Published online Feb 26, 2016. doi: 10.4330/wjc.v8.i2.192
Peer-review started: June 29, 2015
First decision: September 17, 2015
Revised: October 31, 2015
Accepted: December 1, 2015
Article in press: December 2, 2015
Published online: February 26, 2016
Processing time: 239 Days and 23.4 Hours
Aortic stenosis (AS) is a disease that progresses slowly for years without symptoms, so patients need to be carefully managed with appropriate follow up and referred for aortic valve replacement in a timely manner. Development of symptoms is a clear indication for aortic valve intervention in patients with severe AS. The decision for early surgery in patients with asymptomatic severe AS is more complex. In this review, we discuss how to identify high-risk patients with asymptomatic severe AS who may benefit from early surgery.
Core tip: We focused on how to identify high-risk patients in asymptomatic aortic stenosis. Revised American Heart Association/American College of Cardiology guidelines and diagnostic testing for appropriate clinical decision making are discussed in this article.
- Citation: Katayama M, Chaliki HP. Diagnosis and management of patients with asymptomatic severe aortic stenosis. World J Cardiol 2016; 8(2): 192-200
- URL: https://www.wjgnet.com/1949-8462/full/v8/i2/192.htm
- DOI: https://dx.doi.org/10.4330/wjc.v8.i2.192
As a result of the aging population, aortic stenosis (AS) is currently one of the most common valvular heart diseases to need surgical intervention. AS is a slowly progressive chronic condition, but once a patient becomes symptomatic, the prognosis is dismal. Although percutaneous valve technology is now approved for high-risk patients with symptomatic AS, clinical management of asymptomatic patients with severe AS is still difficult. Assessment of symptoms in sedentary elderly patients with severe AS is often challenging. It is common for patients to have nonspecific symptoms such as shortness of breath or general feelings of weakness that can be explained by many reasons other than cardiac diseases. Advances in multiple modality imaging provide additional objective information about subtle functional deterioration of the left ventricle (LV), myocardial tissue damage, and the amount of the valve calcification. Existing and new parameters are investigated to improve the clinical decision-making process.
In this review, we focus on recent advances in diagnostic methods for assessment of AS and discuss how to implement these methods in current clinical practice as it relates to the management of patients with asymptomatic severe AS.
A normal aortic valve is tricuspid and a normal valve opening area is 3 to 4 cm2. Progression from aortic valve sclerosis to AS is reported to be 9% per 5 years[1]. There is some evidence to suggest that NOTCH 1 genetic mutations and specific lipoprotein polymorphism is associated with congenital AS and valve calcification[2]. In AS, it has been reported that an average rate of increase in mean gradient is 7 to 8 mmHg/year; in maximum velocity, 0.2 to 0.4 m/s per year; and in a decrease in valve area, 0.1 to 0.15 cm2/year[3-6]. Hemodynamic progression of AS is gradual and linear, though there is variability and some patients present with rapid progression. Presence of aortic valve calcification, coronary artery disease, advanced age, renal impairment, and baseline AS severity are risk factors for rapid progression[4,7-9].
The hemodynamic progression of AS lead to LV hypertrophy (LVH) as a compensation mechanism of the heart. Morphological changes such as increasing muscle fiber thickness, collagen volume, and interstitial fibrosis occur in AS patients[10]. These changes result in LV diastolic and systolic dysfunction[11]. LV mass regression starts soon after aortic valve replacement (AVR) and may continue through another 8 years postoperatively, while diastolic dysfunction persists up to 2 years due to the relatively increased amount of fibrotic tissue in the myocardium[12-14]. These results may encourage AVR before the fibrotic change becomes too substantial or irreversible to advance postoperative recovery. Thus, the amount of myocardial structural change in AS can be a good parameter to define the severity of AS, and the new imaging technique gains greater prominence to determine the timing of surgical intervention in asymptomatic severe AS[15].
When the valve area is decreased to one-fourth of the normal valve area (0.75-1.00 cm2), in general, patients develop symptoms, although there is high inter-individual variability. A fundamental principle of fluid dynamics is that flow velocity within the conduit depends on volumetric flow rate. Patients with normal LVEF (LV ejection fraction) and normal flow will generally have a mean gradient > 40 mmHg in the setting of severe AS. However, recent studies have identified a new entity, termed “paradoxical low-gradient severe AS”, where the stroke volume is reduced in the setting of increased afterload and concentric LVH, resulting in a low gradient despite severe AS in the setting of normal LVEF. Recent American College of Cardiology/American Heart Association (ACC/AHA) guidelines have recognized this entity and have developed guidelines for management of this new group of AS patients.
AHA/ACC guidelines for the management of patients with valvular heart disease, which was revised in 2014, has a major change for the staging of AS (Table 1)[16].
