INTRODUCTION
Chronic functional mitral regurgitation (FMR) is a frequent complication of ischemic heart disease or less frequently dilated cardiomyopathy (DCM), following left ventricular (LV) dysfunction and remodeling. Various degrees of severity of FMR are commonly described in patients with LV dysfunction despite a structurally normal valve. Indeed, according to Carpentier’s functional classification, FMR can be due to dilated mitral annulus (type I) or more often to a systolic restriction of leaflet motion (type IIIb).
The exact occurrence of FMR is difficult to assess because of different diagnostic approaches and timing of evaluation. The prevalence of FMR is nevertheless considerable, varying from 20% to 50% after myocardial infarction (MI) as assessed by echocardiographic studies[1]. This has been further confirmed by recent studies assessing long-term outcome of patients affected by heart failure associated with FMR treated with standard medical therapy[2].
Both ischemic and non-ischemic FMR are related to an unfavourable outcome in DCM[3,4], independently of the degree of ventricular dysfunction. Additionally the degree of FMR relates directly to the mortality and heart failure events. Actually, FMR is related to a decreased survival rate even if of mild degree, as MR severity positively correlates to increased mortality. An effective regurgitant orifice area > 20 mm2 has been shown to double all-cause mortality and the risk of admission for acute decompensated heart failure. Furthermore, the presence of even moderate MR increased the risk of heart failure and death by more than 3-fold and 2-fold at 5 years respectively[5].
PATHOPHYSIOLOGY
Several pathophysiologic mechanisms are involved in determining FMR. Kaul et al[6] speculated that MR resulted from global LV dysfunction, rejecting the role of dysfunction of papillary muscles and the adjacent LV myocardium in determining FMR. Further studies failed to demonstrate that LV systolic dysfunction in the absence of LV dilatation and remodeling produced significant MR, whereas leaflets tethering was the only independent predictor of MR and LV sphericity was correlated to MR grade. Certainly an imbalance between closing and tethering forces is responsible for FMR due to LV dilatation and reduction of contractility, global LV dyssynchrony, papillary muscles displacement and dyssynchrony, altered systolic mitral annular contraction[7].
Tethering is the principal determinant of FMR, because of LV remodeling associated to apical and posterior papillary muscle displacement, that lead to a reduction in closing forces.
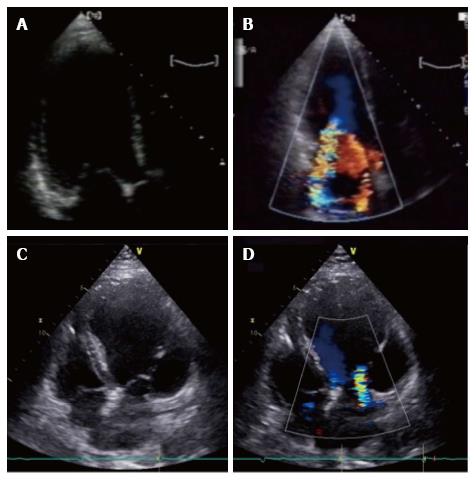
Depending on the type of global or local LV remodeling, two tethering patterns have been described[6]: The asymmetric and symmetric ones, depending on mitral leaflets position and their point of coaptation[8] (Figure 1).
Figure 1 Asymmetric and symmetric tethering pattern.
A, B: Asymmetric tethering pattern. Typical “hockey stick” or ‘‘bent knee’’ configuration. MV coaptation point is moved posteriorly and the anterior leaflet coapts creating a ‘‘pseudo-prolapse’’ appearance with a large regurgitant jet oriented along the posterior wall of the left atrium; C: Symmetric tethering pattern. Both leaflets are apically dislocated and coapt at the same level into the ventricle; D: Color-Doppler shows large central jet.
