Published online Feb 26, 2016. doi: 10.4330/wjc.v8.i2.240
Peer-review started: September 8, 2015
First decision: September 23, 2015
Revised: September 25, 2015
Accepted: November 23, 2015
Article in press: November 25, 2015
Published online: February 26, 2016
Processing time: 172 Days and 0.8 Hours
AIM: To evaluate the premise, that biodegradable polymer drug eluting stents (BD-DES) could improve clinical outcomes compared to second generation permanent polymer drug eluting stents (PP-DES), we pooled the data from all the available randomized control trials (RCT) comparing the clinical performance of both these stents.
METHODS: A systematic literature search of PubMed, Cochrane, Google scholar databases, EMBASE, MEDLINE and SCOPUS was performed during time period of January 2001 to April 2015 for RCT and comparing safety and efficacy of BD-DES vs second generation PP-DES. The primary outcomes of interest were definite stent thrombosis, target lesion revascularization, myocardial infarction, cardiac deaths and total deaths during the study period.
RESULTS: A total of 11 RCT’s with a total of 12644 patients were included in the meta-analysis, with 6598 patients in BD-DES vs 6046 patients in second generation PP-DES. The mean follow up period was 16 mo. Pooled analysis showed non-inferiority of BD-DES, comparing events of stent thrombosis (OR = 1.42, 95%CI: 0.79-2.52, P = 0.24), target lesion revascularization (OR = 0.99, 95%CI: 0.84-1.17, P = 0.92), myocardial infarction (OR = 1.06, 95%CI: 0.86-1.29, P = 0.92), cardiac deaths (OR = 1.07, 95%CI 0.82-1.41, P = 0.94) and total deaths (OR = 0.96, 95%CI: 0.80-1.17, P = 0.71).
CONCLUSION: BD-DES, when compared to second generation PP-DES, showed no significant advantage and the outcomes were comparable between both the groups.
Core tip: No direct comparison has been done so far with biodegradable polymers in drug eluting stent compared to permanent alloy in second-generation drug eluting stent. We explored the efficacy of these two stents via meta-analysis of randomized control trials in terms of definite stent thrombosis, target lesion revascularization, myocardial infarction, cardiac deaths and total deaths.
- Citation: Pandya B, Gaddam S, Raza M, Asti D, Nalluri N, Vazzana T, Kandov R, Lafferty J. Biodegradable polymer stents vs second generation drug eluting stents: A meta-analysis and systematic review of randomized controlled trials. World J Cardiol 2016; 8(2): 240-246
- URL: https://www.wjgnet.com/1949-8462/full/v8/i2/240.htm
- DOI: https://dx.doi.org/10.4330/wjc.v8.i2.240
It’s been more than two decades since the introduction of coronary stents and during this period the stent designs have been modified to improve patient safety. Bare metal stents (BMS) were trailed by first generation permanent polymer drug eluting stents (PP-DES) (Paclitaxel and Sirolimus) then followed by second generation PP-DES (Everolimus and Zotarolimus) and now biodegradable polymer DES (BD-DES) are envisaging potentially improved patient outcomes.
Stent designing is the crux of interventional cardiology research and the changes have been dynamic. Initial BMS used a simple expandable metal alloy frame work, while PP-DES use an anti-proliferative drug coating on the metal platform, glued by a binding durable polymer to hold and elute the drug over time. Beyond any uncertainty, PP-DES are superior to BMS in decreasing restenosis, however PP-DES require longer duration of dual-antiplatelet therapy to avert the risk of stent thrombosis[1]. It is now understood, the metal alloy and the permanent polymer are among the culprits for prolonged inflammation leading to very late stent thrombosis and late restenosis (termed late catch-up phenomena) and henceforth the unremitting search for safer stents[2]. The second generation PP-DES introduced few years ago, have superior metal frame work (cobalt-chromium and platinum-chromium) with thinner metal struts, enhanced biocompatible binding polymer and these stents have proven improved patient outcomes compared to its predecessors[3]. Nevertheless, the potential need for dual-antiplatelet therapy beyond one year is still an apprehension among cardiologists and patients with second generation PP-DES. The BD-DES, unlike second generation PP-DES, will elute the anti-proliferative drug and the biodegradable polymer subsequently dissolves leaving behind a bare metal stent[4]. BD-DES is introduced with an anticipation to decrease the stent thrombosis events (especially very late events) and evading the need for prolonged dual-antiplatelet therapy.
Several randomized control trials and registries have been published in last few years, with most trials comparing first generation PP-DES to BD-DES. As anticipated, long term follow up data has shown superiority of BD-DES in decreasing very late stent thrombosis events when compared with Sirolimus (first generation) PP-DES[5]. However, there are only fewer studies comparing second generation PP-DES to BD-DES. Since second generation PP-DES is current standard of care in United States, it is of immense importance to study if the newer BD-DES offer any better outcomes. We performed a meta-analysis and systematic review of randomized control trials comparing efficacy and safety of BD-DES to second generation PP-DES (Everolimus and Zotarolimus).
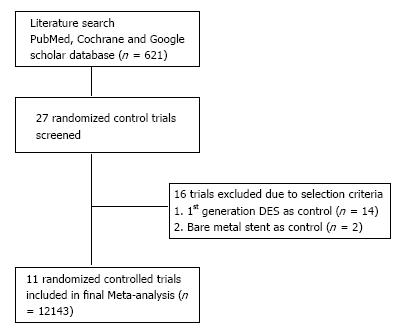
Two independent investigators systematically searched PubMed, Cochrane and Google scholar database from January 2001 to April 2015. We used following keywords: “biodegradable stent”, “biodegradable polymer”, “biodegradable polymer drug eluting”, and “biodegradable stent coronary”. Reference lists from selected studies were manually searched for potentially relevant studies. Whenever available, the most recent follow up data on a study was included. The PRISMA statement was used as guidance for selection of studies to be included in the meta-analysis and is depicted in Figure 1. Randomized control trials comparing BD-DES vs second generation DES with a primary end point of definite stent thrombosis, target lesion revascularization, myocardial infarction, cardiac deaths and total death were included in the study. We found 11 trials comparing BD-DES to second generation (Everolimus or Zotarolimus) PP-DES. In ISAR-TEST 4 trial, both first generation Sirolimus and second generation Everolimus DES were used, but in our meta-analysis we only used data pertinent to second generation Everolimus DES. Given the low incidence of stent thrombosis and other outcomes, a meta-analysis was performed to prove treatment differences between these two stents.
Two authors screened all relevant literature by their abstract and title found by electronic search. Only trails published in English were taken into consideration. Inclusion criteria were (1) randomized control trials; (2) comparing biodegradable polymers to second generation drug eluting stents; and (3) reporting outcomes as a target lesion revascularization [target lesion revascularization (TLR)], definite stent thrombosis (DST), myocardial infarction (MI), cardiac deaths and total deaths. We excluded studies with first generation drug eluting stents and bare metal stents as controls and also studies performed on select population, like complex lesions or on bifurcating lesions. Randomized control trials comparing BD-DES to second generation PP-DES were only included in this study.
Data from all 11 trials were extracted by same two authors in regards to first author, year of publication, total No. of patients and No. of patients in each group (Table 1). Authors also extracted mean age group of patients, patients with DM and HTN, follow up duration and duration of use of dual antiplatelet therapy (Table 2).
| Ref. | Trial acronym | Yr | BD-DES type | PP-DES type | Total patients | BD-DES patients | PP-DES patients |
| Natsuaki et al[8] | NEXT | 2013 | Biolimus | Everolimus | 3235 | 1617 | 1618 |
| Smits et al[9] | COMPARE 2 | 2013 | Biolimus | Everolimus | 2707 | 1795 | 912 |
| Gao et al[10] | TARGET 1 | 2013 | Sirolmus | Everolimus | 458 | 227 | 231 |
| Byrne et al[11] | ISAR-TEST 4 | 2011 | Sirolimus | Everolimus | 2603 | 652 | 1304 |
| Xu et al[12] | 2011 | Sirolimus | Zotarolimus | 324 | 168 | 156 | |
| Separham et al[13] | 2011 | Biolimus | Everolimus | 200 | 100 | 100 | |
| Meredith et al[14] | EVOLVE | 2012 | Biolimus | Everolimus | 192 | 98 | 94 |
| Pilgrim et al[15] | BIOSCIENCE | 2014 | Sirolimus | Everolimus | 2119 | 1063 | 1056 |
| Serruys et al[7] | ABSORB 2 | 2014 | Everolimus | Everolimus | 501 | 335 | 166 |
| Lee et al[16] | 2014 | Biolimus | Everolimus | 500 | 245 | 255 | |
| Windecker et al[17] | BIOFLOW 2 | 2014 | Sirolimus | Everolimus | 452 | 298 | 154 |
| Ref. | Mean age | Male % | Diabetes % | Inclusion criteria | Exclusion criteria | DAPT mo | Follow up mo |
| Natsuaki et al[8] | 69 | 77 | 46 | SA1/ACS2 | Major surgery in 30 d, cardiogenic shock | 3 | 12 |
| Smits et al[9] | 63 | 74 | 22 | SA/ACS | Major surgery in 30 d, cardiogenic chock | 12 | 12 |
| Gao et al[10] | 59 | 69 | 14 | SA/UA3 | AMI4 < 1 wk, CT5, LM6 bifurcation, ISR7 | 12 | 12 |
| Byrne et al[11] | 67 | 75 | 29 | SA/ACS | LM. shock, malignancy, life expectancy < 1 yr | 6 | 36 |
| Xu et al[12] | 57 | 70 | 26 | SA/UA | AMI < 1 wk, LM, CTO | 6 | 24 |
| Separham et al[13] | 61 | 66 | 28 | SA/ACS | Allergy to aspirin, plavix, heparin, stainless steel, everolimus, biolimus or contrast and pregnancy | 12 | 12 |
| Meredith et al[14] | 62 | 80 | 22 | Symp CAD8, Silent Ischemia | AMI, LM CAD, ISR, thrombus in target vessel | 6 | 6 |
| Pilgrim et al[15] | 66 | 77 | 24 | Stable CAD/ACS | Pregnancy, intolerance to aspirin, plavix, planned surgery in 6 mo | 12 | 12 |
| Serruys et al[7] | 61 | 76 | 24 | Evidence of myocardial Ischemia | AMI, unstable arrhythmias, LVEF9 < 30 | NA | 12 |
| Lee et al[16] | 63 | 68 | 32 | SA/UA/NSTEMI10 | STEM11, cardiogenic shock, allergy to aspirin/plavix/heparin/stainless steel/biolimus/everolimus, HD pts, LM CAD | ≥ 12 | 12 |
| Windecker et al[17] | 63 | 78 | 28 | SA/UA/Clinical evidence of myocardial Ischemia | MI within 72 h, LM CAD, triple vessel CAD, LVEF < 30% | ≥ 6 | 9 |
Clinical end points compared were definite stent thrombosis (DST), target lesion revascularization (TLR), myocardial infarction (MI), cardiac deaths and total deaths during the study period.
The results for each trial were obtained on an intention-to-treat analysis. The dichotomous and continuous endpoints from individual trials were analyzed using the odds ratio (OR) and the standard difference in mean (SDM) respectively as a parameter of efficacy with its 95%CI. We assessed heterogeneity with I2 that describes the percentage of total variation across trials due to heterogeneity rather than chance. I2 can be calculated as I2 = 100% × (Qv - df)/Q, where Q is Cochran’s heterogeneity statistics and df the degrees of freedom. Negative values of I2 are put equal to 0, so I2 lies between 0% (no heterogeneity) and 100% (maximal heterogeneity). The continuous outcomes were analyzed using the standard difference in mean. Binary outcomes from individual studies were combined and the summary estimators of treatment effect were calculated using fixed-effect method. Weighting of trial data in the models was based on the inverse variance weight computed as the inverse of the squared standard error value of the effect size. A P value of ≤ 0.05 was regarded as significant. All analyses were performed using Review Manager (RevMan) Version 5.3 for Windows Oxford, England.