| Hemodynamics | LV Function | AVA | Aortic valve | ||
| A | At risk of AS | Vmax < 2 m/s | Normal EF | - | Bicuspid, sclerosis |
| B | Progressive AS | Mild AS: Vmax < 2.0-2.9 m/s or mean ΔP < 20 mmHg Moderate AS: Vmax > 3.0-3.9 m/s or mean ΔP > 20-39 mmHg | Normal EF Early diastolic dysfunction | - | Mild to moderate calcification Reduction in motion Commissural fusion |
| C1 | Asymptomatic severe AS | Vmax≥ 4 m/s or mean ΔP≥ 40 mmHg | Normal EF Diastolic dysfunction | ≤ 1.0 cm2 or ≤ 0.6 cm2/m2 | Severe calcification Severely reduced opening |
| C2 | Asymptomatic severe AS with LV dysfunction | Vmax≥ 4 m/s or mean ΔP≥ 40 mmHg | EF < 50% | ≤ 1.0 cm2 or ≤ 0.6 cm2/m2 | Severe calcification Severely reduced opening |
| D1 | Symptomatic severe high-gradient AS | Vmax≥ 4 m/s or mean ΔP≥ 40 mmHg | EF normal or decreased diastolic dysfunction | ≤ 1.0 cm2 or ≤ 0.6 cm2/m2 Larger with AR/MR | Severe calcification Severely reduced opening |
| D2 | Symptomatic severe low-flow/low-gradient AS with reduced LVEF | Vmax < 4 m/s or mean ΔP < 40 mmHg DOB stress shows Vmax > 4 m/s and AVA ≤ 1.0 cm2 | EF < 50% diastolic dysfunction | ≤ 1.0 cm2 | Severe calcification Severely reduced opening |
| D3 | Symptomatic severe low-gradient AS with normal LVEF or paradoxical low-flow severe AS | Vmax < 4 m/s or mean ΔP < 40 mmHg Stroke volume index < 35 mL/m2 | EF ≥ 50% Small LV chamber Restrictive diastolic filling | ≤ 1.0 cm2 or ≤ 0.6 cm2/m2 | Severe calcification Severely reduced opening |
It should be noted that the stage D3 definition is based on a relatively new concept related to the progression of AS. Specifically, low-flow/low-gradient AS with preserved EF represents a more advanced stage of AS with severe concentric hypertrophy, high peripheral arterial pressure, and low systemic arterial compliance[17-19]. Valvulo-arterial impedance (Zva) calculated as shown below was introduced as a global hemodynamic load on the LV in AS[17,20,21]. Zva = (systolic blood pressure + mean gradient of aortic valve)/stroke volume index.
More importantly, low-flow/low-gradient severe AS is reported to have a poorer prognosis without surgical intervention in some studies[17,22,23], while other studies report a better prognosis-similar to the prognosis of patients with moderate AS[24-26]. Nevertheless, the majority of evidence is in favor of the new entity termed “paradoxical low-gradient AS”, in which LVEF is preserved yet the mean aortic valve gradient is low due to low stroke volume.
This condition must be diagnosed with utmost caution, avoiding measurement errors and, in some cases, establishing additional diagnostic methods such as cardiac catheterization or other imaging studies, including magnetic resonance imaging (MRI) and computed tomographic (CT) scans.
Current ACC/AHA guidelines put much more focus on velocity/pressure gradient findings than on aortic valve area (AVA) given that prior natural history studies show their prognostic importance. Namely, aortic velocity (> 4 m/s) is reported to be one of the most important factors associated with a higher event rate in AS[4,5,27]. Asymptomatic patients with very severe AS with a Vmax≥ 5 m/s or mean gradient ≥ 60 mmHg have an even worse event-free survival[28].
Two-dimensional/Doppler echocardiography plays a fundamental role in the diagnosis of AS. It is important to examine the etiology of AS, visual severity of valve calcification, position of the coronary artery orifice, concomitant myocardial disease, wall motion asynergy, and other valvular heart diseases with echocardiography. Echocardiography can provide systolic and diastolic functions. All parameters referred to in guidelines are available by echocardiography, which sometimes needs careful data interpretation while recognizing limitations.
An important consideration in echocardiography is to detect the highest peak aortic flow velocity using multiple transducer positions (the suprasternal window and right parasternal window with right decubitus position should be used in addition to the apical window). The Pedoff probe, which has a high signal to noise ratio, is ideal to detect the highest velocity. This requires advanced operator skill and, therefore, missing the highest velocity in AS is one of the causes of underestimation of the gradient.
The pressure gradient is calculated according to the modified Bernoulli equation; the pressure gradient = 4 ×ν2. However, if there is an increased velocity (> 1.5 m/s) at the LV outflow tract (LVOT) by septal thickening or by systolic anterior motion of the mitral valve, this simplified equation is less reliable. In those cases, it is recommended that the corrected peak to peak gradient should be used[29].
AVA is calculated by a continuity equation. Measurement of the LVOT size for stroke volume calculation is the second possible error for diagnostic severity. American Society of Echocardiography guidelines recommend the measurement at the same position of the pulse wave sample volume, specifically 0.5 to 1 cm below the aortic annulus[29]. Accurate measurement of LVOT diameter is critical, as the continuity method requires squaring of this measurement. Even an error of a few millimeters in this measurement can lead to large differences in the calculated valve area.
Additionally, appropriate position of the pulse-wave Doppler signal to avoid flow acceleration by calcified valve or outflow obstruction is important. Overestimation or underestimation of stroke volume can lead to an unreliable calculation of AVA. In some patients, one may also encounter dynamic LVOT obstruction with flow acceleration in the LVOT. In these patients, one must calculate stroke volume either by two-dimensional or three-dimensional volumetric methods. One can also use RVOT diameter and Doppler signals at the right ventricular outflow tract to calculate stroke volume.
AVA can also be measured by planimetry, both by transthoracic echocardiography and transesophageal echocardiography[30]. The planimetry method has its own limitations. Shadowing by calcification interferes with the visualization of the valve edge. The anatomical orifice area can be measured larger than the effective orifice area. Nonplanar structures of the valve may cause difficulty in reliable measurement, which is improved by real-time three-dimensional echocardiography[31]. With careful attention to these limitations, planimetry can be considered an alternative/complimentary measure when Doppler measurements are not appropriate.
A low-dose dobutamine stress echocardiography is performed to diagnose true or pseudo AS in low-gradient, reduced EF patients (though usually symptomatic, patients rarely present with low LVEF and gradient without any symptom). In addition, low-dose dobutamine echocardiography can identify high-risk patients who do not have contractile reserve, i.e., an increase in stroke volume ≥ 20%. Loss of contractile reserve suggests a patient may have other myocardial disease or advanced stages of severe AS. A maximum velocity ≥ 4.0 m/s with AVA ≤ 1.0 cm2 at any flow rate during dobutamine stress echocardiography is diagnosed as true severe AS[16,29]. Pseudo AS would show an increase of valve area to > 1.0 cm2. Although suggested, evidence for the use of dobutamine stress evaluation of low-flow/low-gradient AS with preserved EF (“paradoxical low-gradient severe AS”) to diagnose true/pseudo AS is limited.
Diastolic dysfunction is an important parameter in the evaluation of AS. Worsening of diastolic function is related to age and other comorbidities, such as hypertension, that are not uncommon in the elderly. It has been reported by Park et al[32] that echocardiographic markers of diastolic dysfunction, such as increased E/e′ and left atrial volume index, are associated with dyspnea in severe AS patients. Increased E/e′ (> 15) has been shown to predict survival in both asymptomatic and symptomatic patients with AS (adjusted mortality risk = 2.34; 95%CI: 1.27-4.33)[33]. Although echocardiographic measures of diastolic dysfunction are markers of worse AS, current guidelines do not support its use in surgical decision making in patients with either symptomatic or asymptomatic AS.
Recent advances in echocardiography led to the development of newer methods to detect subtle changes in LV function beyond EF. Specifically, two-dimensional speckle-tracking echocardiography has been used in numerous research studies to detect early systolic functional deterioration in cardiomyopathies, including amyloidosis and hypertrophic cardiomyopathy. Global longitudinal strain (GLS) by two-dimensional speckle-tracking echocardiography is decreased in severe AS and can be used as a prognostic measure. Kearney et al[34] reported that decreased GLS (> -15%) in asymptomatic severe AS with preserved EF had poor survival when compared to patients with GLS ≤ -15%, and GLS was a predictor of all-cause mortality (HR = 1.42; 95%CI: 1.27-1.59). Using echocardiography, van Dalen et al[35] and Staron et al[36] reported that increased apical rotation is more common in AS patients than control patients. In general, worsening systolic longitudinal motion, apical rotation, and diastolic untwisting working in concert are manifestations of progressive AS. Further research studies and standardization of analyzing software are necessary to incorporate these measurements into current clinical practice, specifically their role in surgical decision making for patients with asymptomatic severe AS.
Aortic valve calcification (AVC) is a prognostic factor in asymptomatic AS. Rosenhek et al[27] evaluated the degree of AVC using echocardiography and showed moderate and severe AVC related to future death or development of symptoms (RR = 5.2; 95%CI: 2.4-13.5). However, the definition of the degree of AVC by echocardiography has not been established. Therefore, evaluation of AVC by echocardiography is still a qualitative and subjective measure that is dependent on the echocardiographer’s experience. A cardiac CT scan can provide quantitative measure of AVC, and > 1000 Agaston units can be considered severe calcification[16,37]. Currently, a cardiac CT scan can be used as a complementary method for the diagnosis and management of AS[16]. When it is difficult to judge the severity of AS due to discordant measurements in echocardiography or a possible paradoxical low-flow/low-gradient AS, CT imaging can help to provide the calcium score, which relates to stenosis severity and prognosis[38,39]. Due to the recent rapid development of transcatheter aortic valve replacement (TAVR) procedures, the cardiac CT scan has emerged as a key imaging modality not only to assess aortic valve and root calcification, but also for precise measurement of the aortic annulus and peripheral arteries[40-42]. Whether or not three-dimensional LVOT measurements by CT imaging should replace echocardiography in order to resolve the measurement error issue is still uncertain, and warrants further research[43,44].
Magnetic resonance imaging (MRI) has the advantage of providing more accurate anatomical and hemodynamic information, LV mass, and stroke volume than echocardiography. In addition, cardiac MRI with gadolinium contrast can provide information about fibrosis or collagen deposition of the myocardium, which is a consequence of long-term exposure to substantial afterload. The presence or absence of myocardial fibrosis in any cardiac disease is an important prognostic factor[45-48]. Dweck et al[49] performed contrast-enhanced cardiac MRI in 143 AS patients, and the reported late gadolinium enhancement in the mid wall was a predictor of all-cause mortality (HR = 8.59; 95%CI: 1.97-37.38). Further research using more sensitive methods, i.e., T1 mapping, to detect myocardial fibrosis in AS patients, as well as prognostic studies linking fibrosis to better outcomes, are warranted before MRI can be used in routine clinical practice for the management of patients with AS[50].
Brain natriuretic peptide (BNP) is thought to be a good marker of increased wall stress in the myocardium, thus BNP increases with age, the presence of hypertension, valvular heart disease, and other myocardial diseases. It has been reported that BNP increases along with the severity of AS, but considerable overlap between the groups has also been observed. Bergler-Klein et al[51] reported that asymptomatic severe AS patients whose plasma BNP was < 130 pg/mL rarely developed symptoms for 6 to 9 mo. Another study showed that a BNP ≥ 300 pg/mL was a poor prognostic factor in medically followed severe AS patients who were both symptomatic and asymptomatic[33]. More recently, Clavel et al[52] reported that moderate/severe asymptomatic AS patients with BNP clinical activation and an elevated BNP greater than the upper normal range of the same age/sex have a higher rate of mortality (HR = 2.35; 95%CI: 1.57-3.56). Recently published research is summarized in Table 2. Disadvantages of BNP include that fact that it is not disease-specific, and BNP levels vary even in the same patient according to physical activities and loading conditions. Therefore, a single value of BNP may not be helpful in surgical decision making in asymptomatic severe AS patients. However, serial measures and rising levels of BNP can be used for surgical decision making in asymptomatic severe AS patients, as proposed by European Society of Cardiology (ESC) guidelines[53].
| Source | BNP cut-off value | Results | Enrolled patients |
| Bergler-Klein et al[51] | BNP 130 pg/mL | BNP < 130 pg/mL (n = 25) had better symptom-free survival (P < 0.001) | Asymptomatic severe AS, EF ≥ 50% (n = 43) |
| Biner et al[33] | BNP 300 pg/mL | Combined use of BNP > 300 pg/mL and E/e’ > 15 predicted 1-yr mortality (hazard ratio = 2.59; 95%CI: 1.21-5.55, P = 0.014) | Severe AS, symptomatic and asymptomatic, any EF included (n = 79) |
| Berger-Klein et al[54] | BNP 550 pg/mL | BNP ≥ 550 pg/mL showed poorer survival both in medically and surgically treated groups | Indexed effective orifice area ≤ 0.6 cm2/m2 with low-flow/low-gradient AS; symptomatic and asymptomatic, with EF ≤ 40% (n = 69) |
| Clavel et al[52] | BNP ratio: Measured BNP/maximal-normal-BNP for age and sex | Higher BNP ratio showed worse mortality in asymptomatic patients with preserved EF (hazard ratio = 2.35; 95%CI: 1.57-3.56, P < 0.0001) | Total, moderate or severe AS, any EF (n = 1953) Asymptomatic, with EF > 50% (n = 565) |
Symptom onset is the key to referring severe AS patients for AVR because of a poor prognosis without AVR. However, it is challenging in some patients who claim to be asymptomatic yet have severe AS. In order to risk-stratify high-risk asymptomatic patients, an exercise test, such as the standard treadmill test, without imaging is reasonable according to recently published guidelines[16,55-57]. Development of symptoms early on in exercise treadmill testing or an abnormal blood pressure response (below baseline or an inadequate increase of blood pressure < 20 mmHg) are considered indications for surgery in patients with severe AS; however, exercise testing is contraindicated in patients with symptomatic severe AS (Class III)[16]. Although ESC guidelines[53] have suggested the use of an increased mean gradient during exercise testing (> 20 mmHg) as an indication for surgery in asymptomatic patients (Class IIb), it was not supported in the more recent ACC/AHA guidelines[16].
Catheterization has the risk of a small cerebral emboli when the wire crosses the valve[58]; thus, catheterization is recommended only when there is discrepancy between noninvasive testing, clinical examination, and clinical presentation.
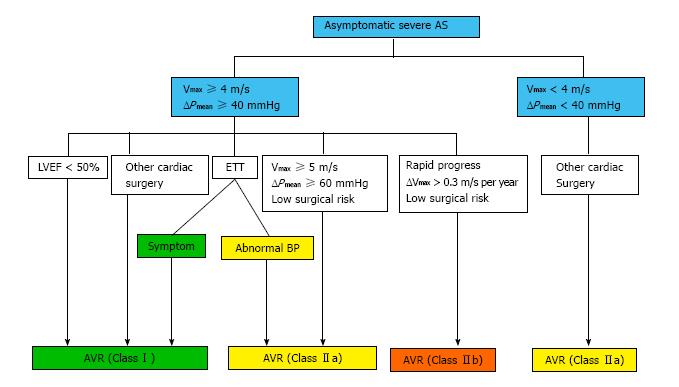
It is clear that AVR is recommended in symptomatic patients with severe AS; however, the decision to recommend early surgery in asymptomatic severe AS patients is still challenging. Indications for AVR in asymptomatic patients are shown in Figure 1, which is based on 2014 AHA/ACC guidelines. Indications for AVR have been consistent between AHA/ACC guidelines and ESC guidelines, though there are slight differences. Asymptomatic patients with severe calcification and a rapid increase in aortic peak transvalvular velocity should be considered for AVR in ESC guidelines with a Class IIa indication, but that is a Class IIb indication according to AHA/ACC guidelines. Patients with elevated BNP levels, an increase in the Doppler mean pressure gradient with exercise, and excessive LVH may be considered for AVR by ESC guidelines (Class IIb), but these are not employed in AHA/ACC guidelines.
Based on the current evidence and guidelines, it is reasonable to consider AVR in severe AS patients when (1) systolic function is decreased (EF < 50%); (2) it is very severe AS (Vmax≥ 5 m/s, ΔP ≥ 60 mmHg); (3) results of the exercise test are abnormal; or (4) there is rapid progression in AS severity (ΔVmax > 0.3 m/s per year) (Figure 1). One must follow patients more closely despite asymptomatic severe AS when there is (1) severe aortic valve calcification; (2) end-stage renal disease; (3) worsening diastolic dysfunction; (4) increased left atrial volume; (5) high brain natriuretic peptide, especially during serial measurements; and (6) new onset of atrial fibrillation or frequent episodes of paroxysmal atrial fibrillation.
Coronary artery disease and AS have similar risk factors. Additionally, AS has an active inflammation that causes valve calcification. Positive results in experimental and clinical studies on the effectiveness of statins to decrease hemodynamic progression have been published[59,60], while randomized clinical trials were performed to validate the effect of statins on AS progression[61]. Although there was the benefit of fewer ischemic cardiovascular events in the treatment groups, no considerable difference in hemodynamic progression was observed between the treatment and placebo groups[6]. However, many patients with AS have known concomitant coronary artery disease or risk factors and hyperlipidemia. Guideline-based statin therapy should be considered in these patients regardless of presence of AS.
For AS, the only effective treatment is valve replacement, but it is important to properly manage comorbidities, especially hypertension. Calcific AS is commonly found in the elderly, thus many patients have already been on antihypertensive medication at the time of diagnosis, including diuretics and vasodilators, though diuretics and vasodilators have been thought to be avoided. The current guidelines recommend following guideline-directed medical therapy for hypertension, starting at a low dose and gradually increasing to achieve appropriate blood pressure control. Effectiveness of angiotensin-converting enzyme inhibitors has been investigated on AS in terms of potential benefit on reducing LV fibrosis[62-64]. Patients with LVOT obstruction caused by discrete upper septal thickening or mid-ventricular obstruction by severely concentric hypertrophy in the setting of AS and hypertension pose a clinical challenge. A β-blocker and appropriate hydration is recommended, and diuretics/vasodilators should be avoided in these patients.
Currently, due to an aging population, AS is one of the most common valvular heart diseases. Recent ACC/AHA guidelines provide a new classification system of categorizing valve diseases in patients, including those with AS, that is similar to the classification used in patients with heart failure. In addition, diagnostic strategies and treatment options for the new entity termed “paradoxical low-gradient severe AS”, despite preserved EF, are given.
Decisions for AVR are based on the presence or absence of symptoms, but proactive investigation with multimodality testing for risk assessment is recommended in patients who are asymptomatic or who have indeterminate symptoms. Exercise stress testing is recommended for asymptomatic severe AS patients in addition to two-dimensional/Doppler echocardiographic testing at rest for risk stratification. If a patient is not physically appropriate for exercise testing, use of a biomarker and multiple imaging modalities, such as CT and MRI with contrast, can complement the risk stratification of asymptomatic severe AS.
Based on the available evidence, it is now reasonable to consider AVR in asymptomatic severe AS patients with (1) decreased EF (< 50%); (2) very severe AS (Vmax > 5 m/s or ΔP ≥ 60 mmHg); (3) an abnormal exercise test; (4) rapid progression of AS (ΔVmax >0.3 m/s per year); and (5) progressively rising BNP.
Careful attention with frequent follow up is necessary in patients with (1) heavy calcification of the aortic valve (especially end-stage renal disease patients); (2) advanced stage of diastolic dysfunction (≥ stage 2); (3) elevated BNP compared to same age/sex; and (4) new onset of atrial fibrillation.
P- Reviewer: Cebi N, Lymperopoulos A, Nunez-Gil IJ, Peteiro J, Skobel E S- Editor: Song XX L- Editor: A E- Editor: Lu YJ
| 1. | Novaro GM, Katz R, Aviles RJ, Gottdiener JS, Cushman M, Psaty BM, Otto CM, Griffin BP. Clinical factors, but not C-reactive protein, predict progression of calcific aortic-valve disease: the Cardiovascular Health Study. J Am Coll Cardiol. 2007;50:1992-1998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 149] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 2. | Otto CM, Prendergast B. Aortic-valve stenosis--from patients at risk to severe valve obstruction. N Engl J Med. 2014;371:744-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 412] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 3. | Otto CM, Pearlman AS, Gardner CL. Hemodynamic progression of aortic stenosis in adults assessed by Doppler echocardiography. J Am Coll Cardiol. 1989;13:545-550. [PubMed] |
| 4. | Rosenhek R, Klaar U, Schemper M, Scholten C, Heger M, Gabriel H, Binder T, Maurer G, Baumgartner H. Mild and moderate aortic stenosis. Natural history and risk stratification by echocardiography. Eur Heart J. 2004;25:199-205. [PubMed] |
| 5. | Otto CM, Burwash IG, Legget ME, Munt BI, Fujioka M, Healy NL, Kraft CD, Miyake-Hull CY, Schwaegler RG. Prospective study of asymptomatic valvular aortic stenosis. Clinical, echocardiographic, and exercise predictors of outcome. Circulation. 1997;95:2262-2270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 733] [Cited by in RCA: 700] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 6. | Rossebø AB, Pedersen TR, Boman K, Brudi P, Chambers JB, Egstrup K, Gerdts E, Gohlke-Bärwolf C, Holme I, Kesäniemi YA. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008;359:1343-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1166] [Cited by in RCA: 1145] [Article Influence: 67.4] [Reference Citation Analysis (0)] |
| 7. | Nassimiha D, Aronow WS, Ahn C, Goldman ME. Rate of progression of valvular aortic stenosis in patients & gt; or = 60 years of age. Am J Cardiol. 2001;87:807-89, A9. [PubMed] |
| 8. | Kearney LG, Ord M, Buxton BF, Matalanis G, Patel SK, Burrell LM, Srivastava PM. Progression of aortic stenosis in elderly patients over long-term follow up. Int J Cardiol. 2013;167:1226-1231. [PubMed] |
| 9. | Perkovic V, Hunt D, Griffin SV, du Plessis M, Becker GJ. Accelerated progression of calcific aortic stenosis in dialysis patients. Nephron Clin Pract. 2003;94:c40-c45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 88] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Villari B, Campbell SE, Hess OM, Mall G, Vassalli G, Weber KT, Krayenbuehl HP. Influence of collagen network on left ventricular systolic and diastolic function in aortic valve disease. J Am Coll Cardiol. 1993;22:1477-1484. [PubMed] |
| 11. | Krayenbuehl HP, Hess OM, Monrad ES, Schneider J, Mall G, Turina M. Left ventricular myocardial structure in aortic valve disease before, intermediate, and late after aortic valve replacement. Circulation. 1989;79:744-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 350] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 12. | Gilchrist IC, Waxman HL, Kurnik PB. Improvement in early diastolic filling dynamics after aortic valve replacement. Am J Cardiol. 1990;66:1124-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Monrad ES, Hess OM, Murakami T, Nonogi H, Corin WJ, Krayenbuehl HP. Time course of regression of left ventricular hypertrophy after aortic valve replacement. Circulation. 1988;77:1345-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 166] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Villari B, Vassalli G, Betocchi S, Briguori C, Chiariello M, Hess OM. Normalization of left ventricular nonuniformity late after valve replacement for aortic stenosis. Am J Cardiol. 1996;78:66-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Burt JR, Zimmerman SL, Kamel IR, Halushka M, Bluemke DA. Myocardial T1 mapping: techniques and potential applications. Radiographics. 2014;34:377-395. [PubMed] |
| 16. | Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Guyton RA, O’Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM, Thomas JD; American College of Cardiology/American Heart Association Task Force on Practice G. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:e57-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1918] [Cited by in RCA: 1865] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 17. | Hachicha Z, Dumesnil JG, Bogaty P, Pibarot P. Paradoxical low-flow, low-gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation. 2007;115:2856-2864. [PubMed] |
| 18. | Herrmann S, Störk S, Niemann M, Lange V, Strotmann JM, Frantz S, Beer M, Gattenlöhner S, Voelker W, Ertl G. Low-gradient aortic valve stenosis myocardial fibrosis and its influence on function and outcome. J Am Coll Cardiol. 2011;58:402-412. [PubMed] |
| 19. | Pibarot P, Dumesnil JG. Improving assessment of aortic stenosis. J Am Coll Cardiol. 2012;60:169-180. [PubMed] |
| 20. | Briand M, Dumesnil JG, Kadem L, Tongue AG, Rieu R, Garcia D, Pibarot P. Reduced systemic arterial compliance impacts significantly on left ventricular afterload and function in aortic stenosis: implications for diagnosis and treatment. J Am Coll Cardiol. 2005;46:291-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 381] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 21. | Hachicha Z, Dumesnil JG, Pibarot P. Usefulness of the valvuloarterial impedance to predict adverse outcome in asymptomatic aortic stenosis. J Am Coll Cardiol. 2009;54:1003-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 267] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 22. | Clavel MA, Dumesnil JG, Capoulade R, Mathieu P, Sénéchal M, Pibarot P. Outcome of patients with aortic stenosis, small valve area, and low-flow, low-gradient despite preserved left ventricular ejection fraction. J Am Coll Cardiol. 2012;60:1259-1267. [PubMed] |
| 23. | Eleid MF, Sorajja P, Michelena HI, Malouf JF, Scott CG, Pellikka PA. Flow-gradient patterns in severe aortic stenosis with preserved ejection fraction: clinical characteristics and predictors of survival. Circulation. 2013;128:1781-1789. [PubMed] |
| 24. | Jander N, Minners J, Holme I, Gerdts E, Boman K, Brudi P, Chambers JB, Egstrup K, Kesäniemi YA, Malbecq W. Outcome of patients with low-gradient “severe” aortic stenosis and preserved ejection fraction. Circulation. 2011;123:887-895. [PubMed] |
| 25. | Tribouilloy C, Rusinaru D, Maréchaux S, Castel AL, Debry N, Maizel J, Mentaverri R, Kamel S, Slama M, Lévy F. Low-gradient, low-flow severe aortic stenosis with preserved left ventricular ejection fraction: characteristics, outcome, and implications for surgery. J Am Coll Cardiol. 2015;65:55-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 158] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 26. | Yamashita E, Takeuchi M, Seo Y, Izumo M, Ishizu T, Sato K, Suzuki K, Akashi YJ, Aonuma K, Otsuji Y. Prognostic value of paradoxical low-gradient severe aortic stenosis in Japan: Japanese Multicenter Aortic Stenosis Study, Retrospective (JUST-R) Registry. J Cardiol. 2015;65:360-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Rosenhek R, Binder T, Porenta G, Lang I, Christ G, Schemper M, Maurer G, Baumgartner H. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med. 2000;343:611-617. [PubMed] |
| 28. | Rosenhek R, Zilberszac R, Schemper M, Czerny M, Mundigler G, Graf S, Bergler-Klein J, Grimm M, Gabriel H, Maurer G. Natural history of very severe aortic stenosis. Circulation. 2010;121:151-156. [PubMed] |
| 29. | Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, Iung B, Otto CM, Pellikka PA, Quiñones M. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr. 2009;22:1-23; quiz 101-102. [PubMed] |
| 30. | Okura H, Yoshida K, Hozumi T, Akasaka T, Yoshikawa J. Planimetry and transthoracic two-dimensional echocardiography in noninvasive assessment of aortic valve area in patients with valvular aortic stenosis. J Am Coll Cardiol. 1997;30:753-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 37] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Nakai H, Takeuchi M, Yoshitani H, Kaku K, Haruki N, Otsuji Y. Pitfalls of anatomical aortic valve area measurements using two-dimensional transoesophageal echocardiography and the potential of three-dimensional transoesophageal echocardiography. Eur J Echocardiogr. 2010;11:369-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 32. | Park SJ, Enriquez-Sarano M, Chang SA, Choi JO, Lee SC, Park SW, Kim DK, Jeon ES, Oh JK. Hemodynamic patterns for symptomatic presentations of severe aortic stenosis. JACC Cardiovasc Imaging. 2013;6:137-146. [PubMed] |
| 33. | Biner S, Rafique AM, Goykhman P, Morrissey RP, Naghi J, Siegel RJ. Prognostic value of E/E’ ratio in patients with unoperated severe aortic stenosis. JACC Cardiovasc Imaging. 2010;3:899-907. [PubMed] |
| 34. | Kearney LG, Lu K, Ord M, Patel SK, Profitis K, Matalanis G, Burrell LM, Srivastava PM. Global longitudinal strain is a strong independent predictor of all-cause mortality in patients with aortic stenosis. Eur Heart J Cardiovasc Imaging. 2012;13:827-833. [PubMed] |
| 35. | van Dalen BM, Tzikas A, Soliman OI, Kauer F, Heuvelman HJ, Vletter WB, ten Cate FJ, Geleijnse ML. Left ventricular twist and untwist in aortic stenosis. Int J Cardiol. 2011;148:319-324. [PubMed] |
| 36. | Staron A, Bansal M, Kalakoti P, Nakabo A, Gasior Z, Pysz P, Wita K, Jasinski M, Sengupta PP. Speckle tracking echocardiography derived 2-dimensional myocardial strain predicts left ventricular function and mass regression in aortic stenosis patients undergoing aortic valve replacement. Int J Cardiovasc Imaging. 2013;29:797-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 37. | Messika-Zeitoun D, Aubry MC, Detaint D, Bielak LF, Peyser PA, Sheedy PF, Turner ST, Breen JF, Scott C, Tajik AJ. Evaluation and clinical implications of aortic valve calcification measured by electron-beam computed tomography. Circulation. 2004;110:356-362. [PubMed] |
| 38. | Clavel MA, Messika-Zeitoun D, Pibarot P, Aggarwal SR, Malouf J, Araoz PA, Michelena HI, Cueff C, Larose E, Capoulade R. The complex nature of discordant severe calcified aortic valve disease grading: new insights from combined Doppler echocardiographic and computed tomographic study. J Am Coll Cardiol. 2013;62:2329-2338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 415] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 39. | Clavel MA, Pibarot P, Messika-Zeitoun D, Capoulade R, Malouf J, Aggarval S, Araoz PA, Michelena HI, Cueff C, Larose E. Impact of aortic valve calcification, as measured by MDCT, on survival in patients with aortic stenosis: results of an international registry study. J Am Coll Cardiol. 2014;64:1202-1213. [PubMed] |
| 40. | Kasel AM, Cassese S, Bleiziffer S, Amaki M, Hahn RT, Kastrati A, Sengupta PP. Standardized imaging for aortic annular sizing: implications for transcatheter valve selection. JACC Cardiovasc Imaging. 2013;6:249-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 194] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 41. | Koos R, Mahnken AH, Dohmen G, Brehmer K, Günther RW, Autschbach R, Marx N, Hoffmann R. Association of aortic valve calcification severity with the degree of aortic regurgitation after transcatheter aortic valve implantation. Int J Cardiol. 2011;150:142-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 131] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 42. | Kodali SK, Williams MR, Smith CR, Svensson LG, Webb JG, Makkar RR, Fontana GP, Dewey TM, Thourani VH, Pichard AD. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med. 2012;366:1686-1695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1741] [Cited by in RCA: 1769] [Article Influence: 136.1] [Reference Citation Analysis (0)] |
| 43. | Kamperidis V, van Rosendael PJ, Katsanos S, van der Kley F, Regeer M, Al Amri I, Sianos G, Marsan NA, Delgado V, Bax JJ. Low gradient severe aortic stenosis with preserved ejection fraction: reclassification of severity by fusion of Doppler and computed tomographic data. Eur Heart J. 2015;36:2087-2096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 44. | Clavel MA, Malouf J, Messika-Zeitoun D, Araoz PA, Michelena HI, Enriquez-Sarano M. Aortic valve area calculation in aortic stenosis by CT and Doppler echocardiography. JACC Cardiovasc Imaging. 2015;8:248-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 149] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 45. | Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, Klocke FJ, Bonow RO, Judd RM. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2376] [Cited by in RCA: 2212] [Article Influence: 88.5] [Reference Citation Analysis (0)] |
| 46. | Moon JC, McKenna WJ, McCrohon JA, Elliott PM, Smith GC, Pennell DJ. Toward clinical risk assessment in hypertrophic cardiomyopathy with gadolinium cardiovascular magnetic resonance. J Am Coll Cardiol. 2003;41:1561-1567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 511] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 47. | Assomull RG, Prasad SK, Lyne J, Smith G, Burman ED, Khan M, Sheppard MN, Poole-Wilson PA, Pennell DJ. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol. 2006;48:1977-1985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 845] [Cited by in RCA: 856] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 48. | Rudolph A, Abdel-Aty H, Bohl S, Boyé P, Zagrosek A, Dietz R, Schulz-Menger J. Noninvasive detection of fibrosis applying contrast-enhanced cardiac magnetic resonance in different forms of left ventricular hypertrophy relation to remodeling. J Am Coll Cardiol. 2009;53:284-291. [PubMed] |
| 49. | Dweck MR, Joshi S, Murigu T, Alpendurada F, Jabbour A, Melina G, Banya W, Gulati A, Roussin I, Raza S. Midwall fibrosis is an independent predictor of mortality in patients with aortic stenosis. J Am Coll Cardiol. 2011;58:1271-1279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 419] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 50. | Iles L, Pfluger H, Phrommintikul A, Cherayath J, Aksit P, Gupta SN, Kaye DM, Taylor AJ. Evaluation of diffuse myocardial fibrosis in heart failure with cardiac magnetic resonance contrast-enhanced T1 mapping. J Am Coll Cardiol. 2008;52:1574-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 587] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 51. | Bergler-Klein J, Klaar U, Heger M, Rosenhek R, Mundigler G, Gabriel H, Binder T, Pacher R, Maurer G, Baumgartner H. Natriuretic peptides predict symptom-free survival and postoperative outcome in severe aortic stenosis. Circulation. 2004;109:2302-2308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 314] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 52. | Clavel MA, Malouf J, Michelena HI, Suri RM, Jaffe AS, Mahoney DW, Enriquez-Sarano M. B-type natriuretic peptide clinical activation in aortic stenosis: impact on long-term survival. J Am Coll Cardiol. 2014;63:2016-2025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 154] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 53. | Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Barón-Esquivias G, Baumgartner H, Borger MA, Carrel TP, De Bonis M, Evangelista A. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J. 2012;33:2451-2496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2682] [Cited by in RCA: 2647] [Article Influence: 203.6] [Reference Citation Analysis (0)] |
| 54. | Bergler-Klein J, Mundigler G, Pibarot P, Burwash IG, Dumesnil JG, Blais C, Fuchs C, Mohty D, Beanlands RS, Hachicha Z. B-type natriuretic peptide in low-flow, low-gradient aortic stenosis: relationship to hemodynamics and clinical outcome: results from the Multicenter Truly or Pseudo-Severe Aortic Stenosis (TOPAS) study. Circulation. 2007;115:2848-2855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 100] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 55. | Amato MC, Moffa PJ, Werner KE, Ramires JA. Treatment decision in asymptomatic aortic valve stenosis: role of exercise testing. Heart. 2001;86:381-386. [PubMed] |
| 56. | Das P, Rimington H, Chambers J. Exercise testing to stratify risk in aortic stenosis. Eur Heart J. 2005;26:1309-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 233] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 57. | Alborino D, Hoffmann JL, Fournet PC, Bloch A. Value of exercise testing to evaluate the indication for surgery in asymptomatic patients with valvular aortic stenosis. J Heart Valve Dis. 2002;11:204-209. [PubMed] |
| 58. | Meine TJ, Harrison JK. Should we cross the valve: the risk of retrograde catheterization of the left ventricle in patients with aortic stenosis. Am Heart J. 2004;148:41-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 59. | Rajamannan NM, Subramaniam M, Springett M, Sebo TC, Niekrasz M, McConnell JP, Singh RJ, Stone NJ, Bonow RO, Spelsberg TC. Atorvastatin inhibits hypercholesterolemia-induced cellular proliferation and bone matrix production in the rabbit aortic valve. Circulation. 2002;105:2660-2665. [PubMed] |
| 60. | Moura LM, Ramos SF, Zamorano JL, Barros IM, Azevedo LF, Rocha-Gonçalves F, Rajamannan NM. Rosuvastatin affecting aortic valve endothelium to slow the progression of aortic stenosis. J Am Coll Cardiol. 2007;49:554-561. [PubMed] |
| 61. | Cowell SJ, Newby DE, Prescott RJ, Bloomfield P, Reid J, Northridge DB, Boon NA; Lipid Lowering Trial IoRI. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med. 2005;352:2389-2397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 767] [Cited by in RCA: 782] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 62. | O’Brien KD, Zhao XQ, Shavelle DM, Caulfield MT, Letterer RA, Kapadia SR, Probstfield JL, Otto CM. Hemodynamic effects of the angiotensin-converting enzyme inhibitor, ramipril, in patients with mild to moderate aortic stenosis and preserved left ventricular function. J Investig Med. 2004;52:185-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 63. | Chockalingam A, Venkatesan S, Subramaniam T, Jagannathan V, Elangovan S, Alagesan R, Gnanavelu G, Dorairajan S, Krishna BP, Chockalingam V; Symptomatic Cardiac Obstruction-Pilot Study of Enalapril in Aortic S. Safety and efficacy of angiotensin-converting enzyme inhibitors in symptomatic severe aortic stenosis: Symptomatic Cardiac Obstruction-Pilot Study of Enalapril in Aortic Stenosis (SCOPE-AS). Am Heart J. 2004;147:E19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 113] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 64. | Nadir MA, Wei L, Elder DH, Libianto R, Lim TK, Pauriah M, Pringle SD, Doney AD, Choy AM, Struthers AD. Impact of renin-angiotensin system blockade therapy on outcome in aortic stenosis. J Am Coll Cardiol. 2011;58:570-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 127] [Article Influence: 9.1] [Reference Citation Analysis (0)] |