Published online Apr 26, 2016. doi: 10.4330/wjc.v8.i4.302
Peer-review started: June 24, 2015
First decision: August 25, 2015
Revised: September 23, 2015
Accepted: January 21, 2016
Article in press: January 22, 2016
Published online: April 26, 2016
Processing time: 299 Days and 10.6 Hours
Hospital volume is regarded amongst many in the medical community as an important quality metric. This is especially true in more complicated and less commonly performed procedures such as structural heart disease interventions. Seminal work on hospital volume relationships was done by Luft et al more than 4 decades ago, when they demonstrated that hospitals performing > 200 surgical procedures a year had 25%-41% lower mortality than those performing fewer procedures. Numerous volume-outcome studies have since been done for varied surgical procedures. An old adage “practice makes perfect” indicating superior operator and institutional experience at higher volume hospitals is believed to primarily contribute to the volume outcome relationship. Compelling evidence from a slew of recent publications has also highlighted the role of hospital volume in predicting superior post-procedural outcomes following structural heart disease interventions. These included transcatheter aortic valve repair, transcatheter mitral valve repair, septal ablation and septal myectomy for hypertrophic obstructive cardiomyopathy, left atrial appendage closure and atrial septal defect/patent foramen ovale closure. This is especially important since these structural heart interventions are relatively complex with evolving technology and a steep learning curve. The benefit was demonstrated both in lower mortality and complications as well as better economics in terms of lower length of stay and hospitalization costs seen at high volume centers. We present an overview of the available literature that underscores the importance of hospital volume in complex structural heart disease interventions.
Core tip: Hospital volume is regarded amongst many in the medical community as an important quality metric. This is especially true in more complicated and less commonly performed procedures such as structural heart disease interventions. We present an overview of the available literature that underscores the importance of hospital volume in complex structural heart disease interventions including transcatheter aortic valve repair, transcatheter mitral valve repair, septal ablation and septal myectomy for hypertrophic obstructive cardiomyopathy, left atrial appendage closure and atrial septal defect/patent foramen ovale closure.
- Citation: Panaich SS, Patel N, Arora S, Patel NJ, Patel SV, Savani C, Singh V, Sonani R, Deshmukh A, Cleman M, Mangi A, Forrest JK, Badheka AO. Influence of hospital volume and outcomes of adult structural heart procedures. World J Cardiol 2016; 8(4): 302-309
- URL: https://www.wjgnet.com/1949-8462/full/v8/i4/302.htm
- DOI: https://dx.doi.org/10.4330/wjc.v8.i4.302
Hospital volume is regarded amongst many in the medical community as an important quality metric. The patients are unlikely to be in a position to choose between hospitals when it comes to emergent procedures. However, in case of non-emergent procedures, volume might be an important quality measure that could guide hospital selection by patients or referring physicians. This is especially true in more complicated and less commonly performed procedures such as structural heart disease interventions. Compelling evidence from a slew of recent publications has highlighted the role of hospital volume in predicting superior post-procedural outcomes following structural heart disease interventions[1,2]. This benefit was demonstrated both in lower mortality and complications as well as better economics in terms of lower length of stay (LOS) and hospitalization costs seen at high volume centers. To address this possible relationship of hospital volume and outcomes of structural heart disease procedures, we performed the search on PubMed and Medline with the following key words: Hospital volume, transcatheter aortic valve repair (TAVR), transcatheter mitral valve repair (TMVR), septal ablation (SA) and septal myectomy for hypertrophic obstructive cardiomyopathy (HOCM), left atrial appendage closure and atrial septal defect (ASD)/patent foramen ovale (PFO) closure and included all the studies with the above key words. We present an overview of the available literature that underscores the importance of hospital volume in complex structural heart disease interventions.
Seminal work on hospital volume relationships was done by Luft et al[3] more than 4 decades ago, when they demonstrated that hospitals performing > 200 surgical procedures a year 25%-41% lower mortality than those performing fewer procedures. Numerous volume-outcome studies have since been done for varied surgical procedures[4-7]. Certain agencies such as the Leapfrog group based in Washington DC have also made attempts to lay down minimal hospital volume requirements for various surgical procedures as a part of quality control[8]. The participating employers can use incentives to motivate their employees to get healthcare in institutions meeting these volume requirements[8]. Such standards for structural heart disease interventions are however not well defined partly because of the novelty of these procedures with lack of substantial evidence regarding volume-outcome relationship.
An old adage “practice makes perfect” indicating superior operator and institutional experience at higher volume hospitals is believed to primarily contribute to the volume outcome relationship[9]. This is further associated with evolution in the process of healthcare, with higher volume hospitals more likely to have better finances to develop more robust standards of care and infrastructure[8]. Hospital volume is thus believed by some to be a surrogate for possibly superior operator experience and availability of better ancillary support[9]. Selective referral with migration of lower risk patients to higher volume hospitals could also provide a healthier patient bias for such institutions[8]. Indeed, physicians might be inclined to refer their patients for elective procedures to larger hospitals with higher procedural volume leaving low volume institutions with more emergent procedures.
Following its approval, TAVR has rapidly evolved into a sought-after service with an increasing number of centers offering this structural intervention. However, TAVR program entails extensive resource utilization in terms of physician and ancillary manpower and other operational needs that newer lower volume centers might struggle with. A complex procedure such as TAVR should be performed in specialty valvular heart disease centers lead by multidisciplinary heart valve teams. As of today, there is paucity of any evidence-based data to formulate clinical competency guidelines for TAVR. As per a sole consensus document[10], TAVR procedures can be introduced in centers that perform > 1000 catheterizations/400 percutaneous coronary interventions (PCIs) annually with TAVR interventionalists who have performed 100 structural procedures over their lifetime or at least 30 left-sided structural procedures per year. Likewise for surgical support, a minimum institutional annual volume of 50 aortic-valve replacements is recommended with surgeons who have completed 100 valve replacements over their career, with at least 10 considered high risk[10].
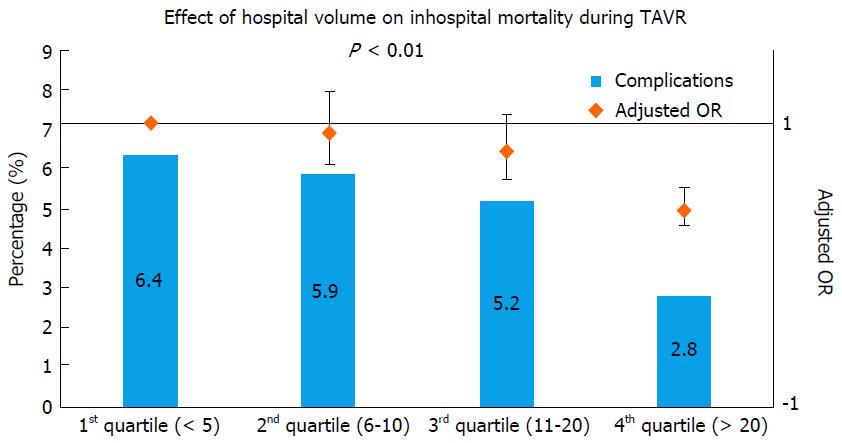
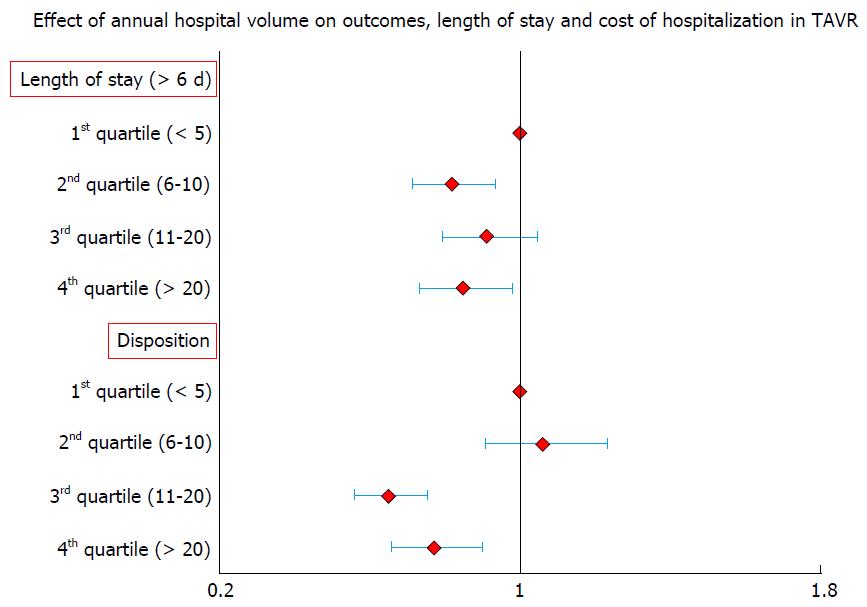
Previous literature on surgical valve replacement has highlighted the importance of institutional volume in predicting post-procedural outcomes[11]. Intuitively, one can reason that a similarly complex, percutaneous valve replacement procedure would also have superior results in higher volume institutions. In a recent analysis from Nationwide Inpatient Sample (NIS), we found hospital volume to be significantly predictive of lower in-hospital mortality following TAVR[12] (Table 1 and Figure 1). When compared to patients treated in lowest quartile of hospital volume, adjusted OR of in-hospital mortality in the highest quartile of hospital volume was 0.38 (0.27-0.54, P≤ 0.001). Increasing hospital volume was also independently predictive of shorter LOS and lower hospitalization costs (Table 1 and Figure 2). A separate spline analysis confirmed the significant hospital volume and outcome relationship with the predicted probability of in-hospital mortality dropping with increasing hospital volume.
| Multivariate simple logistic regression for mortality | Multivariate simple logistic regression for any complications and mortality | Multivariate simple logistic regression for LOS (LOS ≥ 6 d) | Multivariate simple logistic regression for disposition of transfer to short-term hospital/other facilities/home health care | |||||
| Variable | OR (95%CI) | P value | OR (95%CI) | P value | OR (95%CI) | P value | OR (95%CI) | P value |
| Hospital volume quartile | ||||||||
| 1st quartile | 1 | Referent | 1 | Referent | 1 | Referent | 1 | Referent |
| 2nd quartile | 0.92 (0.70-1.21) | 0.550 | 0.86 (0.76-0.99) | 0.029 | 0.82 (0.71-0.94) | 0.004 | 1.06 (0.91-1.23) | 0.451 |
| 3rd quartile | 0.80 (0.60-1.06) | 0.114 | 0.70 (0.61-0.80) | < 0.001 | 0.91 (0.80-1.05) | 0.194 | 0.65 (0.56-0.75) | < 0.001 |
| 4th quartile | 0.38 (0.27-0.54) | < 0.001 | 0.71 (0.62-0.82) | < 0.001 | 0.85 (0.74-0.98) | 0.024 | 0.77 (0.66-0.90) | 0.001 |
| Access | ||||||||
| Transfemoral | 1 | Referent | 1 | Referent | 1 | Referent | 1 | Referent |
| Trans-apical | 1.54 (1.17-2.03) | 0.002 | 1.44 (1.26-1.64) | < 0.001 | 2.27 (1.96-2.63) | < 0.001 | 1.37 (1.18-1.60) | < 0.001 |
| Age (10-yr increment) | 1.26 (1.07-1.47) | 0.005 | 0.97 (0.91-1.03) | 0.316 | 1.07 (1.00-1.14) | 0.051 | 1.57 (1.46-1.69) | < 0.001 |
| Gender | ||||||||
| Male | 1 | Referent | 1 | Referent | 1 | Referent | 1 | Referent |
| Female | 1.09 (0.89-1.36) | 0.392 | 1.21 (1.11-1.33) | < 0.001 | 1.43 (1.30-1.57) | < 0.001 | 1.99 (1.79-2.21) | < 0.001 |
| Charlson score | ||||||||
| 0 | 1 | Referent | 1 | Referent | 1 | Referent | 1 | Referent |
| 1 | 1.29 (0.78-2.14) | 0.321 | 1.13 (0.92-1.38) | 0.236 | 1.23 (1.01-1.50) | 0.038 | 1.40 (1.14-1.72) | 0.002 |
| ≥ 2 | 1.60 (1.01-2.55) | 0.047 | 1.73 (1.44-2.08) | < 0.001 | 2.02 (1.69-2.42) | < 0.001 | 1.72 (1.42-2.07) | < 0.001 |
| Bed size of hospital | ||||||||
| Small | 1 | Referent | 1 | Referent | 1 | Referent | 1 | Referent |
| Medium | 0.43 (0.28-0.67) | < 0.001 | 0.89 (0.70-1.15) | 0.386 | 1.18 (0.91-1.52) | 0.215 | 1.17 (0.88-1.56) | 0.279 |
| Large | 0.42 (0.29-0.61) | < 0.001 | 0.73 (0.58-0.91) | 0.005 | 1.36 (1.09-1.71) | 0.007 | 1.23 (0.96-1.58) | 0.103 |
| Model 2 | ||||||||
| Hospital volume quartile (5 procedures increment) | 0.88 (0.83-0.93) | < 0.001 | 0.94 (0.92-0.95) | < 0.001 | 0.93 (0.91-0.95) | < 0.001 | 0.95 (0.94-0.97) | < 0.001 |
| Model 3 | ||||||||
| Hospital volume quartile (10 procedures increment) | 0.77 (0.69-0.86) | < 0.001 | 0.87 (0.84-0.91) | < 0.001 | 0.87 (0.84-0.90) | < 0.001 | 0.91 (0.87-0.95) | < 0.001 |
The Centers for Medicare and Medicaid (CMS) in their proposal to cover reimbursement for TMVR/Mitra-clip have laid down some operator and institutional requirements. The institution must have had ≥ 25 total mitral valve procedures in the previous year of which at least 10 must be mitral valve repairs. In addition, there should have been ≥ 1000 catheterizations per year performed in that institution, including ≥ 400 PCIs per year. The individual operator should have had ≥ 50 structural procedures per year including ASD and PFO and trans-septal punctures. Besides, it also mandates a comprehensive multi-disciplinary heart team comprised of various cardiologists, surgeons and strong ancillary support along with device-specific training as required by the manufacturer.
The National Institutes of Health in United Kingdom have minimal volume requirements for surgical mitral valve repair[13]. This is considered especially vital due to low volume of this procedure and many low volume centers perform mitral valve replacement more frequently in degenerative MR where mitral valve repair is strongly recommended[13]. Again, TMVR is a relatively new procedure with a steep learning curve and will need further studies to appraise specific volume requirements for involved operators and institutions. A more detailed competency guideline is expected in the forthcoming SCAI/AATS/ACC/STS Multisocietal Consensus Statement: Operator and Institutional Requirements for Transcatheter Valve Repair and Replacement: Part 3: Mitral Valve[13]. In another analysis from NIS (Abstract presented as poster presentation at American Heart Association Scientific Sessions 2014, Chicago, IL), we noted the highest hospital volume tertile to be significantly predictive of lower in-hospital mortality and post-procedural complications following TMVR compared to the lowest volume tertile (OR = 0.12, 95%CI: 0.06-0.23, P < 0.001) (Table 2 and Figure 3A). The predicted probability of mortality and complications was noted to decrease with increasing hospital volume on an additional spline analysis.
| Variable | OR (95%CI) | P value |
| Hospital volume tertile | ||
| 1st tertile | 1 | Referent |
| 2nd tertile | 0.23 (0.12-0.41) | 0.177 |
| 3rd tertile | 0.12 (0.06-0.23) | < 0.001 |
ACCF/AHA HOCM guideline recommends that an operator be labeled experienced in SA only after he/she has performed > 20 procedures in a facility with a cumulative volume of > 50 procedures[14]. However, given the low overall volume of SA, the maintenance of competency requires an annual operator volume of only 5 ablations with no comment on institutional volume[14]. Nonetheless, volume remains one of the many factors required to achieve satisfactory post-procedural outcomes. In a retrospective analysis from NIS, we noted highest hospital volume tertile to be significantly predictive of lower post-procedural complications following SA upon multivariate adjustment (OR = 0.51, 95%CI: 0.26-0.98, P = 0.04). Parallel to septal myectomy, which has been shown to have excellent outcomes when performed at centers of excellence, SA is more likely to be better performed at centers with volume and resources to care for this unique patient population. Indeed, a recent study showed a higher overall in-hospital mortality and post-procedural complication rate following septal myectomy in the real world clinical practice than that reported from selected referral centers[15]. Although, a trend was seen towards higher institutional volume being associated with better outcomes, the final results were non-significant indicating a need for further studies (Table 3).
| Primary outcome | Length of stay | Cost of care | ||||
| OR (95%CI) | P value | OR (95%CI) | P value | OR (95%CI) | P value | |
| Hospital volume (n of procedure per yr) | ||||||
| 1st tertile (< 14) | Referent | Referent | Referent | |||
| 2nd tertile (14-37) | 0.73 (0.57-0.93) | 0.013 | 0.50 (0.36-0.69) | < 0.001 | 0.72 (0.49-1.07) | 0.104 |
| 3rd tertile (> 37) | 0.67 (0.48-0.94) | 0.019 | 0.37 (0.24-0.57) | < 0.001 | 2.55 (1.54-4.20) | < 0.001 |
Some of the initial data suggested that ASD/PFO closure could be performed safely at low-volume hospitals[16]. Relative simplicity of ASD/PFO closure that shares some of the techniques with other, more commonly preformed percutaneous interventions[17] led some to believe that volume-outcome relationship might not hold true for this procedure. The current ACC/AHA/SCAI guidelines recommend a minimal annual volume of > 10 ASD/PFO closure procedures for maintenance of catheterization laboratory proficiency. However, these guidelines also note the lack of sufficient evidence based data for this recommendation.
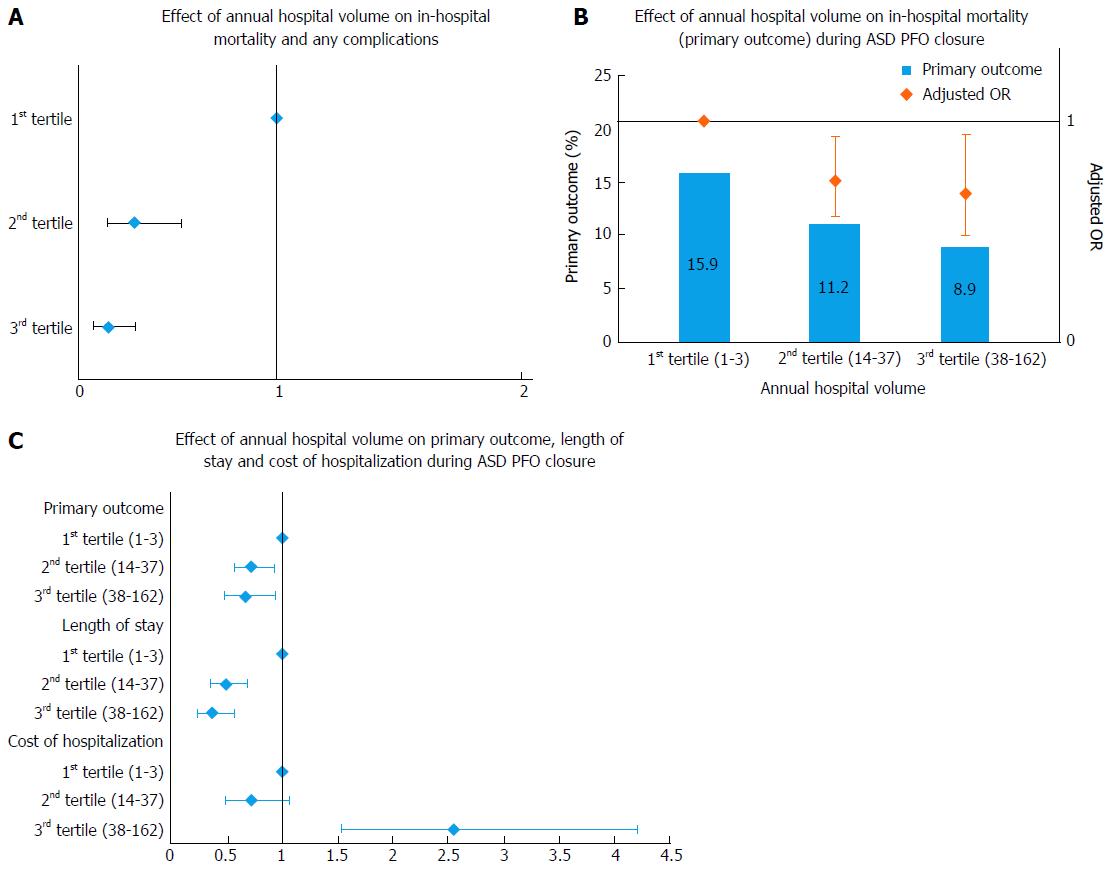
Opotowsky et al[18] showed the inverse hospital volume outcome relationship in an early study from the NIS database. In another study that included a larger sample size, an absolute risk reduction of 4.6% was noted when procedures were performed at hospitals with an annual procedural volume > 10[1]. An additional absolute risk reduction of 2.1% was further noticed if procedures were performed at hospitals with an annual volume > 25 indicating a need for possible revision of competency guidelines. Furthermore nearly 30% of the hospitals performing ASD/PFO closures were observed to be below the recommended threshold of 10 annual procedures (Figure 3B and C and Table 3).
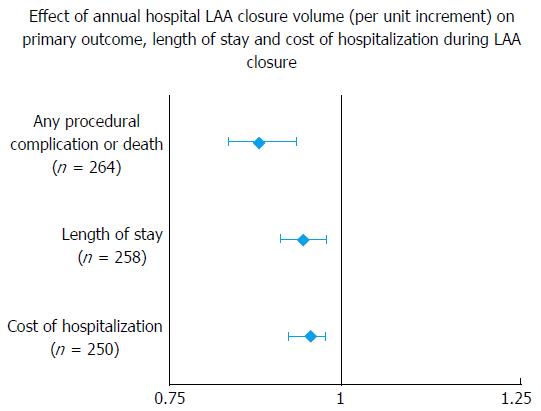
A recent study by Badheka et al[2] showed higher hospital volume to be inversely associated with better post-procedural outcomes as well as lower hospitalization costs and shorter LOS[2] (Figure 4 and Table 4). Hospitals with and annual volume cut-off of > 18 procedures had post-procedural complication rate, which compared favorably with trial data. This study added evidence to inverse operator volume-outcome relationship seen in the CAP registry[19]. Further studies are again needed to determine volume thresholds and lay down minimal competency requirements.
| Any procedural complication or death (n = 264) | OR with 95%CI | P value |
| Hospital annual LAA closure volume (per unit increase) | 0.89 (0.85-0.94) | < 0.001 |
| Length of stay (n = 258) | HR | P value |
| Hospital annual LAA closure volume (per unit increase) | 0.95 (0.92-0.98) | < 0.001 |
| Cost of hospitalization (n = 250) | Estimate ($) | P value |
| Hospital annual LAA closure volume (per unit increase) | 0.96 (0.93-0.98) | < 0.001 |
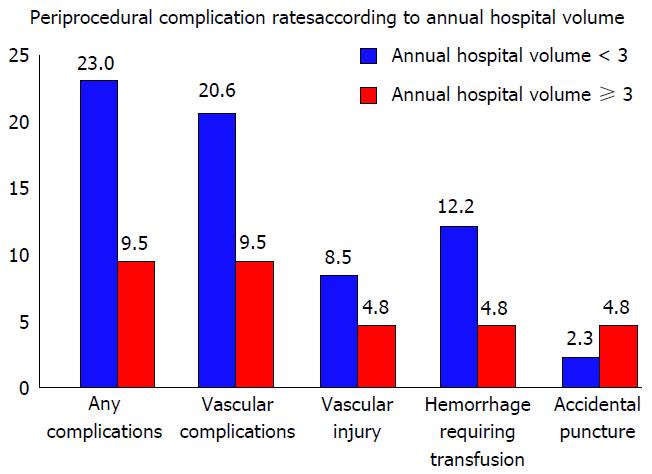
The use of stenting for adult aortic coarctation has been on the rise given the literature on favorable initial and intermediate outcomes. In a retrospective analysis, we observed significantly lower rate of post-procedural complications in hospitals performing more than 3 procedures annually (9.5% vs 23%, P = 0.002) including a lower rate of vascular complications (9.5% vs 20.6%) (Figure 5). Adjusted OR of post-procedural complications in hospitals with annual volume of 3 or more procedures was 0.40 (0.19-0.82, P = 0.013). These were further complemented by lower hospitalization costs at higher volume hospitals.
A volume based referral strategy is not without its limitations. This could restrict the entry of newer hospitals in a highly competitive medical field, which might actually provide contractual leverage to bigger hospitals with potential cost inflation. A procedure-based strategy always has the danger of leading to inappropriate procedures by operators and institutions. Again, in order to keep up higher volumes, many institutions may forego quality improvement activities. Besides, low volume centers play an integral role in healthcare by catering to smaller communities especially in rural areas and in pre-tertiary care. The benefits of selective referral to high volume centers thus must be weighed against a potential lack of access to healthcare resulting from regionalization. However, most of the emerging structural heart disease interventions are elective procedures that could justify transfer to higher volume centers.
Some authors have also suggested the role of operator volume and experience in contributing towards effect of institutional volume on outcomes. Indeed, some studies studying outcomes of surgical procedures have shown that the institutional-volume relationship might be non-significant once operator volume is accounted for. Nonetheless, other studies have also demonstrated persistent hospital volume outcomes relationship even after adjusting for operator volume. Additionally, hospital complexity in terms of range of services and technological provided can be responsible for improved outcomes as shown in a prior study by McCrum et al[20] and thus findings of retrospective studies should be interpreted with caution. But in the absence of detailed information on the quality of surgical procedures at a particular hospital, high hospital volume remains a valid contributor in reducing surgical mortality[21].
Hospital volume cannot be used as a sole quality metric since many low volume centers are known to provide safe and efficient healthcare. It is important to appraise the factors that result in superior outcomes in a subset of low-volume hospitals and further develop programs that allow other hospitals to adopt such practices. Development of newer structural heart programs with their multidisciplinary heart valve teams have lead to improved outcomes of surgical procedures in these hospital irrespective of annual procedural volume. This was demonstrated in recent analysis of improved outcomes of surgical aortic and mitral valve replacement in TAVR and TMVR capable centers respectively (abstract presented as poster presentation at SCAI 2015 Scientific Sessions, San Diego, CA). Risk-adjusted mortality rates, complication and readmission rates when considered together are some of the other important factors that can be used to assess quality of healthcare provided by different hospitals.
It has been previously demonstrated that a high coronary intervention volume does not translate into superior structural heart disease interventions outcomes[22]. Moreover, the procedural volume requirements are difficult to apply to structural disease interventions since these complex, highly specialized interventions are performed in much lower numbers. This further lends support for amendments in training requirements with a focus on procedure specific training with variable proctoring and use of simulators, in depth knowledge of the field besides annual volume recommendations. Additionally, a standardized process of certification and maintenance based on outcomes needs to be developed[20]. A plausible option is the evolution of umbrella training wherein trainees could have the opportunity to rotate through different hospitals and gain knowledge about best clinical practices.
Hospital volume is indeed a genuine predictor of post-procedural outcomes. This is important in the current era of expanding structural heart disease interventions, which are relatively complex with evolving technology and a steep learning curve. However, other quality metrics should also be accounted for in order to avoid labeling any good low-volume hospitals as underperformers. Further studies are mandatory to study the volume-outcome relationship for multiple emerging structural interventions since current data on many such interventions is either extrapolated from other procedures or based on consensus rather than evidence.
P- Reviewer: Bugiardini R, Caceres-Loriga F, Tang JM, Teragawa H S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Singh V, Badheka AO, Patel NJ, Chothani A, Mehta K, Arora S, Patel N, Deshmukh A, Shah N, Savani GT. Influence of hospital volume on outcomes of percutaneous atrial septal defect and patent foramen ovale closure: a 10-years US perspective. Catheter Cardiovasc Interv. 2015;85:1073-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 2. | Badheka AO, Chothani A, Mehta K, Patel NJ, Deshmukh A, Hoosien M, Shah N, Singh V, Grover P, Savani GT. Utilization and adverse outcomes of percutaneous left atrial appendage closure for stroke prevention in atrial fibrillation in the United States: influence of hospital volume. Circ Arrhythm Electrophysiol. 2015;8:42-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 3. | Luft HS, Bunker JP, Enthoven AC. Should operations be regionalized? The empirical relation between surgical volume and mortality. N Engl J Med. 1979;301:1364-1369. [PubMed] |
| 4. | Holt PJ, Poloniecki JD, Gerrard D, Loftus IM, Thompson MM. Meta-analysis and systematic review of the relationship between volume and outcome in abdominal aortic aneurysm surgery. Br J Surg. 2007;94:395-403. [PubMed] |
| 5. | Rathore SS, Epstein AJ, Volpp KG, Krumholz HM. Hospital coronary artery bypass graft surgery volume and patient mortality, 1998-2000. Ann Surg. 2004;239:110-117. [PubMed] |
| 6. | Sollano JA, Gelijns AC, Moskowitz AJ, Heitjan DF, Cullinane S, Saha T, Chen JM, Roohan PJ, Reemtsma K, Shields EP. Volume-outcome relationships in cardiovascular operations: New York State, 1990-1995. J Thorac Cardiovasc Surg. 1999;117:419-428; discussion 428-430. [PubMed] |
| 7. | Wen HC, Tang CH, Lin HC, Tsai CS, Chen CS, Li CY. Association between surgeon and hospital volume in coronary artery bypass graft surgery outcomes: a population-based study. Ann Thorac Surg. 2006;81:835-842. [PubMed] |
| 8. | Wang L. The volume-outcome relationship: busier hospitals are indeed better, but why? J Natl Cancer Inst. 2003;95:700-702. [PubMed] |
| 9. | Pennsylvania Health Care Cost Containment Council. Is hospital volume the way to measure quality outcomes? PHC4 FYI. 2002;1-3. [PubMed] |
| 10. | Tommaso CL, Bolman RM, Feldman T, Bavaria J, Acker MA, Aldea G, Cameron DE, Dean LS, Fullerton D, Hijazi ZM. Multisociety (AATS, ACCF, SCAI, and STS) expert consensus statement: operator and institutional requirements for transcatheter valve repair and replacement, part 1: transcatheter aortic valve replacement. J Thorac Cardiovasc Surg. 2012;143:1254-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Patel HJ, Herbert MA, Drake DH, Hanson EC, Theurer PF, Bell GF, Prager RL. Aortic valve replacement: using a statewide cardiac surgical database identifies a procedural volume hinge point. Ann Thorac Surg. 2013;96:1560-1565; discussion 1565-1566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 12. | Badheka AO, Patel NJ, Panaich SS, Patel SV, Jhamnani S, Singh V, Pant S, Patel N, Patel N, Arora S. Effect of Hospital Volume on Outcomes of Transcatheter Aortic Valve Implantation. Am J Cardiol. 2015;116:587-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 13. | Gammie JS, O’Brien SM, Griffith BP, Ferguson TB, Peterson ED. Influence of hospital procedural volume on care process and mortality for patients undergoing elective surgery for mitral regurgitation. Circulation. 2007;115:881-887. [PubMed] |
| 14. | Harold JG, Bass TA, Bashore TM, Brindis RG, Brush JE, Burke JA, Dehmer GJ, Deychak YA, Jneid H, Jollis JG. ACCF/AHA/SCAI 2013 update of the clinical competence statement on coronary artery interventional procedures: a report of the American College of Cardiology Foundation/American Heart Association/American College of Physicians Task Force on Clinical Competence and Training (writing committee to revise the 2007 clinical competence statement on cardiac interventional procedures). Circulation. 2013;128:436-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 15. | Panaich SS, Badheka AO, Chothani A, Mehta K, Patel NJ, Deshmukh A, Singh V, Savani GT, Arora S, Patel N. Results of ventricular septal myectomy and hypertrophic cardiomyopathy (from Nationwide Inpatient Sample [1998-2010]). Am J Cardiol. 2014;114:1390-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 109] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 16. | Aslam F, Iliadis AE, Blankenship JC. Percutaneous closure of patent foramen ovale: success and outcomes of a low-volume procedure at a rural medical center. J Invasive Cardiol. 2007;19:20-24. [PubMed] |
| 17. | Meier B. Closure of patent foramen ovale: technique, pitfalls, complications, and follow up. Heart. 2005;91:444-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 91] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 18. | Opotowsky AR, Landzberg MJ, Kimmel SE, Webb GD. Percutaneous closure of patent foramen ovale and atrial septal defect in adults: the impact of clinical variables and hospital procedure volume on in-hospital adverse events. Am Heart J. 2009;157:867-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Reddy VY, Holmes D, Doshi SK, Neuzil P, Kar S. Safety of percutaneous left atrial appendage closure: results from the Watchman Left Atrial Appendage System for Embolic Protection in Patients with AF (PROTECT AF) clinical trial and the Continued Access Registry. Circulation. 2011;123:417-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 630] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 20. | McCrum ML, Lipsitz SR, Berry WR, Jha AK, Gawande AA. Beyond volume: does hospital complexity matter?: an analysis of inpatient surgical mortality in the United States. Med Care. 2014;52:235-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Birkmeyer JD, Siewers AE, Finlayson EV, Stukel TA, Lucas FL, Batista I, Welch HG, Wennberg DE. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128-1137. [PubMed] |
| 22. | Ruiz CE, Feldman TE, Hijazi ZM, Holmes DR, Webb JG, Tuzcu EM, Herrmann H, Martin GR. Interventional fellowship in structural and congenital heart disease for adults. JACC Cardiovasc Interv. 2010;3:e1-e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |