Published online Feb 26, 2016. doi: 10.4330/wjc.v8.i2.231
Peer-review started: August 10, 2015
First decision: September 16, 2015
Revised: October 9, 2015
Accepted: December 9, 2015
Article in press: December 11, 2015
Published online: February 26, 2016
Processing time: 203 Days and 7.8 Hours
AIM: To investigate the association of arterial wave reflection with coronary flow reserve (CFR) in coronary artery disease (CAD) patients after successful revascularization.
METHODS: We assessed 70 patients with angiographically documented CAD who had undergone recent successful revascularization. We measured (1) reactive hyperemia index (RHI) using fingertip peripheral arterial tonometry (RH-PAT Endo-PAT); (2) carotid to femoral pulse wave velocity (PWVc-Complior); (3) augmentation index (AIx), the diastolic area (DAI%) and diastolic reflection area (DRA) of the central aortic pulse wave (Arteriograph); (4) CFR using Doppler echocardiography; and (5) blood levels of lipoprotein-phospholipase A2 (Lp-PLA2).
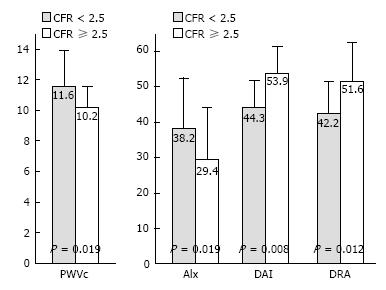
RESULTS: After adjustment for age, sex, blood pressure parameter, lipidemic, diabetic and smoking status, we found that coronary flow reserve was independently related to AIx (b = -0.38, r = 0.009), DAI (b = 0.36, P = 0.014), DRA (b = 0.39, P = 0.005) and RT (b = -0.29, P = 0.026). Additionally, patients with CFR < 2.5 had higher PWVc (11.6 ± 2.3 vs 10.2 ± 1.4 m/s, P = 0.019), SBPc (139.1 ± 17.8 vs 125.2 ± 19.1 mmHg, P = 0.026), AIx (38.2% ± 14.8% vs 29.4% ± 15.1%, P = 0.011) and lower RHI (1.26 ± 0.28 vs 1.50 ± 0.46, P = 0.012), DAI (44.3% ± 7.9% vs 53.9% ± 6.7%, P = 0.008), DRA (42.2 ± 9.6 vs 51.6 ± 11.4, P = 0.012) and LpPLA2 (268.1 ± 91.9 vs 199.5 ± 78.4 ng/mL, P = 0.002) compared with those with CFR ≥ 2.5. Elevated LpPLA2 was related with reduced CFR (r = -0.33, P = 0.001), RHI (r = -0.37, P < 0.001) and DRA (r = -0.35, P = 0.001) as well as increased PWVc (r = 0.34, P = 0.012) and AIx (r = 0.34, P = 0.001).
CONCLUSION: Abnormal arterial wave reflections are related with impaired coronary flow reserve despite successful revascularization in CAD patients. There is a common inflammatory link between impaired aortic wall properties, endothelial dysfunction and coronary flow impairment in CAD.
Core tip: The present study is a contribution to investigate the association between the abnormalities in arterial wave reflections and coronary flow reserve. We demonstrated that augmentation of the systolic component of the central aortic pulse wave instead of diastolic is related with impaired coronary flow reserve after adjustment for several other factors potentially influencing coronary microcirculatory function. Furthermore, endothelial dysfunction as assessed by reactive hyperemia index and an inflammatory process as assessed by increased levels of lipoprotein-associated Phospholipase A2 are related with increased arterial stiffness and abnormal wave reflections in coronary artery disease patients.
- Citation: Tritakis V, Tzortzis S, Ikonomidis I, Dima K, Pavlidis G, Trivilou P, Paraskevaidis I, Katsimaglis G, Parissis J, Lekakis J. Association of arterial stiffness with coronary flow reserve in revascularized coronary artery disease patients. World J Cardiol 2016; 8(2): 231-239
- URL: https://www.wjgnet.com/1949-8462/full/v8/i2/231.htm
- DOI: https://dx.doi.org/10.4330/wjc.v8.i2.231
Atherosclerosis is a complex process with many faces which include impaired coronary microcirculatory function, endothelial dysfunction, increased arterial stiffness and discrete plaque formation within epicardial coronary tree.
The measurement of peripheral vasodilator response using fingertip peripheral arterial tonometry (PAT) provides a useful method for assessing arterial endothelial function[1-3]. Previous studies have shown an independent association of reactive hyperemia (RH-PAT) index with coronary endothelial function[3] and cardiovascular risk in patients with coronary artery disease (CAD)[4].
Coronary flow reserve assessed by Doppler echocardiography (CFR) is a reliable, non-invasive method to identify epicardial coronary patency as well as coronary microcirculatory integrity[5-8]. The scaling values of decreasing CFR constitute a comprehensive indicator of cardiovascular risk even in the presence of critical epicardial coronary stenosis[6].
Pulse wave velocity (PWV)[9] a valid marker of arterial stiffness, is independently related with the impairment of coronary microcirculation as assessed by CFR in patients with CAD[10,11]. Increased arterial stiffness causes an early arrival of wave reflection in systole instead of diastole and thus reduces coronary perfusion. Augmentation index (AIx), aortic diastolic reflection area (DRA) and index (DAI), derived by pulse wave analysis, are non-invasive markers of wave reflections[9,11-13]. However, the association between the abnormalities in wave reflections and coronary flow reserve in CAD patients after successful revascularization has not been fully investigated.
Lipoprotein-associated Phospholipase A2 (Lp-PLA2) is an inflammatory biomarker related with endothelial dysfunction, carotid atherosclerosis, impaired coronary flow reserve and increased arterial stiffness in CAD patients[14]. However its association with abnormal wave reflections has not been clarified.
In the present study we hypothesized that abnormal arterial wave reflections may determine coronary flow reserve. Thus, we examined the association of abnormal wave reflections, as assessed by AIx, DRA and DAI with coronary flow reserve using Doppler echocardiography after successful revascularization in CAD patients. Finally we examined the association of wave reflection with endothelial dysfunction as assessed by RHI and with inflammatory process assessed by circulating levels of LpPLA2.
We enrolled 70 patients (84.3% men, mean age 60.2 ± 9.8 years) with (1) exercise- and/or stress-related angina (2) evidence of reversible ischemia during stress echocardiography or thallium scintigraphy (3) stenosis of ≥ 50% in the left main coronary artery and or ≥ 70% in one or several of the major coronary arteries before inclusion in the study as defined in the ESC guidelines[15] (Table 1). All the patients had undergone successful revascularization (PCI, n = 64 or CABG, n = 6) into their LAD within a year before inclusion in the study. PCI was considered successful when there was remained reduction in the caliber of the stenotic artery to < 20% with a final TIMI flow grade 3 without side branch loss, flow-limiting dissection, or angiographic thrombus (as visually assessed by angiography[16]). All participants attended our preventive medicine laboratory. Using valid questionnaire, we recorded pharmaceutical regimens and other cardiovascular risk factors (smoking, hypertension, diabetes mellitus, dyslipidemia, family history of CAD).
| Variables | Values (n = 70) |
| Clinical | |
| Age (yr) | 60.2 ± 9.8 |
| Gender (males), n (%) | 59 (84.3) |
| Hypertension, n (%) | 38 (54.2) |
| DM, n (%) | 23 (32.8) |
| Dyslipidemia, n (%) | 57 (81.4) |
| Smoking, n (%) | 43 (61.5) |
| FH of CAD, n (%) | 25 (35.7) |
| SBP (mmHg) | 128 ± 18 |
| DBP (mmHg) | 77 ± 10 |
| Medications | |
| ASA n (%) | 70 (100) |
| Nitrates n (%) | 38 (54.3) |
| ACEIs/ARBS n (%) | 59 (84.2) |
| CCBs n (%) | 12 (17.1) |
| Statins n (%) | 65 (92.8) |
| β-blockers n (%) | 60 (85.5) |
| Biochemical | |
| Chol (mg/dL) | 198.8 ± 40.8 |
| TG (mg/dL) | 148.2 ± 79.9 |
| HDL (mg/dL) | 40.9 ± 11.4 |
| LDL (mg/dL) | 134.5 ± 35.9 |
| Glu (mg/dL) | 106.5 ± 32.5 |
| CRP (mg/L) | 2.44 ± 1.66 |
| Lp-PLA2 (ng/mL) | 231.9 ± 90.9 |
| Vascular markers | |
| CFR | 2.65 ± 0.94 |
| RHI-PAT | 1.37 ± 0.43 |
| PWVc (m/s) | 10.32 ± 2.39 |
| AIx (%) | 35.8 ± 15.4 |
| SAI (%) | 50.6 ± 8.7 |
| DAI (%) | 49.4 ± 8.7 |
| DRA | 45.4 ± 12.6 |
| RT (ms) | 115.1 ± 22.5 |
| SBPc (mm Hg) | 133.2 ± 19.6 |
| DBPc (mmHg) | 83.3 ± 12.4 |
Exclusion criteria were: The presence of acute infection, malignancy, chronic heart failure (class NYHA III and IV), chronic obstructive pulmonary disease, recent major surgery, and severe chronic auto-immune diseases, liver and renal impairment. We also excluded patients with recent (within 6 mo) acute cardiovascular events.
Blood sampling for measurement of Lp-PLA2 was performed on the morning before we performed echocardiography and vascular tests in all patients.
The study protocol was approved by the Local Ethics Committee, conducted in compliance with the Declaration of Helsinki and written informed consent was obtained from all patients before study entrance.
Measurement of peripheral vasodilator response with fingertip peripheral arterial tonometry (PAT) technology (EndoPAT; Itamar Medical Ltd, Caesarea, Israel) is increasingly being used as an alternative measure of endothelium-dependent dilation in response to reactive hyperemia[3]. The EndoPAT device records digital pulse wave amplitude (PWA) using fingertip plethysmography and consists of two finger-mounted probes, which include a system of inflatable latex air-cushions within a rigid external case. A blood pressure cuff is placed on one upper arm (study arm), while the contralateral arm serves as a control (control arm)[2]. PWA is measured continuously during three phases: A quiet baseline period, 5-min forearm occlusion (with inflation of the arterial pressure cuff to supra-systemic pressure), and reactive hyperemia following cuff release.
The reactive hyperaemia index (RHI) is calculated as follows: The ratio of the average amplitude of the PAT signal over a 1-min time interval starting 1 min after cuff deflation divided by the average amplitude of the PAT signal of a 3.5 min time period before cuff inflation (baseline)[3]. The result is further divided by the same ratio from the control arm, which allows the device to account for potential effects of systemic changes in vascular tone during testing. The final ratio is then multiplied by a proprietary baseline correction factor.
The reactive hyperemia index (RHI) measures nitric-oxide dependent changes in vascular tone[17]. An RHI < 1.35 has been related with impaired coronary endothelial function[3]. All studies were stored digitally and were analyzed by personnel blinded to clinical and laboratory data, using a computerized station.
Assessment of arterial wave reflections was performed non-invasively with the commercially available Arteriograph apparatus (TensioMed Budapest Hungary, Ltd) by analysis of the oscillometric pressure curves registered on the upper arm with a single pressure cuff. The principle of the oscillometric method is based on plethysmography and registers oscillometric pulsatile pressure changes in the brachial artery[18]. An upper arm cuff was applied to the patient and after a first simple BP measurement, the cuff was over-inflated with 35-40 mmHg beyond the systolic BP. During systole, the blood volume having been ejected into the aorta generates pulse wave (early systolic peak, P1). This pulse wave runs down and reflects from the bifurcation of aorta, creating a second wave (late systolic peak, P2). Both early and late systolic peak were obtained and recorded on the computer as pulse waves. The software of Arteriograph decomposes the early, late systolic and diastolic waves and also determines the onset and peaks of the waves, measuring noninvasively and other hemodynamic parameters as central systolic and diastolic blood pressure (SBPc, DBPc mmHg), augmentation index (AIx%), return time (RT in sec.) of the wave reflection, systolic area index (SAI%), diastolic area index (DAI %) and diastolic reflection area (DRA)[18].
The AIx is defined as the ratio of the difference between the second (P2, appearing because of the reflection of the first pulse wave) and first systolic peaks (P1 induced by the heart systole) to pulse pressure (PP), and it is expressed as a percentage of the ratio [Aix = 100 × (P2-P1)/PP]. DRA is derived by duration of the diastole and the area between the expected (theoretical) diastolic pressure curve without reflection and the truly measured diastolic curve with reflection and reflects the quality of the coronary arterial diastolic filling. SAI and DAI are the areas of systolic and diastolic portions under the pulse wave curve of a complete cardiac cycle, respectively. Thus, the higher the DAI and DRA are, the better the coronary perfusion is. Furthermore, RT is the time of the pulse wave travelling from the aortic root to the bifurcation and back, so this value is smaller as the aortic wall is stiffer[18].
All studies were stored digitally and were analyzed by personnel blinded to clinical and laboratory data, using a computerized station.
Studies were conducted using a Vivid 7 (GE Medical Systems, Horten Norway) phased array ultrasound system using second harmonic imaging. Dr Ignatios Ikonomidis, counting more than 5500 CFR echo studies the last 10 years, has performed the echocardiographic examinations and the CFR measurements for this study[5,8,14]. All studies were stored digitally and were analyzed by two observers blinded to clinical and laboratory data, using a computerized station (Echopac GE, Horten Norway). All patients had adequate quality of images for analysis.
We assessed transthoracic Doppler Echocardiographic-derived coronary flow reserve by obtaining the color-guided pulse-wave Doppler signals. In the long axis apical projections using a 7 MHz transducer, we recorded the maximal velocity and velocity-time integral in the distal LAD at baseline and during hyperaemic conditions after the intravenous administration of adenosine (0.14 mg/kg per minute)[5-8] for 3 min. Measurements of three cardiac cycles were averaged. CFR was calculated as the ratio of hyperemic to resting maximal diastolic velocity. The feasibility of the method was greater than 98% for all indices in our study cohort (initially 71 patients were recruited, but one patient was excluded due to unfeasible CFR study).
The mean CFR value of our cohort (< 2.5) was used for subgroup analysis after previously published cutoff values for impaired CFR in CAD patients[6,19].
The carotid-femoral PWV (PWVc) was assessed by measuring the pulse transit time and the distance travelled between the two recording sites. For pulse wave recording we used a validated noninvasive device (Complior SP®, Alam Medical, France) with capability of online wave recording. A simultaneous recording was performed by two pressure-sensitive transducers of two different pulse waves based over the right common carotid artery and the right femoral artery, respectively. Measurement of the distance between the transducers over the body surface allowed obtaining PWVc. Measurements were performed by a single observer, blinded to clinical and laboratory data, and the whole procedure has been internally validated in our laboratory[8,20].
Serum levels of Lp-PLA2 were measured in our biochemistry laboratory with a commercially available enzyme-linked immunoassay (ELISA) (PLAC test, diaDexus, Inc, San Francisco, CA) with minimum detection limit of 0.34 ng/mL[14]. The inter- and intra assay variations were < 5% and 8%. An Lp-PLA2 concentration of 235 ng/mL has been suggested to use as a clinical decision threshold[21]. Analyses were performed by personnel blinded to clinical and laboratory data.
All variables are expressed as mean ± SD. Statistical analysis was performed using SPSS 21.0 statistical software package (SPSS Inc, Illinois, United States). Categorical data were analysed using the standard chi-square test. Variables were tested by the Kolmogorov-Smirnov test to assess the normality of distribution. Parameters without normal distribution were transformed into ranks for further analysis. Patients were categorised into equal subgroups, according to the median value of CFR in our study cohort. Mean values of continuous variables were compared between groups using unpaired Student’s t-test or the Mann-Whitney U-test, where applicable.
Simple linear regression was used to investigate relations between variables. Multiple linear relations were checked by multiple linear regression analysis using forward or backward procedure. Associations are presented by means of standardized regression coefficient (b). All covariates included in the final models were tested for interactions. Tolerance values for each covariate was > 0.5 in the multivariate models.
Clinical and biochemical characteristics of our study population are presented in Table 1. The mean values of the vascular parameters and the pharmaceutical regimen of the study cohort are shown in Table 1.
In univariate analysis, a decreasing CFR was related with increasing PWVc (r = -0.38, P = 0.015), SBPc (r = -0.34, P = 0.022), AIx (r = -0.50, P = 0.003), SAI (r = -0.49, P = 0.006) as well as decreasing RT (r = 0.45, P = 0.009), DAI (r = 0.49, P = 0.006) DRA (r = 0.55, P < 0.001) and RHI (r = 0.47, P = 0.002). Furthermore, RHI was related to AIx (r = 0.48, P < 0.001), RT (r = -0.29, P = 0.024) and SBPc (r = 0.40, P = 0.001).
In multivariate analysis, after adjustment of age, sex, blood pressure parameter, lipidemic, diabetic and smoking status, we found that coronary flow reserve was independently related to AIx (b = -0.38, r = 0.009), DAI (b = 0.36, P = 0.014), DRA (b = 0.39, P = 0.005) and RT (b = -0.29, P = 0.026).
Patients were categorised in high and low CFR according to the median value of CFR. Patients with CFR < 2.5 had similar clinical characteristics with those with CFR ≥ 2.5 with the exception of higher cholesterol level, (Table 2, P < 0.05). However, patients with CFR < 2.5 had higher PWVc, SBPc, AIx, SAI and lower RT, DAI and DRA compared with those with CFR ≥ 2.5 after adjustment for cholesterol levels (Table 2, P < 0.05 and Figure 1).
| CFR < 2.5(n = 34) | CFR≥2.5(n = 36) | P | |
| Clinical | |||
| Age (yr) | 62.1 ± 9.2 | 58.4 ± 10.5 | 0.265 |
| Males, n (%) | 29 (85.2) | 30 (83.3) | 0.869 |
| Hypertension, n (%) | 20 (58.8) | 18 (50) | 0.368 |
| Diabetes, n (%) | 13 (38.2) | 10 (27.7) | 0.631 |
| Dyslipidemia, n (%) | 28 (82.3) | 29 (80.5) | 0.307 |
| Smoking, n (%) | 23 (67.6) | 20 (55.5) | 0.449 |
| FH of CAD | 14 (41.1) | 11 (30.5) | 0.334 |
| SBP (mmHg) | 130.9 ± 20.3 | 120.4 ± 14.8 | 0.011 |
| DBP (mmHg) | 77.4 ± 10.4 | 74.8 ± 8.8 | 0.058 |
| Medications | |||
| ASA, n (%) | 33 (97) | 34 (94.4) | 0.942 |
| Nitrates, n (%) | 23 (67.6) | 27 (75) | 0.131 |
| ACEIs/ARBS, n (%) | 33 (97) | 34 (94.4) | 0.956 |
| CCBs, n (%) | 5 (14.7) | 7 (19.4) | 0.597 |
| Statins, n (%) | 32 (94.1) | 33 (91.6) | 0.547 |
| β-blockers, n (%) | 29 (85.2) | 31 (86.1) | 0.765 |
| Biochemical | |||
| Chol (mg/dL) | 206.7 ± 44.1 | 190.6 ± 38.9 | 0.078 |
| TG (mg/dL) | 147.0 ± 57.1 | 143.9 ± 69.7 | 0.824 |
| HDL (mg/dL) | 39.6 ± 8.6 | 40.9 ± 12.8 | 0.567 |
| LDL (mg/dL) | 141.6 ± 37.8 | 126.6 ± 32.9 | 0.055 |
| Glu (mg/dL) | 100.9 ± 2 2.7 | 112.4 ± 39.9 | 0.126 |
| CRP (mg/L) | 2.5 ± 1.8 | 2.4 ± 1.5 | 0.279 |
| Lp-PLA2 (ng/mL) | 268.1 ± 91.9 | 199.5 ± 78.4 | 0.002 |
| Vascular markers | |||
| RHI-PAT | 1.26 ± 0.28 | 1.50 ± 0.46 | 0.012 |
| PWVc (m/s) | 11.6 ± 2.3 | 10.2 ± 1.4 | 0.019 |
| AIx (%) | 38.2 ± 14.8 | 29.4 ± 15.1 | 0.011 |
| SAI (%) | 55.7 ± 7.9 | 46.1 ± 6.7 | 0.008 |
| DAI (%) | 44.3 ± 7.9 | 53.9 ± 6.7 | 0.008 |
| DRA | 42.2 ± 9.6 | 51.6 ± 11.4 | 0.012 |
| RT (ms) | 106.1 ± 20.8 | 123.0 ± 22.1 | 0.015 |
| SBPc (mm Hg) | 139.1 ± 17.8 | 125.2 ± 19.1 | 0.026 |
| DBPc (mmHg) | 84.7 ± 12.1 | 80.0 ± 11.0 | 0.118 |
Furthermore, these patients with CFR < 2.5 had higher LpPLA2 compared with those with CFR ≥ 2.5 (Table 2, P = 0.002).
Elevated LpPLA2 was related with reduced CFR (r = -0.331, P = 0.001), RHI (r = -0.371, P < 0.001) and DRA (r = -0.35, P = 0.001) as well as increased PWVc (r = 0.34, P = 0.012) and AIx (r = 0.34, P = 0.001).
In the present study, we found a close association between arterial wave reflection markers, as assessed by AIx, DRA and DAI, and decreasing CFR in CAD patients after successful revascularization. Furthermore, we demonstrated that diastolic component of central aortic pulse wave as expressed with DRA and DAI is an independent determinant of impaired coronary flow reserve after adjustment for several other factors potentially influencing coronary microcirculatory function. Finally we have shown that endothelial dysfunction as assessed by RHI and the inflammatory process as assessed by LpPLA2 are associated with abnormal wave reflection and increased arterial stiffness.
Coronary flow reserve (CFR) represents the capacity of the coronary circulation to dilate following an increase in myocardial metabolic demands and can be expressed by the difference between the hyperemic flow and the resting flow curve. Impaired CFR constitutes a marker of coronary microcirculatory dysfunction and reflects the impairment of the epicardial coronary artery flow in the presence of significant coronary stenosis[6], as well as coronary microcirculatory dysfunction[11,14]. CFR entails strong prognostic significance in stable patients with known or suspected ischemic heart disease, independently of other risk factors[22-26]. Thus, the scaling values of decreasing CFR constitute a comprehensive indicator of cardiovascular risk even in the presence of critical epicardial coronary stenosis[6].
The association of increased PWV with the presence and prognosis of angiographic CAD has been extensively demonstrated[11,27,28]. Experimental studies have shown that low aortic compliance is associated with a reduction in coronary blood flow[29], particularly subendocardial flow[30,31]. In a human study, Leung et al[32] have shown that a compliant aorta, as measured by PWV, is associated with a greater improvement in hyperemic coronary blood flow from successful PCI than a stiff aorta and this relationship persisted for PWV even after accounting for stenosis severity. Furthermore, exercise-induced rise in coronary blood flow, related to ischemic threshold, could be determined by aortic stiffness. This is supported by the findings of Kingwell et al[33] who found indexes of arterial stiffness were stronger independent predictors of the exercise-induced ischemic threshold than maximum coronary stenosis assessed angiographically.
In the present study, we confirm the above mentioned close relation of PWV with CFR. PWV is a marker of aortic stiffness, whereas AIx, which is largely determined by wave reflections, represents much more the vasomotor tone in the small medium-sized muscular vessels downstream in the circulation[9,12]. In our study, we demonstrated for the first time that AIx is related to CFR, indicating that not only stiffness of the large elastic arteries impairs CFR, but stiffening of the smaller muscular arteries contributes as well. However, the net effect of increased systemic arterial stiffness on coronary vasodilatory reserve is thought to be mediated by reduced coronary perfusion during diastole.
Increased arterial stiffness increases the velocity of both forward and reflected pulse wave[9]. This increase in velocity of wave of pulse causes arrival of reflected waves at the aorta during systole and not during diastole as it occurs under conditions of normal aortic elastic properties. The early arrival of the reflected waves (1) augments the systolic aortic pressure and thus increase of LV afterload, wall stress and cardiac workload leading to increased myocardial oxygen demands; (2) reduces the diastolic aortic pressure resulting in reduced myocardial perfusion[9,34]. Thus, arterial stiffness causes a mismatch between myocardial oxygen demands and myocardial perfusion resulting in reduction of coronary flow reserve after hyperemia[10,19,22]. Additionally, stiffening of the large arteries, results in reduction of their capacity to function as an elastic reservoir resulting in a greater peripheral runoff of stroke volume during systole[13,29,31]. Together with the reduced elastic recoil, the diastolic blood pressure and hence coronary blood flow is decreased.
Indeed, in our study, we found that DAI and DRA, two markers that reflect the contribution of reflected waves to perfusion of the coronary circulation, were closely associated with CFR, even after adjustment for other factors influencing CFR. This finding supports the above mentioned pathophysiological mechanism.
Besides the above mentioned arterio-coronary coupling that may explain the lower coronary flow reserve associated with a stiff arterial tree, arterial stiffness may be a marker of a more generalized vascular disease process which among others, includes endothelial dysfunction. Previous studies have shown that large artery stiffness itself is influenced by endothelial function via basal release of nitric oxide[35] as well as that aortic stiffness is associated with brachial artery endothelial dysfunction[36]. On the other side, adenosine-induced CFR is also thought to be at least partly endothelium dependent[8]. Thus, endothelial function through NO production is an important determinant of coronary flow response to physiological or pharmacological stimuli[10,19].
Reactive hyperaemia peripheral arterial tonometry (RHI-PAT) is a method to assess peripheral microvascular endothelial function and is linked to coronary microvascular endothelial dysfunction[3], as this parameter is predominantly determined by the bioavailability of NO[16]. Both impaired CFR and reduced RHI-PAT have proven prognostic value in CAD patients[4,6,7]. In the present study we document an independent association of peripheral endothelial dysfunction, assessed by RH-PAT, with coronary endothelial dysfunction, assessed by CFR after successful revascularization in patients with CAD. It is possible that coronary endothelial dysfunction may coexist with aortic stiffness and may contribute to abnormal coronary microcirculatory response to hyperemia, as well as impaired aortic wall properties. Furthermore, the association of RHI-PAT with AIx and RT indicates that peripheral endothelial dysfunction contribute to impaired aortic wall properties, as well as that determines at least partly, stiffening of both large elastic arteries and smaller muscular arteries.
On the other hand increased PWV is associated with enhanced vascular inflammation and injury[20,27]. Indeed, in our study we measured LpPLA2 as a marker of vascular inflammation and we found that patients with high LpPLA2 levels had higher PWVc, AIx, and reduced DRA, DAI, CFR and RHI. These findings indicate a common effect of LpPLA2 in all vascular territories, indicating a generalized vascular disease process which causes reduced CFR directly and/or indirectly through arterial stiffness and impaired endothelial function as we mentioned above.
Our results establish a close relation between increasing PWVc, AIx, DAI, DRA, RHI-PAT and CFR in CAD patients. However, this study was not designed to verify whether this relation is causative or secondary to endothelial dysfunction and interstitial fibrosis within aortic and coronary wall in CAD patients. It is possible that the generalized vascular damage was the link between PWVc, AIx and CFR in our study.
In summary, in the present study, we demonstrated that augmentation of the systolic component of the central aortic pulse wave, as expressed by augmentation index and reduced diastolic component of central aortic pulse wave as expressed by diastolic reflection area and index are related with impaired coronary flow reserve after adjustment for several other factors potentially influencing coronary microcirculatory function. Furthermore, endothelial dysfunction as assessed by RHI and an inflammatory process as assessed by increased levels of Lp-PLA2 are related with increased arterial stiffness and abnormal wave reflections in CAD patients. These findings underscore the need to assess arterial wall properties in CAD patients to better stratify the risk of future events after successful revascularization.
Atherosclerosis is a complex process with many faces which include impaired coronary microcirculatory function, endothelial dysfunction, increased arterial stiffness and discrete plaque formation within epicardial coronary tree.
Pulse wave velocity (PWV) a valid marker of arterial stiffness, is independently related with the impairment of coronary microcirculation as assessed by coronary flow reserve in patients with coronary artery disease (CAD).
The authors demonstrated that augmentation of the systolic component of the central aortic pulse wave, as expressed by augmentation index and reduced diastolic component of central aortic pulse wave as expressed by diastolic reflection area and index are related with impaired coronary flow reserve after adjustment for several other factors potentially influencing coronary microcirculatory function.
These findings underscore the need to assess arterial wall properties in CAD patients to better stratify the risk of future events after successful revascularization.
The authors measured (1) reactive hyperemia index (RHI) using fingertip peripheral arterial tonometry (RH-PAT Endo-PAT); (2) carotid to femoral pulse wave velocity (PWVc-Complior); (3) augmentation index (AIx), the diastolic area (DAI%) and diastolic reflection area (DRA) of the central aortic pulse wave (Arteriograph); (4) CFR using Doppler echocardiography and 5) blood levels of Lipoprotein-phospholipase A2 (Lp-PLA2).
The authors studied a group of 70 patients with CAD by means of coronary flow reserve and several indexes related to arteriosclerosis (peripheral arterial tonometry, pulse waveform analysis, carotid to femoral pulse wave velocity) and to inflamation (Lp-PLA2). As expected these indexes were impaired in patients with lower coronary flow reserve.
P- Reviewer: Kettering K, Peteiro J, Said SAM, Sun Z S- Editor: Qiu S L- Editor: A E- Editor: Lu YJ
| 1. | Kuvin JT, Patel AR, Sliney KA, Pandian NG, Sheffy J, Schnall RP, Karas RH, Udelson JE. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J. 2003;146:168-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 114] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 2. | Lekakis J, Abraham P, Balbarini A, Blann A, Boulanger CM, Cockcroft J, Cosentino F, Deanfield J, Gallino A, Ikonomidis I. Methods for evaluating endothelial function: a position statement from the European Society of Cardiology Working Group on Peripheral Circulation. Eur J Cardiovasc Prev Rehabil. 2011;18:775-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 221] [Article Influence: 15.8] [Reference Citation Analysis (1)] |
| 3. | Bonetti PO, Pumper GM, Higano ST, Holmes DR, Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44:2137-2141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 686] [Cited by in RCA: 748] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 4. | Rubinshtein R, Kuvin JT, Soffler M, Lennon RJ, Lavi S, Nelson RE, Pumper GM, Lerman LO, Lerman A. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur Heart J. 2010;31:1142-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 545] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 5. | Ikonomidis I, Tzortzis S, Paraskevaidis I, Triantafyllidi H, Papadopoulos C, Papadakis I, Trivilou P, Parissis J, Anastasiou-Nana M, Lekakis J. Association of abnormal coronary microcirculatory function with impaired response of longitudinal left ventricular function during adenosine stress echocardiography in untreated hypertensive patients. Eur Heart J Cardiovasc Imaging. 2012;13:1030-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Cortigiani L, Rigo F, Gherardi S, Bovenzi F, Picano E, Sicari R. Implication of the continuous prognostic spectrum of Doppler echocardiographic derived coronary flow reserve on left anterior descending artery. Am J Cardiol. 2010;105:158-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Sicari R, Rigo F, Cortigiani L, Gherardi S, Galderisi M, Picano E. Additive prognostic value of coronary flow reserve in patients with chest pain syndrome and normal or near-normal coronary arteries. Am J Cardiol. 2009;103:626-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Ikonomidis I, Lekakis J, Papadopoulos C, Triantafyllidi H, Paraskevaidis I, Georgoula G, Tzortzis S, Revela I, Kremastinos DT. Incremental value of pulse wave velocity in the determination of coronary microcirculatory dysfunction in never-treated patients with essential hypertension. Am J Hypertens. 2008;21:806-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 9. | Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588-2605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4068] [Cited by in RCA: 4300] [Article Influence: 226.3] [Reference Citation Analysis (0)] |
| 10. | Fukuda D, Yoshiyama M, Shimada K, Yamashita H, Ehara S, Nakamura Y, Kamimori K, Tanaka A, Kawarabayashi T, Yoshikawa J. Relation between aortic stiffness and coronary flow reserve in patients with coronary artery disease. Heart. 2006;92:759-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Ikonomidis I, Makavos G, Lekakis J. Arterial stiffness and coronary artery disease. Curr Opin Cardiol. 2015;30:422-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 12. | Vlachopoulos C, O’rourke M. Genesis of the normal and abnormal arterial pulse. Curr Probl Cardiol. 2000;25:303-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 74] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Nemes A, Takács R, Gavallér H, Várkonyi TT, Wittmann T, Forster T, Lengyel C. Correlations between Arteriograph-derived pulse wave velocity and aortic elastic properties by echocardiography. Clin Physiol Funct Imaging. 2011;31:61-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Ikonomidis I, Kadoglou NN, Tritakis V, Paraskevaidis I, Dimas K, Trivilou P, Papadakis I, Tzortzis S, Triantafyllidi H, Parissis J. Association of Lp-PLA2 with digital reactive hyperemia, coronary flow reserve, carotid atherosclerosis and arterial stiffness in coronary artery disease. Atherosclerosis. 2014;234:34-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, Bugiardini R, Crea F, Cuisset T, Di Mario C. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949-3003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2772] [Cited by in RCA: 2989] [Article Influence: 249.1] [Reference Citation Analysis (0)] |
| 16. | Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol. 2011;58:e44-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1654] [Cited by in RCA: 1744] [Article Influence: 124.6] [Reference Citation Analysis (0)] |
| 17. | Nohria A, Gerhard-Herman M, Creager MA, Hurley S, Mitra D, Ganz P. Role of nitric oxide in the regulation of digital pulse volume amplitude in humans. J Appl Physiol (1985). 2006;101:545-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 346] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 18. | McQueen JM. The influence of the lexicon on phonetic categorization: stimulus quality in word-final ambiguity. J Exp Psychol Hum Percept Perform. 1991;17:433-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 234] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 19. | Rigo F. Coronary flow reserve in stress-echo lab. From pathophysiologic toy to diagnostic tool. Cardiovasc Ultrasound. 2005;3:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 89] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 20. | Ikonomidis I, Kadoglou N, Tsiotra PC, Kollias A, Palios I, Fountoulaki K, Halvatsiotis I, Maratou E, Dimitriadis G, Kremastinos DT. Arterial stiffness is associated with increased monocyte expression of adiponectin receptor mRNA and protein in patients with coronary artery disease. Am J Hypertens. 2012;25:746-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Lanman RB, Wolfert RL, Fleming JK, Jaffe AS, Roberts WL, Warnick GR, McConnell JP. Lipoprotein-associated phospholipase A2: review and recommendation of a clinical cut point for adults. Prev Cardiol. 2006;9:138-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Galderisi M, Capaldo B, Sidiropulos M, D’Errico A, Ferrara L, Turco A, Guarini P, Riccardi G, de Divitiis O. Determinants of reduction of coronary flow reserve in patients with type 2 diabetes mellitus or arterial hypertension without angiographically determined epicardial coronary stenosis. Am J Hypertens. 2007;20:1283-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Tuccillo B, Accadia M, Rumolo S, Iengo R, D’Andrea A, Granata G, Sacra C, Guarini P, Al-Kebsi M, De Michele M. Factors predicting coronary flow reserve impairment in patients evaluated for chest pain: an ultrasound study. J Cardiovasc Med (Hagerstown). 2008;9:251-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Pirat B, Bozbas H, Simsek V, Yildirir A, Sade LE, Gursoy Y, Altin C, Atar I, Muderrisoglu H. Impaired coronary flow reserve in patients with metabolic syndrome. Atherosclerosis. 2008;201:112-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Ascione L, De Michele M, Accadia M, Rumolo S, Sacra C, Alberta Ortali V, Inserviente L, Petti M, Russo G, Tuccillo B. Effect of acute hyperhomocysteinemia on coronary flow reserve in healthy adults. J Am Soc Echocardiogr. 2004;17:1281-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Rigo F, Sicari R, Gherardi S, Djordjevic-Dikic A, Cortigiani L, Picano E. Prognostic value of coronary flow reserve in medically treated patients with left anterior descending coronary disease with stenosis 51% to 75% in diameter. Am J Cardiol. 2007;100:1527-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Ikonomidis I, Stamatelopoulos K, Lekakis J, Vamvakou GD, Kremastinos DT. Inflammatory and non-invasive vascular markers: the multimarker approach for risk stratification in coronary artery disease. Atherosclerosis. 2008;199:3-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 105] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 28. | Orlova IA, Nuraliev EY, Yarovaya EB, Ageev FT. Prognostic value of changes in arterial stiffness in men with coronary artery disease. Vasc Health Risk Manag. 2010;6:1015-1021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Bouvrain Y, Lévy B. [“Windkessel” and coronary debit]. Arch Mal Coeur Vaiss. 1981;74:635-639. [PubMed] |
| 30. | Ohtsuka S, Kakihana M, Watanabe H, Sugishita Y. Chronically decreased aortic distensibility causes deterioration of coronary perfusion during increased left ventricular contraction. J Am Coll Cardiol. 1994;24:1406-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 132] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 31. | Watanabe H, Ohtsuka S, Kakihana M, Sugishita Y. Coronary circulation in dogs with an experimental decrease in aortic compliance. J Am Coll Cardiol. 1993;21:1497-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 208] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 32. | Leung MC, Meredith IT, Cameron JD. Aortic stiffness affects the coronary blood flow response to percutaneous coronary intervention. Am J Physiol Heart Circ Physiol. 2006;290:H624-H630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 69] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 33. | Kingwell BA, Waddell TK, Medley TL, Cameron JD, Dart AM. Large artery stiffness predicts ischemic threshold in patients with coronary artery disease. J Am Coll Cardiol. 2002;40:773-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 193] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 34. | Vinereanu D, Nicolaides E, Boden L, Payne N, Jones CJ, Fraser AG. Conduit arterial stiffness is associated with impaired left ventricular subendocardial function. Heart. 2003;89:449-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 35. | Wilkinson IB, Qasem A, McEniery CM, Webb DJ, Avolio AP, Cockcroft JR. Nitric oxide regulates local arterial distensibility in vivo. Circulation. 2002;105:213-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 348] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 36. | Nigam A, Mitchell GF, Lambert J, Tardif JC. Relation between conduit vessel stiffness (assessed by tonometry) and endothelial function (assessed by flow-mediated dilatation) in patients with and without coronary heart disease. Am J Cardiol. 2003;92:395-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 114] [Article Influence: 5.2] [Reference Citation Analysis (0)] |