dual termination. Their origin was NCS in 21 (54%), RCS in 10 (26%), left coronary sinus (LCS) in 4 (10%) and the thoracic aorta in 4 (10%) of the subjects. The termination was into RA in 29 (74%), left atrium (LA) in 6 (15%), RV in 2 (5%), pulmonary artery in 1 (3%) and right ventricular outflow tract in 1 (3%). Four subjects (11%) were asymptomatic. In the symptomatic subjects (89%), the most common presentations were dyspnea (21 ×), followed by congestive heart failure (6 ×), chest pain (7 ×), palpitations (7 ×), IE (6 ×) with Streptococcus mitis[14] and Staphylococcus epidermidis[15]. Although syncope, hematuria, hemoptysis and recurrent respiratory tract infection are rarely reported, sudden death has also been observed[16].
Diagnosis was established by multidiagnostic approach in 23 (60%), a single method in 14 (37%) (echocardiography in 12 and catheterization in 2), and at autopsy in 2 (3%)[17] of the subjects. IE was found in 6 (16%) subjects, all originating from the NCS and communicating with the RA in 5 and the LA in one. Treatment included percutaneous transcatheter closure in 12 (30%) and surgical correction in 24 (63%) and there were 3 mortalities (7%) (Table 1).
An aortic-atrial fistula is an aortocameral fistula presenting as an extracardiac vascular communication that may be congenital[1] or acquired[2]. ACF is an uncommon complication of native AV endocarditis, which is associated with high morbidity and mortality. In 1963, Kuipers et al[18] reported spontaneous ruptured aortic dissection into the right atrium. Congenital fistulas are less commonly reported than the acquired types. ACF may originate from any of the 3 sinuses of Valsalva. Acquired ACF may occur following bacterial endocarditis[19], acute aortic dissection[2], ruptured sinus of Valsalva aneurysm (RSVA)[19], and post-cardiovascular surgical procedures associated with[5] or without infective endocarditis (IE)[20-22]. Furthermore, ACF may occur after coronary artery bypass grafting[23], after mitral valve replacement[23], following repeat AV replacement[24] or secondary to iatrogenic endovascular injury during an invasive diagnostic procedure[4].
ACF may incidentally be found during routine preoperative examination[13] or presented with severe heart failure[2]. The surgical correction of ACF is the treatment of choice but percutaneous transcatheter device intervention has recently been successfully introduced for the closure of ACF[5,6,8,9].
In 1831, Hope[25] described a ruptured aneurysm of a sinus of Valsalva into the right atrium. Congenital or acquired aortic-atrial fistulas are rare anomalies. In 1924, a large autopsy series (n = 4000) revealed aorta to atrium fistula as an incidental finding; rupture was found in 1197, of which 13 were into the RA due to infectious, traumatic and atherosclerotic causes[26]. ACF may be congenital[1] or acquired complicating acute aortic dissection[2] following an intimal tear in the vicinity and proximity of the aortic root or after AV replacement[3] (Table 2). ACF may occur in patients with infective endocarditis[19], as was the case in the current patient and in 16% of the reviewed subjects.
| Etiology | Condition/references |
| Congenital | Congenital RCS-RA fistula[1] and aortic isthmus-RA fistula[46] |
| Acquired-iatrogenic (post-surgical and non-surgical intervention/infectious/diagnostic procedures) | Iatrogenic aorta-right atrial fistula: late (14 years) post-surgical repair of VSD and ASD[45] |
| Post-corrective surgery of sinus of Valsalva aneurysm[21] | |
| Post-CABG[23,48] | |
| Post-AVR[3,8] | |
| Post-MVR[23] | |
| Post-ARR, after operating on a type A dissection[20,47] | |
| Following ASO closure of the secundum ASDII[30] | |
| NVE[10], RCS/NCS-right atrial fistula (current case) secondary to NVE | |
| PVE[5,15] | |
| ACF associated with diagnostic cardiac catheterization (NCS-RAA)[4] | |
| Acquired-accidental/traumatic | ACF post-non-penetrating thoracic injury[49] has been reported |
| Spontaneous | RSVA[27] |
| Rupture of ascending aorta aneurysm[18] |
Audible continuous murmur may raise suspicion for the presence of ACF[4]. The clinical manifestations of ACF may include exertional dyspnea[2,5], chest pain[6,7], palpitation[6,8], congestive heart failure[9,10] and recurrent respiratory tract infection[11,12]. Our patient presented with reduced physical fitness as the only symptom, occurring late after the index native valve endocarditis. Among the 38 reviewed subjects, four (11%) were asymptomatic and the majority (89%) were symptomatic.
ACF may originate from any of the three sinuses of Valsalva, but origin from the NCS was infrequently reported[27]. Congenital aneurysms (origin RCS 65%-85%, NCS 10%-30% and LCS < 5%) of the sinus of Valsalva have a tendency to rupture, mainly into the right cardiac chambers (termination RV 63%, RA 32%), resulting in an ACF[28,29]. Congenital aneurysms of the sinus of Valsalva may be associated with other defects including bicuspid AV, ventricular septal defect and coarctation of the aorta[21].
ACF may occur between the aorta and right atrium[2], as was the case in our current patient, or left atrium[24]. Congenital ACF may be incidentally found in asymptomatic adult subjects[13]. There have been a few reports of iatrogenic acquired fistula formation associated with the percutaneous device closure of atrial septal defects with an Amplatzer septal occluder[30-33].
Congenital aortic-atrial fistulas are extremely rare. Acquired ACF are related to prosthetic valve disorders after aortic root repair associated with[5,15] or without infective endocarditis[22]. The current patient had a prior IE of the native AV. ACF may appear as an early[34], immediate[22] (10 d) or late (4 years)[5] postoperative complication.
Echocardiography [transthoracic (TTE), transesophageal (TEE) and 3-D TEE][7,32,35] is the first diagnostic modality of choice to precisely delineate the fistula components. With complete right and left cardiac catheterization and aortography of the aortic root, the fistula can be appropriately evaluated and the exact location indicated[21,36]. TTE, TEE and 3-D TEE comprise a useful non-invasive diagnostic modality with which to delineate the fistula characteristics. With 2-D echocardiography, TEE, the clinical diagnosis of ACF may be established but ascending aortography is essential for confirmation and to differentiate from other disorders such as ruptured sinus of Valsalva aneurysm[36], aorta-right atrial tunnel[11] and acquired[37] or congenital[38] coronary cameral fistulas.
A multimodality imaging strategy confirms the diagnosis of ACF. Echocardiography (TTE and TEE), selective coronary angiography and retrograde aortography are used for visualization of the coronary ostia and demonstration of the course of the fistula[12,36]. This was the chosen approach in two-third (60%) of the reviewed subjects and in the presented case.
Computed tomography (CT) scan and cardiovascular magnetic resonance imaging (MRI): These diagnostic modalities were not widely applied among the reviewed subjects. In only few cases, CT scan[1,5,6] was performed and MRI technique was found in the case reported by Chung[20]. In our current case, CT and cardiovascular magnetic resonance were not available at that time and moreover, echocardiography and aortography provided adequate imaging quality of the ACF making further investigations unnecessary.
The most common termination sites of “spontaneously” ruptured aneurysms of coronary sinus of Valsalva are into the RA or RV[27,39]; more rarely, the left ventricle[27] may be involved, ensuing acute volume overload of the involved cardiac chamber. Our patient had an acquired aortic-right atrial connection.
The origin of congenital aneurysm is generally related to the right coronary sinus (65%-85%)[1,28,29,39] and those associated with infective endocarditis ensue from the left coronary sinus[40], RCS[41] or NCS[42].
The first successful surgical correction of ruptured sinus Valsalva aneurysm (RSVA) was reported in 1957[43]. In 1966, Temple et al[44] described the successful surgical repair of aortic-right atrial fistula in an adult symptomatic male. ACF may be closed by surgical intervention[2] or by transcatheter device[5]. The treatment of choice is early surgical repair, which is necessary to prevent the development of severe symptoms and complications. Untreated ACF may cause significant morbidity and early mortality. Recently, percutaneous transcatheter treatment of ACF has been reported which is considered a novel method for selected cases[5]. Percutaneous transcatheter closure of ACF, using the Amplatzer duct occluder, Gianturco coil or Amplatzer septal occluder, has proven to be a safe technique which is gaining territory in the non-surgical management of ACF[5,6,8,9]. Our patient had a successful surgical repair with uneventful postoperative recovery.
Our current patient survived infective endocarditis of the AV occurring years prior to presentation. He remains well 7 years following the surgical correction.
The assistance of the librarian of the medical library of Hospital Group Twente, Mrs. A. Geerdink during the preparation of the manuscript, catheterization laboratory personnel Almelo-Hengelo and personnel of Thorax Center Twente, Enschede are greatly acknowledged.
Aortocameral fistulas (ACF) may be congenital or acquired complicating acute aortic dissection following an intimal tear in the vicinity and proximity of the aortic root or after aortic valve (AV) replacement. ACF is an uncommon complication of native AV endocarditis, which is associated with high morbidity and mortality.
ACF may originate from any of the three sinuses of Valsalva. Audible continuous murmur may raise suspicion for the presence of ACF. The clinical manifestations of ACF may include exertional dyspnea, chest pain, palpitation, congestive heart failure and recurrent respiratory tract infection. ACF may incidentally be found during routine preoperative examination. Untreated ACF may cause significant morbidity and early mortality.
The surgical correction of ACF is the treatment of choice but percutaneous transcatheter device intervention has recently been successfully introduced for the closure of ACF. Acquired ACF is an infrequent entity which may occur late after an episode of endocarditis of the native AV.
This paper presents a case of acquired aortic-atrial fistulas occurring late after infective endocarditis of the aortic valve, the author reviewed 30 suitable papers and summarized the clinical feature, diagnostic modalities and management of such a disease.
The authors reviewed the published literature on the aortic-atrial fistulae, but also included several cases of similar connections that occurred between the aorta and other chambers including the ventricles, the left atrium and the pulmonary artery. The interesting side of the manuscript is the review rather than the clinical case. The review is well written and reports a total of 38 cases, presented in different clinical scenario.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: The Netherlands
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Al-Mohammad A, Formica F, Hua P S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ
| 1. | Chandra S, Vijay S, Kaur D, Dwivedi S. Congenital aorta right atrial fistula: successful transcatheter closure with the Amplatzer occluder. Pediatr Cardiol. 2011;32:1057-1059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Hsu RB, Chien CY, Wang SS, Chu SH. Aorto-right artrial fistula: a rare complication of aortic dissection. Tex Heart Inst J. 2000;27:64-66. [PubMed] |
| 3. | Berman AD, Come PC, Riley MF, Weintraub RM, Johnson RG, Aroesty JM. Two-dimensional and Doppler echocardiographic diagnosis of an aortic to right atrial fistula complicating aortic dissection. J Am Coll Cardiol. 1987;9:228-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Ladowski JS, Hardesty RL. Repair of an iatrogenic aortoatrial fistula. Cathet Cardiovasc Diagn. 1984;10:43-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Estévez-Loureiro R, Salgado Fernández J, Vázquez-González N, Piñeiro-Portela M, López-Sainz Á, Bouzas-Mosquera A, Pombo F, Castro-Beiras A. Percutaneous closure of an aorto-atrial fistula after surgery for infective endocarditis. JACC Cardiovasc Interv. 2012;5:e15-e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Szkutnik M, Kusa J, Glowacki J, Fiszer R, Bialkowski J. Transcatheter closure of ruptured sinus of valsalva aneurysms with an Amplatzer occluder. Rev Esp Cardiol. 2009;62:1317-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Grayburn PA, Schwartz B, Anwar A, Hebeler RF. Migration of an amplatzer septal occluder device for closure of atrial septal defect into the ascending aorta with formation of an aorta-to-right atrial fistula. Am J Cardiol. 2005;96:1607-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Chang CW, Chiu SN, Wu ET, Tsai SK, Wu MH, Wang JK. Transcatheter closure of a ruptured sinus of valsalva aneurysm. Circ J. 2006;70:1043-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 9. | Onorato E, Casilli F, Mbala-Mukendi M, Perlasca E, Santoro F, Bortone F, Arena V. Sudden heart failure due to a ruptured posterior Valsalva sinus aneurysm into the right atrium: feasibility of catheter closure using the Amplatzer duct occluder. Ital Heart J. 2005;6:603-607. [PubMed] |
| 10. | Akowuah EF, Casula R, Thanos A, Cooper GJ. Aorto-right atrial fistula associated with native tricuspid valve endocarditis. J Cardiovasc Surg (Torino). 2002;43:841-842. [PubMed] |
| 11. | Gajjar T, Voleti C, Matta R, Iyer R, Dash PK, Desai N. Aorta-right atrial tunnel: clinical presentation, diagnostic criteria, and surgical options. J Thorac Cardiovasc Surg. 2005;130:1287-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 56] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Moiduddin N, Cheatham JP, Hoffman TM, Phillips AB, Kovalchin JP. Amplatzer septal occluder associated with late pulmonary venous obstruction requiring surgical removal with acquired aorta to left atrial fistula. Am J Cardiol. 2009;103:1039-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Bouchez S, Wouters PF, Vandenplas G. Asymptomatic aorto-atrial fistula identified with intraoperative transesophageal echocardiography. J Cardiothorac Vasc Anesth. 2012;26:e76-e77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Mundo-Sagardía JA, Johnson C, Calderón R, Quintana C. Coexistent congenital aortic defects, aneurysm of sinus of valsalva, atrial septal defect and infective endocarditis: a case report. P R Health Sci J. 2006;25:273-278. [PubMed] |
| 15. | Darwazah A, Kiswani M, Ismail H, Hawari M, Awad S. Aorto-right atrial fistula: a complication of prosthetic aortic valve endocarditis. A case report. J Heart Valve Dis. 2006;15:142-145. [PubMed] |
| 16. | Noureddine M, Raquim S, Elhattaoui M, Tahiri A, Chraibi N. [Aneurysm of the posterior sinus of Valsalva ruptured into right atrium]. Ann Cardiol Angeiol (Paris). 2001;50:211-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Oram S, East T. Rupture of aneurysm of aortic sinus (of Valsalva) into the right side of the heart. Br Heart J. 1955;17:541-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 78] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Kuipers FM, Schatz IJ. Prognosis in dissecting aneurysm of the aorta. Circulation. 1963;27:658-661. [RCA] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Jung TE, Kim JH, Do HD, Lee DH. Simultaneous Aortic and Tricuspid Valve Endocarditis due to Complication of Sinus of Valsalva Rupture. Korean J Thorac Cardiovasc Surg. 2011;44:240-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 20. | Chung DA, Page AJ, Coulden RA, Nashef SA. Aorto-atrial fistula after operated type A dissection. Eur J Cardiothorac Surg. 2000;17:617-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Raufi A, Khan IA, Nair VM, Rahmatullah SI, Rodriguez C, Sacchi TJ, Sahni G, Vasavada BC. Rupture of a surgically repaired sinus of Valsalva aneurysm. J Clin Basic Cardiol. 2002;5:199-200. |
| 22. | Haddad FG, El-Nemnoum R, Haddad F, Maalouly G, El-Rassi I. Giant cell arteritis of the aorta: catastrophic complications without a preexisting aneurysm. Eur J Intern Med. 2008;19:e59-e60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Scalia D, Rizzoli G, Scomparin MA, Testolin L, Isabella GB, Casarotto D. Aorto-right atrial fistula: a rare complication of aortic dissection type A. A report of two cases. J Cardiovasc Surg (Torino). 1997;38:619-622. [PubMed] |
| 24. | Ananthasubramaniam K. Clinical and echocardiographic features of aorto-atrial fistulas. Cardiovasc Ultrasound. 2005;3:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Hope J. A treatise on the diseases of the heart and great vessels. 3rd ed. Germany: Nabu Press 1839; 466-471. |
| 26. | Boyd LJ. A study of four thousand reported cases of aneurysm of the thoracic aorta. Am J Med Sci. 1924;168:654-668. [RCA] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 97] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Babacan KM, Tasdemir O, Zengin M, Karagöz HY, Zorlutuna YI, Ozer C, Sagban M, Yakut C, Bayazit K. Fistulous communication of aortic sinuses into the cardiac chambers. Fifteen years surgical experience and a report of 23 patients. Jpn Heart J. 1986;27:865-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 28. | Katz ES, Cziner DG, Rosenzweig BP, Attubato M, Feit F, Kronzon I. Multifaceted echocardiographic approach to the diagnosis of a ruptured sinus of Valsalva aneurysm. J Am Soc Echocardiogr. 1991;4:494-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 29. | Chow LC, Dittrich HC, Dembitsky WP, Nicod PH. Accurate localization of ruptured sinus of Valsalva aneurysm by real-time two-dimensional Doppler flow imaging. Chest. 1988;94:462-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Chun DS, Turrentine MW, Moustapha A, Hoyer MH. Development of aorta-to-right atrial fistula following closure of secundum atrial septal defect using the Amplatzer septal occluder. Catheter Cardiovasc Interv. 2003;58:246-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Jang GY, Lee JY, Kim SJ, Shim WS, Lee CH. Aorta to right atrial fistula following transcatheter closure of an atrial septal defect. Am J Cardiol. 2005;96:1605-1606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Knirsch W, Dodge-Khatami A, Balmer C, Peuster M, Kadner A, Weiss M, Prêtre R, Berger F. Aortic sinus-left atrial fistula after interventional closure of atrial septal defect. Catheter Cardiovasc Interv. 2005;66:10-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Mello DM, Fahey J, Kopf GS. Repair of aortic-left atrial fistula following the transcatheter closure of an atrial septal defect. Ann Thorac Surg. 2005;80:1495-1498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 34. | Patsouras D, Argyri O, Siminilakis S, Michalis L, Sideris D. Aortic dissection with aorto-left atrial fistula formation soon after aortic valve replacement: A lethal complication diagnosed by transthoracic and transesophageal echocardiography. J Am Soc Echocardiogr. 2002;15:1409-1411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 35. | Mujanovic E, Kabil E, Bergsland J, Stanimirovic-Mujanovic S, Caluk J. Ruptured aneurysm of the noncoronary sinus of valsalva into the right atrium. Med Arh. 2010;64:307-308. [PubMed] |
| 36. | Vydt T, Smolders W, Rademakers F. A massive left-to-right shunt due to a ruptured giant aneurysm of the sinus of Valsalva. Acta Cardiol. 2002;57:449-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 37. | Said SA, Schiphorst RH, Derksen R, Wagenaar LJ. Coronary-cameral fistulas in adults: Acquired types (second of two parts). World J Cardiol. 2013;5:484-494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 38. | Said SA, Nijhuis RL, Akker JW, Takechi M, Slart RH, Bos JS, Hoorntje CR, Houwelingen KG, Bakker-de Boo M, Braam RL. Unilateral and multilateral congenital coronary-pulmonary fistulas in adults: clinical presentation, diagnostic modalities, and management with a brief review of the literature. Clin Cardiol. 2014;37:536-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 39. | Przybojewski JZ, Blake RS, de Wet Lubbe JJ, Rossouw J, van der Walt JJ. Rupture of sinus of Valsalva aneurysm into both right atrium and right ventricle. A case report. S Afr Med J. 1983;63:616-625. [PubMed] |
| 40. | Ebringer A, Goldstein G, Sloman G. Fistula between aorta and left atrium due to bacterial endocarditis. Br Heart J. 1969;31:133-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 41. | Farouque HM, Worthley SG, Yeend RA. Aortico-atrial fistula secondary to bacterial endocarditis. Heart. 2001;86:498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 42. | Chen MY, Zhong DD, Ying ZQ. Aorta-to-right atrium fistula, an unusual complication of endocarditis. J Zhejiang Univ Sci B. 2009;10:230-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 43. | Lillehei CW, Stanley P, Varco RL. Surgical treatment of ruptured aneurysms of the sinus of Valsalva. Ann Surg. 1957;146:459-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 118] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 44. | Temple TE, Rainey RL, Anabtawi IN. Aortico-atrial shunt due to rupture of a dissecting aneurysm of the ascending aorta. J Thorac Cardiovasc Surg. 1966;52:249-254. [PubMed] |
| 45. | Ozay B, Okmen AS, Idiz M, Okmen E, Ketenci B, Yekeler I. Aorta-right atrial fistula after VSD operation. Thorac Cardiovasc Surg. 2007;55:122-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 46. | Elwatidy AF, Galal AN, Rhydderch D, Ashmeg AK. Aorto-right atrial fistula. Ann Thorac Surg. 2003;76:929-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 47. | Russo C, De Chiara F, Bruschi G, Ciliberto GR, Vitali E. Aorto-atrial fistula through the septum in recurrent aortic dissection. Ann Thorac Surg. 2001;72:921-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 48. | Hurley DV, Nishimura RA, Schaff HV, Edwards WD. Aortic dissection with fistula to right atrium. Noninvasive diagnosis by two-dimensional and Doppler echocardiography with successful repair. Case report and review of the literature. J Thorac Cardiovasc Surg. 1986;92:953-957. [PubMed] |
| 49. | Chang H, Chu SH, Lee YT. Traumatic aorto-right atrial fistula after blunt chest injury. Ann Thorac Surg. 1989;47:778-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |