Published online Mar 26, 2016. doi: 10.4330/wjc.v8.i3.258
Peer-review started: June 6, 2015
First decision: August 16, 2015
Revised: November 22, 2015
Accepted: December 17, 2015
Article in press: December 18, 2015
Published online: March 26, 2016
Processing time: 294 Days and 5.5 Hours
Alteration in breathing patterns characterized by cyclic variation of ventilation during rest and during exercise has been recognized in patients with advanced heart failure (HF) for nearly two centuries. Periodic breathing (PB) during exercise is known as exercise oscillatory ventilation (EOV) and is characterized by the periods of hyperpnea and hypopnea without interposed apnea. EOV is a non-invasive parameter detected during submaximal cardiopulmonary exercise testing. Presence of EOV during exercise in HF patients indicates significant impairment in resting and exercise hemodynamic parameters. EOV is also an independent risk factor for poor prognosis in HF patients both with reduced and preserved ejection fraction irrespective of other gas exchange variables. Circulatory delay, increased chemosensitivity, pulmonary congestion and increased ergoreflex signaling have been proposed as the mechanisms underlying the generation of EOV in HF patients. There is no proven treatment of EOV but its reversal has been noted with phosphodiesterase inhibitors, exercise training and acetazolamide in relatively small studies. In this review, we discuss the mechanistic basis of PB during exercise and the clinical implications of recognizing PB patterns in patients with HF.
Core tip: Alteration in breathing patterns in patients with advanced heart failure (HF) characterized by cyclic variation of ventilation with a period of approximately one minute is known as periodic breathing. Periodic breathing during exercise, known as exercise oscillatory ventilation (EOV), is an oscillatory ventilatory pattern during exercise that persists for at least 60% of the exercise test with an amplitude ≥ 15% of the average resting value. Circulatory delay, pulmonary congestion and chemoreceptor sensitivity has been proposed to cause generation of EOV. EOV is found to be an independent predictor of worse outcome irrespective of other gas exchange variables in HF patients.
- Citation: Dhakal BP, Lewis GD. Exercise oscillatory ventilation: Mechanisms and prognostic significance. World J Cardiol 2016; 8(3): 258-266
- URL: https://www.wjgnet.com/1949-8462/full/v8/i3/258.htm
- DOI: https://dx.doi.org/10.4330/wjc.v8.i3.258
Impaired cardiac filling or ejection of the blood are the cardinal features of heart failure (HF) which leads to multiple organ systems dysfunctions[1] with dyspnea on exertion and exercise intolerance being the most common. Alteration in breathing patterns with cyclic variation of breathing secondary to instability in respiratory control has been a recognized feature of HF for almost two centuries[2,3]. Cheyne[2] (1818) first described a severe form of disordered breathing during rest characterized by alternating hyperpnea and hypopnea with intervals of apnea lasting almost a minute in a patient with HF and similar case was described by Stokes[3] nearly three decades later (1854) after which the condition was named Cheyne-Stokes breathing.
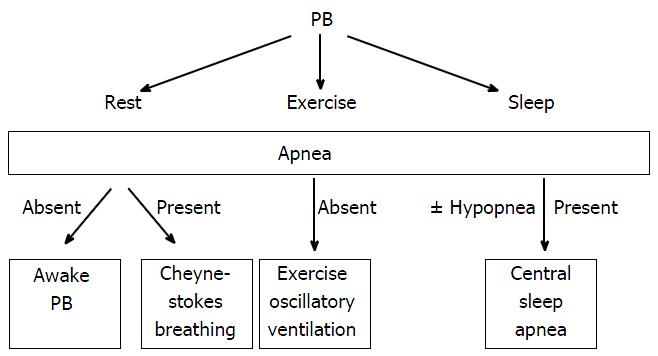
Periodic breathing (PB) characterized by cyclic variation of ventilation with or without interposed apnea have been observed at rest[4], during sleep[4-7] and during exercise[8-10] (Figure 1) in HF patients. Sleep disordered breathing such as obstructive sleep apnea (OSA) and central sleep apnea (CSA) has been observed in nearly 50% of stable HF patients[6] with CSA being significantly more prevalent (40%) than OSA. In one study, the presence of sleep disordered breathing at night was accurately predicted by concomitant daytime PB (AUC 0.821, P < 0.01 at receiver operating characteristic analysis, sensitivity 75%, specificity 75%)[4].
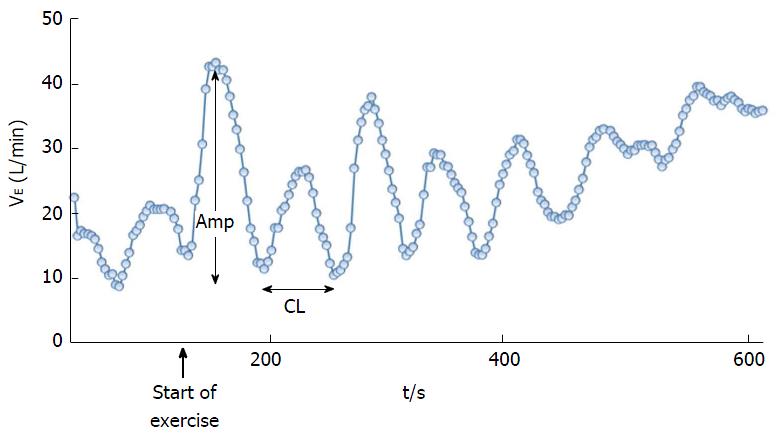
An unusual crescendo-decrescendo ventilatory response to exercise in patients with heart disease without resting Cheyne-Stokes breathing was initially reported by Weber[11] and further described by Kremser et al[12] in 1987. This phenomenon of periodic oscillatory breathing during exertion without interposed apnea is now known as exercise PB or exercise oscillatory ventilation (EOV) (Figure 2). EOV has recently been recognized in significant percentage of symptomatic HF patients, both with reduced[4,9,10,12-17] and preserved[18] left ventricular ejection fraction (LVEF). Despite the frequent occurrence of PB in patients with HF, pathophysiologic mechanisms that induce irregular breathing as well as the therapeutic modalities to reverse this condition in HF still remain incompletely understood. In this review, we focus specifically on EOV discerned in the context of measuring expired gas exchange variables during exercise through cardiopulmonary exercise testing.
Cardiopulmonary exercise testing (CPET) provides a unique opportunity to evaluate patient’s aerobic capacity with breath-by-breath expired gas parameters[19]. Besides providing information about patient’s functional capacity with peak oxygen uptake (VO2)[20], CPET is also helpful in delineating pulmonary vascular abnormalities in HF patients. Studies have shown that ventilatory efficiency (VE/VCO2 slope)[21,22] is even better predictor of HF outcomes than peak VO2. EOV on the other hand is discerned in HF patients during submaximal exercise which makes it a very attractive CPET parameter in those patients who are not able to complete maximal effort exercise testing.
Presence of EOV during CPET is identified by ventilatory oscillations with a typical cycle length and amplitude but there are a lot of variations on its definition[23]. Cycle length of an oscillation in VE is the time between nadirs of two ventilatory oscillations and the amplitude of oscillation is the difference between the peak VE during an oscillation and the nadirs in VE (Figure 2)[24]. Some of the definitions used for EOV are: (1) Kremser et al[12] and Corrà et al[10,13]: Oscillations in VE with a cycle length of approximately 1 min, amplitude > 15% of resting VE, and duration > 60% (> 66%[12]) of exercise duration; (2) Ben-Dov et al[25]: 3 or more consecutive regular oscillations in VE with oscillation amplitude > 25% of average VE and cycle length 30-60 s; (3) Leite et al[15]: Three or more cycles of regular oscillation in VE with standard deviation of 3 consecutive cycle lengths within 20% of the average and minimal average amplitude of oscillation > 5 L/min; and (4) Sun et al[24]: Three or more consecutive cyclic fluctuations in VE, amplitude > 30% of concurrent mean VE, oscillation of ≥ 3 gas exchange variables, cycle length of 40-140 s.
The American Heart Association consensus statement has defined EOV as an oscillatory ventilatory pattern that persists for at least 60% of the exercise test at amplitude 15% or more of the average resting value[19]. Due to the lack of automated measurement methods, presence of EOV during CPET is usually analyzed manually which may have lead to variations in its definitions and appropriate identification. More recently custom software has been used to identify EOV during exercise[26,27].
The prevalence of EOV has been different based on the severity and type of HF patient population studied. Patients with HF with reduced ejection fraction (HFrEF) has been found to have EOV prevalence of 12%-58%[8-10,12,13,15,16,18,24,28]. We found EOV prevalence of 45% in a subset of patients with HFrEF (n = 56, mean ± SD: LVEF = 30% ± 6%, peak VO2 = 12.4 ± 0.5 mL/kg per minute)[8]. EOV is similarly common in patients with HF and preserved ejection fraction (HFpEF)[18,29-31] with one previous study reported prevalence of 31%[18]. Olson et al[29] found that 41% of HF patients with EOV had LVEF ≥ 40%, and in the study by Matsuki et al[30] the mean LVEF in HF patients with EOV was 41.3 ± 16.3.
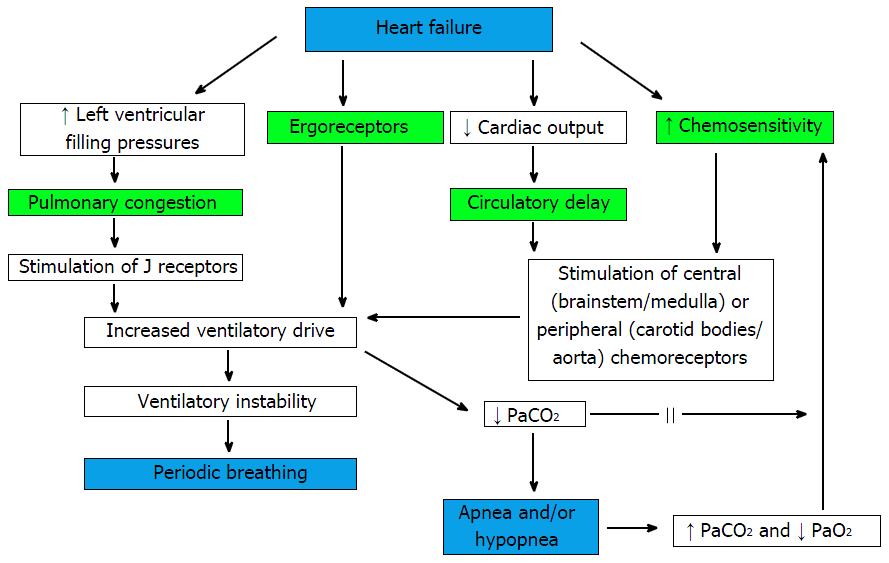
There is limited data regarding the mechanistic basis for EOV despite its significant association with poor outcomes in HF patients[32]. The control of the normal ventilation is through the feedback loop between pulmonary gas exchanging capillaries and peripheral chemoreceptors located in the carotid bodies and the central chemoreceptors located in the medulla (Figure 3)[33-37]. Any instability of this ventilatory regulation can lead to generation of oscillatory respiratory pattern. The generation of crescendo and decrescendo respiratory pattern can be caused by: (1) Circulatory delay (i.e., increased circulation time from the lung to the brain and chemoreceptors due to reduced cardiac index leading to delay in information transfer)[15,36,37]; (2) increase in controller gain (i.e., increased central and peripheral chemoreceptor sensitivity to PaCO2 and PaO2)[14,35,38]; or (3) reduction in system damping (i.e., baroreflex impairment) (Figure 3). The possible mechanisms responsible for generation of PB during exercise (i.e., EOV) have largely been extrapolated from studies of PB at rest[39] and during sleep[15,40] even though there has been limited overlap between PB during exercise and during sleep[13].
Circulatory delay: Reduced cardiac output in patients with HF increases the circulation time from lungs to chemoreceptors and respiratory centers. This delayed transfer of information has been postulated to generate late feedback signals leading to oscillations in ventilation[41]. Hypotension and circulatory delay has been shown to induce cardiorespiratory oscillations in experimental rat models[42]. Similarly reduced resting CI and prolonged lung-to ear circulation time (LECT) were the major determinants of PB at rest in HF patients in one previous study[43]. LVEF has also been noted to be significantly lower in HF with PB compared to those without PB[44]. Delayed generation of respiratory and pulmonary blood flow oscillations during exercise compared to LVEF fluctuations in HF patients also supports delayed circulation causing alterations in respiratory feedback mechanisms[45].
In a study of 56 HFrEF patients, those with EOV demonstrated a greater degree of hemodynamic impairment both at rest and during exercise and had 25% lower cumulative CI compared to HF patients without EOV[8]. The amplitude and duration of oscillations were inversely related to exercise CI, and the changes in cycle length and amplitude of EOV after 12 wk of treatment with sildenafil were inversely related to changes in CI[8]. In another small study (n = 17, age 68 ± 12 years), patients with advanced HF, as reflected by a lower peak VO2 and higher VE/VCO2 slope, had a longer cycle length of ventilatory oscillations and a longer phase difference between oscillating VO2 and VE[46]. Attenuation of EOV during high-intensity exercise could be due to increased CI during exercise leading to reduced circulation time which supports circulatory delay as an important determining factor for the generation of EOV. However, some investigators have argued against contribution of circulatory delay to EOV but did not directly measure cardiac output or circulation time[45].
Increased chemosensitivity: Increased carotid and aortic chemoreceptor sensitivity to minimal changes in arterial O2 and CO2 may contribute to sympathetic overactivity which leads to excessive and irregular ventilation during exercise[47]. Enhanced hypoxic and central hypercapneic chemosensitivity may cause increased ventilatory response (VE/VCO2) to exercise in HF patients[48]. Such chronically increased ventilation causes reduction in arterial concentration of both CO2 and bicarbonate[49] which weakens the blood’s ability to buffer against changes in CO2 levels leading to overly sensitive ventilatory control system. Pitt et al[50] in 1907 observed that a modest increase in partial pressure of CO2 triggers a cycle of hyperventilation-induced reduction in PaCO2 until the apnea threshold is reached leading to Cheyne-Stokes breathing. In a quantitative algebraic analysis of the dynamic cardiorespiratory physiology, circulatory delay and increased chemoreflex gain were found to be the primary factors causing EOV[47]. In both experimental cat models and stable HF patients, inhalation of 100% O2 decreased the peripheral chemoreceptor discharge and thus oscillatory ventilation[34,42]. Steens et al[51] noticed that inhalation of 3% CO2 virtually eradicated Cheyne Stokes Respiration in HFrEF patients with stable NYHA class III-IV symptoms. Similarly dihydrocodeine attenuated PB by reducing chemosensitivity in 42% of HF patients[34].
Despite the proposed mechanism of increased peripheral chemoreceptor sensitivity causing EOV, there may be other non-peripheral chemoreceptor mediated mechanisms involved in mediating increased ventilatory response to exercise[52]. In one study of HFrEF patients, arterial blood gases (PaCO2 and PaO2) at rest and average values across the first 6 min of exercise in HF patients had no relationship with EOV[8]. The amplitude and duration of EOV was also not related to mean PaCO2 which argues against a PaCO2 set point close to the apnea threshold, serving as a major determinant of the presence of EOV in HF patients[8].
Pulmonary congestion: Pulmonary congestion[53] and decreased lung compliance[54] has been postulated to cause overstimulation of the ventilatory control center which leads to hyperventilation and decrease in PCO2[55] and thus generating PB. Elevated pulmonary capillary wedge pressure, a surrogate marker for pulmonary congestion, stretches pulmonary C fibers (J receptors)[56] which in turn stimulates the medullary respiratory center via vagal afferents[57], leading to rapid shallow breathing, hypocapnia, and initiation of PB at rest. The damping effects of O2 and CO2 stores which prevent oscillations are also reduced by pulmonary congestion and a small fluctuation in CO2 level makes the respiratory control unstable in HF patients with pulmonary congestion[37]. In 1943, Christie et al[58] were able to induce PB due to pulmonary congestion by occluding a pulmonary vein. Recent findings of increased resting and exercise cardiac filling pressures[8,30] and higher NT-proBNP[30] levels in HF patients with EOV compared to those without EOV extends their findings. Despite these findings suggestive of role of pulmonary congestion as the etiology for EOV, this mechanism has been questioned by some investigators[45] which noticed disappearance of EOV during later exercise in HF patients despite an increase in PCWP.
Ergoreflex signaling: HF causes metabolic and structural abnormalities in the skeletal muscles which may also lead to enhanced ergoreflex signaling during exercise which has been postulated as an etiologic factor for generation of PB. Increased ergoreflex may be associated with worse NYHA class, decreased exercise tolerance, and hyperventilation during exercise in HF patients[59-61]. In a study by Pardaens et al[62], ergoreflex activity contributed to hyperventilation in HF patients with a history of recent decompensation or persistent symptoms. Oscillations in output of neurologic stimuli from the medullary vasomotor center may explain disappearance of respiratory oscillations found at rest or at low levels of exercise during more intense exercise[43]. Decreased activation of both CO2 chemoreflex and the ergoreflex has recently been shown to decrease ventilatory drive after cardiac resynchronization therapy[63]. Despite the proposed contribution of ergoreceptors to the autonomic, hemodynamic, and respiratory responses to exercise in HF patients, further investigation is needed to establish its relationship to hyperventilation and EOV in HF patients.
It has been well known that the prevalence of EOV tracks with the metrics of HF severity such as higher NYHA class, lower peak VO2, higher VE/VCO2 slopes and lower PETCO2[8,12,13,15,16,24,28-30,64-69] (Table 1). EOV actually provides strong independent prognostic information regarding the severity of HF even after adjustment for these variables. The initial study describing the prognostic significance of PB by Ponikowski et al[34] predicted poor 2-year survival in HF patients with abnormal breathing patterns which was independent of peak VO2 and NYHA class. Similarly Bard et al[17] also observed resting ventilatory variation to be the best predictor of mortality in 44 matched HFrEF patients. Leite et al[15] and Corrà et al[10,13] both found that HF patients with EOV had 3-fold higher mortality compared to those without EOV (Table 1). When EOV is present along with other abnormal ventilatory patterns either during sleep or during exercise, the risk of mortality increases even further as those observed by Corrà et al[13] in a group of HF patients who had abnormal breathing patterns during sleep and EOV during exercise (54% adverse events in patients with EOV and apnea hypopnea index > 30/h vs 17% with EOV alone, OR = 6.65, 95%CI: 2.6-17.1, P < 0.01). Similarly the odds of dying in 6 mo increased by 4-fold (9.4 to 38.9) when EOV was present along with elevated VE/VCO2 slope in another group of HF patients[24]. EOV is not only known to be the independent predictor of overall mortality and sudden cardiac death in HFrEF patients but also the strongest predictor of mortality in HFpEF patients in multivariate models[9]. Ingle et al[28] observed EOV to be the predictor of mortality independent of peak VO2, VE/VCO2 slope, LVEF, age, and 6-min walking distance. EOV has recently been recognized as a potent prognostic indicator in patients with congenital heart disease as EOV along with the percentage of maximum predicted HR were independent predictors of the combined outcome of death, transplantation or cardiovascular hospitalization in patients who underwent Fontan procedure[27].
| Ref. | No. of patients | NYHA class, LVEF | Prevalence of PB | Clinical and prognostic significance of EOV | Significant mortality predictors |
| Corrà et al[10], (2002) | 323 | NYHA 2.2 ± 0.9 LVEF 24 ± 8 | 12% | EOV present in 28% of nonsurvivors vs 9% survivors, follow-up period 22 ± 11 mo | NYHA class, LVEF, peak VO2 |
| Leite et al[15], (2003) | 84 | NYHA 2-4 LVEF 35 ± 7 | 30% | EOV independently increased the risk of death by 2.97 fold, median follow-up period of 11.3 mo | Peak VO2, NYHA class, VE/VCO2 slope |
| Corrà et al[13], (2006) | 133 | NYHA 2.3 ± 0.7 LVEF 23 ± 7 | 21% | 42% mortality in EOV patients vs 15% in non EOV, follow-up period 39 ± 11 mo | NYHA class, peak VO2, VE/VCO2 slope, AHI, LVEF, lower rate of beta blocker use, peak HR |
| Guazzi et al[9], (2007) | 156 | NYHA 1-4 LVEF 35 ± 11 | 33% | EOV was the strongest predictor of overall and SCD mortality. EOV present in 100% arrhythmic and 47% nonarrhythmic deaths, follow-up period 28 ± 25 mo | LV mass, LVESV. VE/VCO2 slope maintained a predictive value as to overall cardiac mortality and pump failure death outperforming EOV as predictor of pump failure mortality |
| Guazzi et al[18], (2008) | 556 (405 HFrEF, 151 HFpEF) | NYHA 2.4 ± 0.8 in HFrEF, 2.0 ± 0.9 in HFpEF | 35% in HFrEF, 31% in HFpEF | EOV was strongest predictor of mortality in HFpEF compared to HFrEF in multivariate models; EOV was similar predictor of mortality in both HFrEF and HFpEF without LVAD or transplant | VE/VCO2 slope in multivariate model, peak VO2 in univariate model |
| Arena et al[16], (2008) | 154 | NYHA 2.2 LVEF 30 ± 14 | 36% | Event (death, transplant or LVAD) free survival 55% in EOV vs 82% in non EOV patients, follow-up period 3 yr | VE/VCO2 slope, LVEF |
| Bard et al[17], (2008) | 44 | LVEF 19 ± 7 | 13% | Death or transplant rate 68% in patients with PB vs 52% without PB | Resting ventilatory variation more powerful predictor of mortality than peak VO2 and VE/VCO2 slope |
| Olson et al[29], (2008) | 47 | NYHA 2.6 ± 0.8 LVEF 37 ± 17 | 7% | EOV associated with higher VE/VCO2 slope, VD/VT, lower PETCO2, higher NYHA class | |
| Ingle et al[28], (2009) | 240 | LVEF 34 ± 6 | 31% by Leite and 25% by Corrá Criteria | 50% of patients diagnosed with EOV by Corrá criteria and 58% diagnosed by Leite criteria died within 1 yr | |
| Sun et al[24], (2010) | 580 | NYHA 2-4 LVEF 26 ± 7 | 51% | EOV combined with elevated VE/VCO2 (≥155% predicted) resulted in an OR of 39 for 6 mo mortality | Peak VO2, AT, peak oxygen pulse significantly worse in nonsurvivors |
| Ueshima et al[68], (2010) | 50 | NYHA 1-3 | 28% | EOV associated with lower peak VO2 and higher VD/VT | |
| Murphy et al[8], (2011) | 56 | NYHA 2-4 LVEF 30 ± 6 | 45% | EOV related to ↓exercise cardiac output and ↑cardiac filling pressures | |
| Scardovi et al[31], (2012) | 370 | NYHA 1-3 LVEF 41% (range 34%-50%) | 58% | EOV, VE/VCO2 slope and its ratio to peak VO2 predicted all-cause mortality independent of LVEF | Hemoglobin level, creatinine, BMI, HF admissions in the previous year |
| Matsuki et al[30], 2013 | 46 | NYHA 3 LVEF 41 ± 16 | 44% | EOV patients had ↑cardiac filling pressures, higher NT-proBNP value, ↑VE/VCO2 slope, low PETCO2 and greater Borg dyspnea score | |
| Nathan et al[27], (2015) | 253 | NYHA 1-3 | 38% | 5 yr rate of death or transplant 14.1% in Fontan patients with EOV vs 4.1% of those without EOV | NYHA class, peak HR |
The superior prognostic value of EOV and VE/VCO2 slope compared to peak VO2 has been observed in multiple studies examining the relative predictive values of various CPET variables (Table 1). EOV along with other CPET derived variables (VE/VCO2 slope, oxygen uptake efficiency slope and ventilatory equivalent for CO2 nadir) has been shown to outperform the traditional Heart Failure Survival Score in predicting outcomes in patients with mild-to-moderate HF[70]. Guazzi et al[71] recently characterized EOV in patients with broader cardiovascular risk factors and found the EOV to be an indicator of worse CV risk factor profile in patients even without clinical manifestations of HF. The feasibility of EOV measurements during submaximal exercise during CPET makes it particularly attractive in HF population who are unable to do maximum effort exercise testing.
Various pharmacological or surgical interventions has been performed in HF patients to identify the potential reversibility of EOV but there has not been any large scale clinical trial with EOV as the primary endpoint. In a small randomized double-blind placebo controlled trial of HFrEF patients, serial assessment of EOV before and after 12 wk of sildenafil treatment showed reduction in EOV cycle length and oscillatory amplitude and increase in exercise CI in the sildenafil group compared to placebo[8]. The changes in oscillatory cycle length and amplitude after sildenafil treatment were inversely related to changes in exercise CI[8]. This finding was further supported by another study from Guazzi et al[18] who noted resolution of EOV in the majority of patients treated with sildenafil, although EOV was not a pre-specified endpoint in these trials with small number of study subjects (n < 40).
Attenuation of PB has been observed with valvular[72] and open heart surgeries, and cardiac transplantation[73]. There are few other studies involving small number of patients that showed resolution of EOV with different therapeutic interventions. For example, Ribeiro et al[74] noticed reduction in EOV with phosphodiesterase-3 inhibitor milrinone in three patients and Castro et al[75] reported reversal of EOV and improvement in NYHA class with exercise training in one HF patient despite no change in LVEF. Reversal of EOV in 71% of stable HFrEF patients has also been observed after 3 mo of outpatient exercise training program[76]. This highlights the importance of exercise therapy in both HFrEF and HFpEF patients. Recent studies have shown that inhalation of CO2[77] and acetazolamide[77,78] treatment significantly reduced PB during exercise in HF patients. Kazimierczak et al[67] noticed reversal of EOV in more than 85% of the HF patients after three months of nocturnal adaptive servoventilation even though it was a very small study (n = 8). Finally, in an experimental study of pacing induced-CHF rabbit models, carotid body chemoreceptor denervation reduced disordered breathing patterns[79].
EOV is a significant prognostic indicator of adverse outcomes in HF patients. EOV identification at submaximal levels of exercise during CPET and the possibility of EOV reversal with HF interventions makes it a potential surrogate end point of interest for HF clinical trials focused on improvement in gas exchange variables and exercise hemodynamics. There is still a need for HF studies with specific EOV endpoint to identify whether HF interventions such as diuretic therapy, exercise training, phosphodiesterase inhibitors, cardiac resynchronization therapy, intensification of neurohormonal blockade, cardiac surgery or other emerging therapies such as neprilysin inhibitors will successfully attenuate EOV, and if that modification translates into improvement in underlying cardiac dysfunction and clinical outcome of HF patients.
EOV is a noninvasive and reproducible exercise para-meter which is easily recognizable during submaximal cardiopulmonary exercise testing. EOV has been proven to be a strong predictor of reduced survival in HF patients irrespective of the echocardiographic and gas exchange variables. Presence of EOV in a HF patient indicates significant impairment in resting and exercise cardiac hemodynamic parameters, especially when the cycle length of EOV is longer than one minute and when EOV occurs early during exercise. HF patients presenting with EOV may therefore need an intensification of therapy to optimize cardiac hemodynamics, and improve overall symptoms and functional capacity.
P- Reviewer: den Uil CA, Falconi M S- Editor: Gong ZM L- Editor: A E- Editor: Li D
| 1. | Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147-e239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4116] [Cited by in RCA: 4654] [Article Influence: 387.8] [Reference Citation Analysis (1)] |
| 2. | Cheyne J. A case of apoplexy in which the fleshy part of the heart was converted in fat. Dublin Hosp Rep 1818; 2: 216-219. . |
| 3. | Stokes W. The Disease of the Heart and Aorta. Dublin: Hodges and Smith, 1854. . |
| 4. | Poletti R, Passino C, Giannoni A, Zyw L, Prontera C, Bramanti F, Clerico A, Piepoli M, Emdin M. Risk factors and prognostic value of daytime Cheyne-Stokes respiration in chronic heart failure patients. Int J Cardiol. 2009;137:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Sin DD, Fitzgerald F, Parker JD, Newton G, Floras JS, Bradley TD. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med. 1999;160:1101-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 769] [Cited by in RCA: 739] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 6. | Javaheri S, Parker TJ, Liming JD, Corbett WS, Nishiyama H, Wexler L, Roselle GA. Sleep apnea in 81 ambulatory male patients with stable heart failure. Types and their prevalences, consequences, and presentations. Circulation. 1998;97:2154-2159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 777] [Cited by in RCA: 746] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 7. | Bradley TD, Floras JS. Sleep apnea and heart failure: Part I: obstructive sleep apnea. Circulation. 2003;107:1671-1678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 363] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 8. | Murphy RM, Shah RV, Malhotra R, Pappagianopoulos PP, Hough SS, Systrom DM, Semigran MJ, Lewis GD. Exercise oscillatory ventilation in systolic heart failure: an indicator of impaired hemodynamic response to exercise. Circulation. 2011;124:1442-1451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 9. | Guazzi M, Raimondo R, Vicenzi M, Arena R, Proserpio C, Sarzi Braga S, Pedretti R. Exercise oscillatory ventilation may predict sudden cardiac death in heart failure patients. J Am Coll Cardiol. 2007;50:299-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 97] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 10. | Corrà U, Giordano A, Bosimini E, Mezzani A, Piepoli M, Coats AJ, Giannuzzi P. Oscillatory ventilation during exercise in patients with chronic heart failure: clinical correlates and prognostic implications. Chest. 2002;121:1572-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 136] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 11. | Weber KT. Cardiopulmonary exercise testing: physiologic principles and clinical applications. 1986. . |
| 12. | Kremser CB, O’Toole MF, Leff AR. Oscillatory hyperventilation in severe congestive heart failure secondary to idiopathic dilated cardiomyopathy or to ischemic cardiomyopathy. Am J Cardiol. 1987;59:900-905. [RCA] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 79] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Corrà U, Pistono M, Mezzani A, Braghiroli A, Giordano A, Lanfranchi P, Bosimini E, Gnemmi M, Giannuzzi P. Sleep and exertional periodic breathing in chronic heart failure: prognostic importance and interdependence. Circulation. 2006;113:44-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 186] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 14. | Ponikowski P, Chua TP, Anker SD, Francis DP, Doehner W, Banasiak W, Poole-Wilson PA, Piepoli MF, Coats AJ. Peripheral chemoreceptor hypersensitivity: an ominous sign in patients with chronic heart failure. Circulation. 2001;104:544-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 229] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 15. | Leite JJ, Mansur AJ, de Freitas HF, Chizola PR, Bocchi EA, Terra-Filho M, Neder JA, Lorenzi-Filho G. Periodic breathing during incremental exercise predicts mortality in patients with chronic heart failure evaluated for cardiac transplantation. J Am Coll Cardiol. 2003;41:2175-2181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 138] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 16. | Arena R, Myers J, Abella J, Peberdy MA, Pinkstaff S, Bensimhon D, Chase P, Guazzi M. Prognostic value of timing and duration characteristics of exercise oscillatory ventilation in patients with heart failure. J Heart Lung Transplant. 2008;27:341-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Bard RL, Gillespie BW, Patel H, Nicklas JM. Prognostic ability of resting periodic breathing and ventilatory variation in closely matched patients with heart failure. J Cardiopulm Rehabil Prev. 2008;28:318-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Guazzi M, Myers J, Peberdy MA, Bensimhon D, Chase P, Arena R. Exercise oscillatory breathing in diastolic heart failure: prevalence and prognostic insights. Eur Heart J. 2008;29:2751-2759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Balady GJ, Arena R, Sietsema K, Myers J, Coke L, Fletcher GF, Forman D, Franklin B, Guazzi M, Gulati M. Clinician’s Guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122:191-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1144] [Cited by in RCA: 1441] [Article Influence: 96.1] [Reference Citation Analysis (0)] |
| 20. | Arena R, Sietsema KE. Cardiopulmonary exercise testing in the clinical evaluation of patients with heart and lung disease. Circulation. 2011;123:668-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 157] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 21. | Arena R, Myers J, Aslam SS, Varughese EB, Peberdy MA. Peak VO2 and VE/VCO2 slope in patients with heart failure: a prognostic comparison. Am Heart J. 2004;147:354-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 386] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 22. | Lewis GD, Shah RV, Pappagianopolas PP, Systrom DM, Semigran MJ. Determinants of ventilatory efficiency in heart failure: the role of right ventricular performance and pulmonary vascular tone. Circ Heart Fail. 2008;1:227-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 128] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 23. | Cornelis J, Beckers P, Vanroy C, Volckaerts T, Vrints C, Vissers D. An overview of the applied definitions and diagnostic methods to assess exercise oscillatory ventilation--a systematic review. Int J Cardiol. 2015;190:161-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Sun XG, Hansen JE, Beshai JF, Wasserman K. Oscillatory breathing and exercise gas exchange abnormalities prognosticate early mortality and morbidity in heart failure. J Am Coll Cardiol. 2010;55:1814-1823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 110] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 25. | Ben-Dov I, Sietsema KE, Casaburi R, Wasserman K. Evidence that circulatory oscillations accompany ventilatory oscillations during exercise in patients with heart failure. Am Rev Respir Dis. 1992;145:776-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Olson TP, Johnson BD. Quantifying oscillatory ventilation during exercise in patients with heart failure. Respir Physiol Neurobiol. 2014;190:25-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Nathan AS, Loukas B, Moko L, Wu F, Rhodes J, Rathod RH, Systrom DM, Ubeda Tikkanen A, Shafer K, Lewis GD. Exercise oscillatory ventilation in patients with Fontan physiology. Circ Heart Fail. 2015;8:304-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Ingle L, Isted A, Witte KK, Cleland JG, Clark AL. Impact of different diagnostic criteria on the prevalence and prognostic significance of exertional oscillatory ventilation in patients with chronic heart failure. Eur J Cardiovasc Prev Rehabil. 2009;16:451-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Olson LJ, Arruda-Olson AM, Somers VK, Scott CG, Johnson BD. Exercise oscillatory ventilation: instability of breathing control associated with advanced heart failure. Chest. 2008;133:474-481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Matsuki R, Kisaka T, Ozono R, Kinoshita H, Sada Y, Oda N, Hidaka T, Tashiro N, Takahashi M, Sekikawa K. Characteristics of patients with severe heart failure exhibiting exercise oscillatory ventilation. Clin Exp Hypertens. 2013;35:267-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Scardovi AB, De Maria R, Ferraironi A, Gatto L, Celestini A, Forte S, Parolini M, Sciarretta S, Ricci R, Guazzi M. A case for assessment of oscillatory breathing during cardiopulmonary exercise test in risk stratification of elderly patients with chronic heart failure. Int J Cardiol. 2012;155:115-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 32. | Dhakal BP, Murphy RM, Lewis GD. Exercise oscillatory ventilation in heart failure. Trends Cardiovasc Med. 2012;22:185-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | Cherniack NS, Longobardo GS. Cheyne-Stokes breathing. An instability in physiologic control. N Engl J Med. 1973;288:952-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 129] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 34. | Ponikowski P, Anker SD, Chua TP, Francis D, Banasiak W, Poole-Wilson PA, Coats AJ, Piepoli M. Oscillatory breathing patterns during wakefulness in patients with chronic heart failure: clinical implications and role of augmented peripheral chemosensitivity. Circulation. 1999;100:2418-2424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 148] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 35. | Khoo MC, Kronauer RE, Strohl KP, Slutsky AS. Factors inducing periodic breathing in humans: a general model. J Appl Physiol Respir Environ Exerc Physiol. 1982;53:644-659. [PubMed] |
| 36. | Millar TW, Hanly PJ, Hunt B, Frais M, Kryger MH. The entrainment of low frequency breathing periodicity. Chest. 1990;98:1143-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 37. | Dowell AR, Buckley CE, Cohen R, Whalen RE, Sieker HO. Cheyne-Stokes respiration. A review of clinical manifestations and critique of physiological mechanisms. Arch Intern Med. 1971;127:712-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 38. | Javaheri S. A mechanism of central sleep apnea in patients with heart failure. N Engl J Med. 1999;341:949-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 320] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 39. | Andreas S, Hagenah G, Moller C, Werner GS, Kreuzer H. Cheyne-Stokes respiration and prognosis in congestive heart failure. Am J Cardiol. 1996;78:1260-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 99] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 40. | Naughton M, Benard D, Tam A, Rutherford R, Bradley TD. Role of hyperventilation in the pathogenesis of central sleep apneas in patients with congestive heart failure. Am Rev Respir Dis. 1993;148:330-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 263] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 41. | Mortara A, Sleight P, Pinna GD, Maestri R, Capomolla S, Febo O, La Rovere MT, Cobelli F. Association between hemodynamic impairment and Cheyne-Stokes respiration and periodic breathing in chronic stable congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1999;84:900-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 42. | Lahiri S, Hsiao C, Zhang R, Mokashi A, Nishino T. Peripheral chemoreceptors in respiratory oscillations. J Appl Physiol (1985). 1985;58:1901-1908. [PubMed] |
| 43. | Yajima T, Koike A, Sugimoto K, Miyahara Y, Marumo F, Hiroe M. Mechanism of periodic breathing in patients with cardiovascular disease. Chest. 1994;106:142-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 44. | Koike A, Shimizu N, Tajima A, Aizawa T, Fu LT, Watanabe H, Itoh H. Relation between oscillatory ventilation at rest before cardiopulmonary exercise testing and prognosis in patients with left ventricular dysfunction. Chest. 2003;123:372-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 45. | Agostoni P, Apostolo A, Albert RK. Mechanisms of periodic breathing during exercise in patients with chronic heart failure. Chest. 2008;133:197-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 46. | Kato J, Koike A, Hoshimoto-Iwamoto M, Nagayama O, Sakurada K, Sato A, Yamashita T, Wasserman K, Aonuma K. Relation between oscillatory breathing and cardiopulmonary function during exercise in cardiac patients. Circ J. 2013;77:661-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 47. | Francis DP, Willson K, Davies LC, Coats AJ, Piepoli M. Quantitative general theory for periodic breathing in chronic heart failure and its clinical implications. Circulation. 2000;102:2214-2221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 135] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 48. | Chua TP, Clark AL, Amadi AA, Coats AJ. Relation between chemosensitivity and the ventilatory response to exercise in chronic heart failure. J Am Coll Cardiol. 1996;27:650-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 197] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 49. | Hanly P, Zuberi N, Gray R. Pathogenesis of Cheyne-Stokes respiration in patients with congestive heart failure. Relationship to arterial PCO2. Chest. 1993;104:1079-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 111] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 50. | Pitt GN, Pembrey MS, Allen RW. Observations upon Cheyne-Stokes’ Respiration. Med Chir Trans. 1907;90:49-82.15. [PubMed] |
| 51. | Steens RD, Millar TW, Su X, Biberdorf D, Buckle P, Ahmed M, Kryger MH. Effect of inhaled 3% CO2 on Cheyne-Stokes respiration in congestive heart failure. Sleep. 1994;17:61-68. [PubMed] |
| 52. | Chua TP, Ponikowski PP, Harrington D, Chambers J, Coats AJ. Contribution of peripheral chemoreceptors to ventilation and the effects of their suppression on exercise tolerance in chronic heart failure. Heart. 1996;76:483-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 59] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 53. | Solin P, Bergin P, Richardson M, Kaye DM, Walters EH, Naughton MT. Influence of pulmonary capillary wedge pressure on central apnea in heart failure. Circulation. 1999;99:1574-1579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 304] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 54. | Agostoni P, Pellegrino R, Conca C, Rodarte JR, Brusasco V. Exercise hyperpnea in chronic heart failure: relationships to lung stiffness and expiratory flow limitation. J Appl Physiol (1985). 2002;92:1409-1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 55. | Lorenzi-Filho G, Azevedo ER, Parker JD, Bradley TD. Relationship of carbon dioxide tension in arterial blood to pulmonary wedge pressure in heart failure. Eur Respir J. 2002;19:37-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 102] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 56. | Paintal AS. Mechanism of stimulation of type J pulmonary receptors. J Physiol. 1969;203:511-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 354] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 57. | Roberts AM, Bhattacharya J, Schultz HD, Coleridge HM, Coleridge JC. Stimulation of pulmonary vagal afferent C-fibers by lung edema in dogs. Circ Res. 1986;58:512-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 99] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 58. | Christie RV, Hayward GW. Periodic changes in respiratory depth, produced by changes in the lung. J Physiol. 1943;102:88-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 59. | Olson TP, Joyner MJ, Johnson BD. Influence of locomotor muscle metaboreceptor stimulation on the ventilatory response to exercise in heart failure. Circ Heart Fail. 2010;3:212-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 60. | Scott AC, Davies LC, Coats AJ, Piepoli M. Relationship of skeletal muscle metaboreceptors in the upper and lower limbs with the respiratory control in patients with heart failure. Clin Sci (Lond). 2002;102:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 61. | Ponikowski PP, Chua TP, Francis DP, Capucci A, Coats AJ, Piepoli MF. Muscle ergoreceptor overactivity reflects deterioration in clinical status and cardiorespiratory reflex control in chronic heart failure. Circulation. 2001;104:2324-2330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 168] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 62. | Pardaens S, Vanderheyden M, Calders P, Willems AM, Bartunek J, de Sutter J. Activation of the ergoreceptors in cardiac patients with and without heart failure. J Card Fail. 2014;20:747-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 63. | Cundrle I, Johnson BD, Rea RF, Scott CG, Somers VK, Olson LJ. Modulation of ventilatory reflex control by cardiac resynchronization therapy. J Card Fail. 2015;21:367-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 64. | Kitzman DW, Brubaker PH, Morgan TM, Stewart KP, Little WC. Exercise training in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single-blind trial. Circ Heart Fail. 2010;3:659-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 302] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 65. | Guazzi M, Arena R, Ascione A, Piepoli M, Guazzi MD. Exercise oscillatory breathing and increased ventilation to carbon dioxide production slope in heart failure: an unfavorable combination with high prognostic value. Am Heart J. 2007;153:859-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 66. | Wang JS, Fu TC, Wang CH, Chou SL, Liu MH, Cherng WJ. Exertional periodic breathing potentiates erythrocyte rheological dysfunction by elevating pro-inflammatory status in patients with anemic heart failure. Int J Cardiol. 2013;167:1289-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 67. | Kazimierczak A, Krzyżanowski K, Wierzbowski R, Ryczek R, Smurzyński P, Michałkiewicz D, Orski Z, Gielerak G. Resolution of exercise oscillatory ventilation with adaptive servoventilation in patients with chronic heart failure and Cheyne-Stokes respiration: preliminary study. Kardiol Pol. 2011;69:1266-1271. [PubMed] |
| 68. | Ueshima K, Kobayashi N, Yamazaki T, Saitoh M, Nakamura M, Nakao K. Clinical significance of awake oscillatory ventilation in patients with heart failure and effects of open-heart surgery. Clin Cardiol. 2010;33:E20-E23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 69. | Schmid JP, Apostolo A, Antonioli L, Cattadori G, Zurek M, Contini M, Agostoni P. Influence of exertional oscillatory ventilation on exercise performance in heart failure. Eur J Cardiovasc Prev Rehabil. 2008;15:688-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 70. | Ingle L, Rigby AS, Sloan R, Carroll S, Goode KM, Cleland JG, Clark AL. Development of a composite model derived from cardiopulmonary exercise tests to predict mortality risk in patients with mild-to-moderate heart failure. Heart. 2014;100:781-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 71. | Guazzi M, Arena R, Pellegrino M, Bandera F, Generati G, Labate V, Alfonzetti E, Villani S, Gaeta MM, Halle M. Prevalence and characterization of exercise oscillatory ventilation in apparently healthy individuals at variable risk for cardiovascular disease: A subanalysis of the EURO-EX trial. Eur J Prev Cardiol. 2016;23:328-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 72. | Tomcsányi J, Karlócai K, Papp L. Disappearance of periodic breathing after heart operations. J Thorac Cardiovasc Surg. 1994;107:317-318. [PubMed] |
| 73. | Murdock DK, Lawless CE, Loeb HS, Scanlon PJ, Pifarré R. The effect of heart transplantation on Cheyne-Stokes respiration associated with congestive heart failure. J Heart Transplant. 1986;5:336-337. [PubMed] |
| 74. | Ribeiro JP, Knutzen A, Rocco MB, Hartley LH, Colucci WS. Periodic breathing during exercise in severe heart failure. Reversal with milrinone or cardiac transplantation. Chest. 1987;92:555-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 75. | Castro RR, Antunes-Correa LM, Ueno LM, Rondon MU, Negrão CE, Nóbrega AC. Reversal of periodic breathing after aerobic training in heart failure. Eur Respir J. 2010;35:1409-1411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 76. | Zurek M, Corrà U, Piepoli MF, Binder RK, Saner H, Schmid JP. Exercise training reverses exertional oscillatory ventilation in heart failure patients. Eur Respir J. 2012;40:1238-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 77. | Apostolo A, Agostoni P, Contini M, Antonioli L, Swenson ER. Acetazolamide and inhaled carbon dioxide reduce periodic breathing during exercise in patients with chronic heart failure. J Card Fail. 2014;20:278-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 78. | Fontana M, Emdin M, Giannoni A, Iudice G, Baruah R, Passino C. Effect of acetazolamide on chemosensitivity, Cheyne-Stokes respiration, and response to effort in patients with heart failure. Am J Cardiol. 2011;107:1675-1680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 79. | Marcus NJ, Del Rio R, Schultz EP, Xia XH, Schultz HD. Carotid body denervation improves autonomic and cardiac function and attenuates disordered breathing in congestive heart failure. J Physiol. 2014;592:391-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 131] [Article Influence: 10.9] [Reference Citation Analysis (0)] |