A total of 11 RCT’s with a total of 12644 patients were included in the meta-analysis, with 6598 patients in BD-DES vs 6046 patients in second generation PP-DES. The mean follow up period was 16 mo. Table 1 shows the main characteristics of the included studies. Table 2 shows the main characteristics of the BD-DES patients included in the studies. The final summary of clinical end points is depicted in Table 3.
| Events | BD-DES (n = 4459) | PP-DES (n = 4221) | ODD S RATIO (95%CI) | P-value |
| Definite stent thrombosis | 34 | 24 | 1.42 (0.79-2.52) | 0.24 |
| Target lesion revascularization | 294 | 350 | 0.99 (0.84-1.17) | 0.92 |
| Myocardial infarction | 202 | 238 | 1.06 (0.86-1.29) | 0.59 |
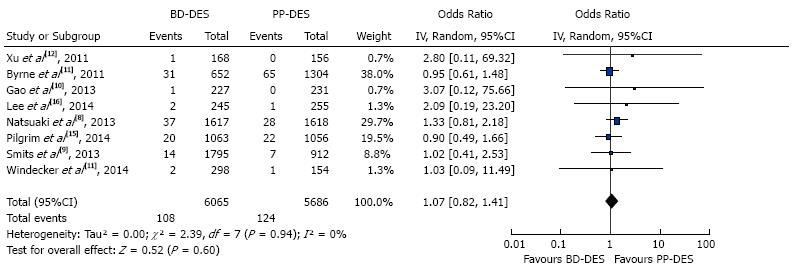
| Cardiac deaths | 108 | 124 | 1.07 (0.82-1.41) | 0.6 |
| Total deaths | 229 | 236 | 0.96 (0.80-1.17) | 0.71 |
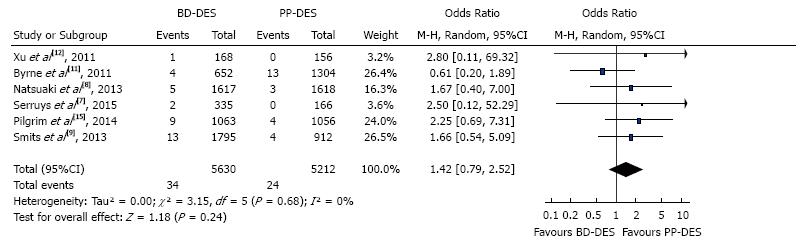
The forest plot for summary effect is shown in Figure 2. There were a total of 34 (0.6%) stent thrombosis events in BD-DES group and 24 (0.46%) stent thrombosis in PP-DES group. The forest plot is shown in the Figure 2, with pooled OR of 1.42 (95%CI: 0.79-2.52), P = 0.24, and I2 for heterogeneity 0%.
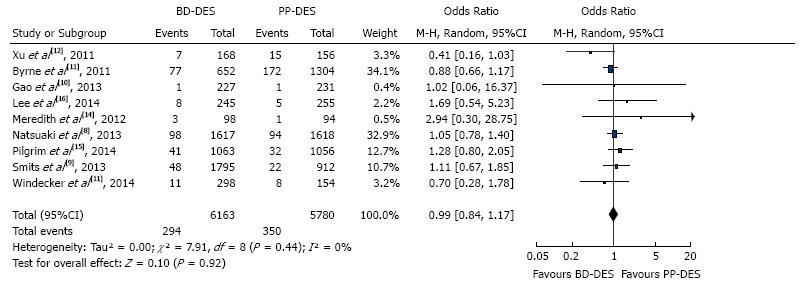
The forest plot for summary effect is shown in Figure 3. There were a total of 294 (4.77%) TLR in BD-DES group vs 350 (6.05%) TLR in PP-DES group. The forest plot is shown in the figure, with pooled OR of 0.99 (95%CI: 0.84-1.17), P = 0.92, and I2 for heterogeneity 0%.
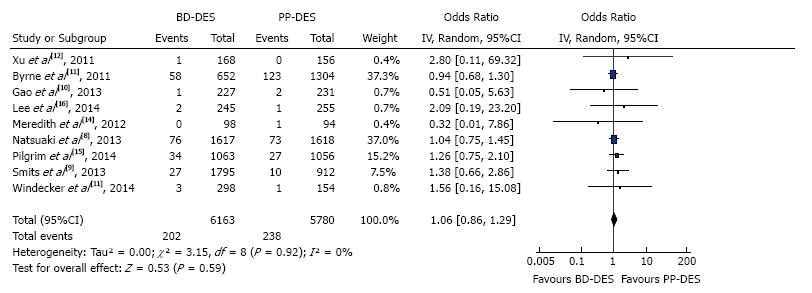
The forest plot for summary effect is shown in Figure 4. There were a total of 202 (3.27%) myocardial infarctions in BD-DES group vs 238 (4.11%) myocardial infarctions in PP-DES group. The forest plot is shown in the Figure 4, with pooled OR of 1.06 (95%CI: 0.86-1.29), P = 0.59, and I2 for heterogeneity 0%.
The forest plot for summary effect is shown in Figure 4. There were a total of 108 (1.78%) cardiac deaths in BD-DES group vs 124 (2.18%) cardiac deaths in PP- DES group. The forest plot is shown in the figure, with pooled OR of 1.07 (95%CI: 0.82-1.41, P = 0.60), and I2 for heterogeneity 0%.
The forest plot for summary effect is shown in Figure 5. There were a total of 229 (3.65%) deaths in BD-DES group vs 236 (4.01%) deaths in PP-DES group. The forest plot is shown in the figure, with pooled OR of 0.96 (95%CI: 0.80-1.17, P = 0.71), and I2 for heterogeneity 0%.
From our study, at a mean follow up of 16 mo, BD-DES use did not significantly decrease mortality (OR = 0.96, P = 0.71) or myocardial infarction events (OR = 1.06, P = 0.59). Rates of stent thrombosis (OR = 1.42, P = 0.24) and target lesion revascularization (OR = 0.99, P = 0.92) were comparable between both the stents. In this study the results for BD-DES, against contrary belief, failed to show any significant decrease in stent thrombosis. Looking at individual study results, except for ISAR-TEST 4, all trials showed non-significant increase in odds of stent thrombosis compared to second generation PP-DES. However it should be remembered, BD-DES are anticipated to have decreased stent thrombosis events at long term follow up, especially after the biodegradable polymer dissolves and leaves behind a bare metal stent. It is important to wait for long term follow up data on these trials, to observe if the very late stent thrombosis rates are lower, as seen in long term follow up data of ISAR-TEST 4. It is also crucial and remains to be observed, if the stent thrombosis events would be lower even after discontinuing dual anti-platelet therapy and the when event rates are adjusted for dual-antiplatelet therapy use between both the groups. Henceforth, it is too early to come to any firm conclusions in regards to superiority of BD-DES to currently used second generation PP-DES. A recent network meta-analysis comparing BD-DES, first and second generation PP-DES and bare metal stents, concluded BD-DES are not superior to second generation BD-DES[6]. In our study, we systematically reviewed all the studies and believe long term follow up of the trials are needed before we can make any such firm conclusions.
A pooled analyses comparing ISAR-TEST3, ISAR-TEST-4 and LEADERS trial showed decreased risk of stent thrombosis with BD-DES at 4 year follow up[5]. It is likely because those trials used first generation Sirolimus DES, and the first generation stents are known to have increased late restenosis and stent thrombosis events. However, the second generation DES, use different metal alloy framework with thin struts and the binding polymer is biocompatible and hence the results of such studies cannot be extrapolated to second generation PP-DES. The time for dual anti-platelet therapy with the biodegradable stent is shorter. In fact, in some trials (Table 2), the time for DAPT is reduced to 3-6 mo, while for the drug-eluted stents can be longer. This can be an advantage in any case, since patients on DAPT may have an increased risk of bleeding, especially if unplanned surgery is needed or in case oral anticoagulation is needed for concomitant disease (atrial fibrillation or deep venous thrombosis).
Interventional cardiologists have always been welcoming to newer technology and novel stent designs. The early enthusiasm of most stents, introduced in the past, could not meet the expectations during long-term follow up. With new BD-DES being studied across the globe, we need to analyze the data more closely before drawing conclusions on their superiority to currently used second generation PP-DES.
The major limitation of this study is the wide variation of follow-up period. In articular, the ISAR-TEST 4 study had the longest mean follow-up period (36 mo), and the odds ratio was completely opposite to all other included studies as pointed out. The results may change with long-term follow-up. Second, Serruys et al[7] included a study investigating a bioresorbable scaffold into the analysis because you aimed to compare BD-DES and PP-DES. Other minor issues are described below. BD-DES used in the RCT was of various types (Biolimus and Sirolimus) and the results should be interpreted with caution in generalizing our results to all types of BD-DES. The patient population in all these studies did vary to some degree (as described in Table 2). Also, the lesions treated and characteristics of stents used- like length and diameter along with lesion complexity could have affected the outcomes.
BD-DES when compared to second generation PP-DES, showed no significant advantage and the outcomes were comparable between both the groups. Long term follow up data is needed, to demonstrate any decrease in very late stent thrombosis events with BD-DES compared to second generation PP-DES.
Biodegradable polymer stent are currently used in Europe for PCI. Despite that there is no clear-cut evidence in literature comparing the efficacy of these two types of stent.
Now a day every effort is made to find the new design of stents, which will minimize the need for longer duration of dual antiplatelet therapy, which can be responsible for their notorious side effect in some situations.
In present study, the authors compared the efficacy of novel biodegradable dug eluting stent with the standard of care second-generation drug eluting stents in the form of meta-analysis of current randomized control trials.
The present results allow authors to think the role of biodegradable drug eluting stent in stent thrombosis, interests them in further investigating the long term outcomes in form of late stent thrombosis and duration of dual antiplatelet therapy.
The present meta-analysis provides more insight into clinical practice in regards to usage of different stent designs.
P- Reviewer: De Ponti R, Kettering K, Petix NR, Said SA, Vermeersch P S- Editor: Qiu S L- Editor: A E- Editor: Lu YJ
| 1. | James SK, Stenestrand U, Lindbäck J, Carlsson J, Scherstén F, Nilsson T, Wallentin L, Lagerqvist B. Long-term safety and efficacy of drug-eluting versus bare-metal stents in Sweden. N Engl J Med. 2009;360:1933-1945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 178] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 2. | Cook S, Ladich E, Nakazawa G, Eshtehardi P, Neidhart M, Vogel R, Togni M, Wenaweser P, Billinger M, Seiler C. Correlation of intravascular ultrasound findings with histopathological analysis of thrombus aspirates in patients with very late drug-eluting stent thrombosis. Circulation. 2009;120:391-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 370] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 3. | Dores H, Raposo L, Campante Teles R, Machado C, Leal S, Araújo Gonçalves P, Mesquita Gabriel H, Sousa Almeida M, Mendes M. Stent thrombosis with second- versus first-generation drug-eluting stents in real-world percutaneous coronary intervention: analysis of 3806 consecutive procedures from a large-volume single-center prospective registry. J Invasive Cardiol. 2013;25:330-336. [PubMed] |
| 4. | Vorpahl M, Finn AV, Nakano M, Virmani R. The bioabsorption process: tissue and cellular mechanisms and outcomes. EuroIntervention. 2009;5 Suppl F:F28-F35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Stefanini GG, Byrne RA, Serruys PW, de Waha A, Meier B, Massberg S, Jüni P, Schömig A, Windecker S, Kastrati A. Biodegradable polymer drug-eluting stents reduce the risk of stent thrombosis at 4 years in patients undergoing percutaneous coronary intervention: a pooled analysis of individual patient data from the ISAR-TEST 3, ISAR-TEST 4, and LEADERS randomized trials. Eur Heart J. 2012;33:1214-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 313] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 6. | Bangalore S, Toklu B, Amoroso N, Fusaro M, Kumar S, Hannan EL, Faxon DP, Feit F. Bare metal stents, durable polymer drug eluting stents, and biodegradable polymer drug eluting stents for coronary artery disease: mixed treatment comparison meta-analysis. BMJ. 2013;347:f6625. [PubMed] |
| 7. | Serruys PW, Chevalier B, Dudek D, Cequier A, Carrié D, Iniguez A, Dominici M, van der Schaaf RJ, Haude M, Wasungu L. A bioresorbable everolimus-eluting scaffold versus a metallic everolimus-eluting stent for ischaemic heart disease caused by de-novo native coronary artery lesions (ABSORB II): an interim 1-year analysis of clinical and procedural secondary outcomes from a randomised controlled trial. Lancet. 2015;385:43-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 462] [Article Influence: 46.2] [Reference Citation Analysis (0)] |
| 8. | Natsuaki M, Kozuma K, Morimoto T, Kadota K, Muramatsu T, Nakagawa Y, Akasaka T, Igarashi K, Tanabe K, Morino Y. Biodegradable polymer biolimus-eluting stent versus durable polymer everolimus-eluting stent: a randomized, controlled, noninferiority trial. J Am Coll Cardiol. 2013;62:181-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 173] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 9. | Smits PC, Hofma S, Togni M, Vázquez N, Valdés M, Voudris V, Slagboom T, Goy JJ, Vuillomenet A, Serra A. Abluminal biodegradable polymer biolimus-eluting stent versus durable polymer everolimus-eluting stent (COMPARE II): a randomised, controlled, non-inferiority trial. Lancet. 2013;381:651-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 238] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 10. | Gao RL, Xu B, Lansky AJ, Yang YJ, Ma CS, Han YL, Chen SL, Li H, Zhang RY, Fu GS. A randomised comparison of a novel abluminal groove-filled biodegradable polymer sirolimus-eluting stent with a durable polymer everolimus-eluting stent: clinical and angiographic follow-up of the TARGET I trial. EuroIntervention. 2013;9:75-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 11. | Byrne RA, Kastrati A, Massberg S, Wieczorek A, Laugwitz KL, Hadamitzky M, Schulz S, Pache J, Fusaro M, Hausleiter J. Biodegradable polymer versus permanent polymer drug-eluting stents and everolimus- versus sirolimus-eluting stents in patients with coronary artery disease: 3-year outcomes from a randomized clinical trial. J Am Coll Cardiol. 2011;58:1325-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 12. | Xu B, Dou KF, Han YL, Lü SZ, Yang YJ, Huo Y, Wang LF, Chen YD, Wang HC, Li WM. A prospective multicenter parallel-controlled trial of TIVOLI biodegradable-polymer-based sirolimus-eluting stent compared to ENDEAVOR zotarolimus-eluting stent for the treatment of coronary artery disease: 8-month angiographic and 2-year clinical follow-up results. Chin Med J (Engl). 2011;124:811-816. [PubMed] |
| 13. | Separham A, Sohrabi B, Aslanabadi N, Ghaffari S. The twelve-month outcome of biolimus eluting stent with biodegradable polymer compared with an everolimus eluting stent with durable polymer. J Cardiovasc Thorac Res. 2011;3:113-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 14. | Meredith IT, Verheye S, Dubois CL, Dens J, Fajadet J, Carrié D, Walsh S, Oldroyd KG, Varenne O, El-Jack S. Primary endpoint results of the EVOLVE trial: a randomized evaluation of a novel bioabsorbable polymer-coated, everolimus-eluting coronary stent. J Am Coll Cardiol. 2012;59:1362-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 164] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 15. | Pilgrim T, Heg D, Roffi M, Tüller D, Muller O, Vuilliomenet A, Cook S, Weilenmann D, Kaiser C, Jamshidi P. Ultrathin strut biodegradable polymer sirolimus-eluting stent versus durable polymer everolimus-eluting stent for percutaneous coronary revascularisation (BIOSCIENCE): a randomised, single-blind, non-inferiority trial. Lancet. 2014;384:2111-2122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 225] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 16. | Lee JY, Park DW, Kim YH, Ahn JM, Kim WJ, Kang SJ, Lee SW, Lee CW, Park SW, Yun SC. Comparison of biolimus A9-eluting (Nobori) and everolimus-eluting (Promus Element) stents in patients with de novo native long coronary artery lesions: a randomized Long Drug-Eluting Stent V trial. Circ Cardiovasc Interv. 2014;7:322-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Windecker S, Haude M, Neumann FJ, Stangl K, Witzenbichler B, Slagboom T, Sabaté M, Goicolea J, Barragan P, Cook S. Comparison of a novel biodegradable polymer sirolimus-eluting stent with a durable polymer everolimus-eluting stent: results of the randomized BIOFLOW-II trial. Circ Cardiovasc Interv. 2015;8:e001441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 164] [Article Influence: 16.4] [Reference Citation Analysis (0)] |