The asymmetric pattern is caused by an an asymmetrical shift of the posterior papillary muscle, determining a greater tenting of the posterior leaflet compared to the anterior one. Papillary muscle tethers the body of the anterior leaflet generating a “hockey stick” or ‘‘bent knee’’ configuration (Figure 1A and B). MV coaptation point is moved posteriorly and the anterior leaflet coapts creating a ‘‘pseudo-prolapse’’ appearance. The associated MR jet is typically eccentric, directed posteriorly in the left atrium (LA). Conversely in the symmetric pattern MV leaflet coaptation point is displaced towards the apex and both leaflets are tethered, generating a typically central MR jet (Figure 1C and D). This usually occurs in the context of a large anterior myocardial infarction, multiple infarcted area or idiopathic DCM.
Chronic FMR cause progressive LV dilation and papillary muscles displacement, leading to a further increase of tethering forces acting on mitral leaflets and, therefore, to a worsening of MR in vicious cycle.
Also conduction abnormalities, caused either by right ventricular pacing or bundle branch block, predispose to FMR. In fact the presence of intraventricular conduction determines mechanical dyssynchrony and mitral valve deformation[9].
Cardiac mechanical dyssynchrony can be distinguished in atrioventricular, inter- and intraventricular. Prolongation of the atrioventricular conduction time delays systolic ventricular contraction, hampering early diastolic filling when atrial suddenly decrease. Accordingly LV diastolic pressure exceeds atrial pressure causing diastolic mitral regurgitation. The reduction in LV preload determines a decrease in its contractility, according to Starling law.
As for inter- and intraventricular dyssynchrony, the former refers to delayed activation of LV relative to the right one, whereas the latter indicates differences in the timing of contraction of distinct myocardial segments. Both types of conduction delays cause an asynchronous contraction of LV wall (ventricular dyssynchrony), reducing stroke volume.
Mauer et al[10] first proved that significant differences in MR existed depending on the site of cardiac pacing. In particular they demonstrated in dogs that artificial stimulation through a right ventricular apical pacemaker generated a severe MR compared to a basal LV pacing within the coronary sinus.
Mechanical dyssynchrony may contribute to FMR as follows. First a decrease in MV closing forces can be determined by LV global dyssynchrony that may decrease the efficacy of LV systolic contraction[11,12]. Secondly, a geometric distortion of mitral valve apparatus may be induced by dyssynchronous contraction of the papillary muscle insertion sites[13]. Third, impaired leaflet coaptation can be enhanced by dyssynchronous contraction of LV basal segments, that may cause a papillary muscles asynchronous contraction[14] (Figure 2). The prolonged QRS duration correlates with both FMR severity and duration in patients with DCM[15,16]. Supporting this, several studies have shown that one of the positive effects of cardiac resynchronization therapy (CRT) is a decrease in FMR grade[17-20]. Soyama et al[21] analysed 32 patients affected by DCM with Tissue Doppler echocardiography showing that a dyssynchronous activation of myocardial segments adjacent to the papillary muscles could cause MR determining a non-synchronized closure of mitral leaflets. Donal et al[22] reported that MR in patients with DCM is a multifactorial and complex phenomenon, thus its accurate description should take into account LV contraction abnormalities and dyssynchrony, LV geometry and mitral orifice.
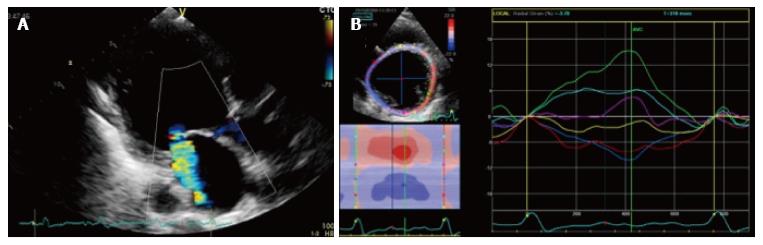
Figure 2 Impaired leaflet coaptation can be enhanced by dyssynchronous contraction of left ventricle basal segments, that may cause a papillary muscles asynchronous contraction.
A: 2D radial strain of a papillary muscles short axis in a patient with functional MR; B: During systole, myocardial segments adjacent to postero-medial papillary muscles (red and blue segments) show negative radial strain values, whereas antero-lateral papillary muscles (light blue and green segments) show a positive strain values, resulting in a significant papillary dyssynchrony. 2D: 2 dimensional; MR: Magnetic resonance.
Considering LV reverse remodeling after CRT, certainly the changes in MV apparatus influence the improvement of FMR. Konstantinou et al[23] studied FMR secondary to ischemic (n = 55) and non-ischemic DCM (n = 48) and found that FMR severity is mainly determined by the degree of mitral apparatus distortion; furthermore the authors found that in these patients, a quick estimation of FMR severity could be obtained observing coaptation height. Finally, additional determinants of FMR included the presence of global LV dyssynchrony and reduced myocardial systolic velocities of the posteromedial papillary muscle insertion site.
FMR is a dynamic condition, changing dramatically with loading conditions, because of phasic fluctuations in the balance between tethering and closing forces. The increase in afterload (i.e., hypertension, exercise) worsens MR, further deforming the infarcted papillary muscles bearing segments because they promptly deforms in response to increased intraventricular pressure. On the contrary diuretic therapy, afterload decrease by vasodilators or general anaesthesia reduce FMR severity. FMR is therefore a dynamic lesion, varying through the cardiac cycle, as the regurgitant volume is greater in the early and late systolic phases, lower in the mid systole, having a beat to beat variation[24]. In addition, Ennezat et al[25] showed that rest LV dyssynchrony is associated with worsening of FMR during exertion. In this study, 20% of patients with significant LV dyssynchrony developed an exercise-induced EROA increase, whereas the rest did not have a decrease in mitral EROA during exercise[25].
DEFORMATION IMAGING
Myocardial deformation imaging is a novel echocardiographic tool that can be used to evaluate global and regional myocardial function.
The evaluation of contractile function with echocardiography has traditionally been limited to volume-based assessment of global systolic function with ejection fraction (EF) and of segmental wall motion or visual estimation of regional thickening. These methods have suffered from lack of reproducibility and standardization and are generally considered to be extremely sensitive to loading conditions. These limitations have led to an interest in techniques that provide more objective and reproducible measures of contractile function.
During systolic phase, ventricular myocardium shortens in the longitudinal and circumferential planes, while getting thicker in the radial plane. Deformation imaging allows for a more direct evaluation of myocardial changes through the cardiac cycle by speckle tracking analysis.
Myocardial deformation imaging with echocardiography can be performed with the use of either tissue Doppler-based or 2-dimensional (2D) speckle tracking-based methods. Doppler methods suffer from limitations similar to those of traditional Doppler because it can only accurately assess deformation in the plane incident with the ultrasound beam and requires prospective acquisition of dedicated images at high frame rate.
Speckle tracking analysis is obtained assessing the spatial dislocation (tracking) of speckles (spots created by the interplay between ultrasounds and myocardial fibers) on bidimensional echo. This tool offers the advantage of an objective quantitative assessment of regional and global myocardial function, not affected by insonation angle, cardiac translational movements[26-28], with a good interobserver and intraobserver reproducibility, because of its semi-automated feature[29]. Furthermore, although initially proposed only for the LV functional assessment, many authors have showed its utility for the evaluation of other cardiac structures, in particular of the LA. A recent comparison between speckle tracking derived measures and jagged MRI showed feasibility and reproducibility of this echocardiographic tool[30].
Speckle tracking analysis evaluates strain, that can be described as the systolic change in length of a myocardial segment relatively to its length at rest, so expressed as a percentage. Strain rate is also calculated as the rate of this deformation[31].
This technique has some limitations, based on the need of a good definition of the endocardial borders, therefore being highly dependent on the quality of 2D images and frame rate.
Myocardial strain can be assessed in four principal planes of deformation: Radial strain (myocardial thickening) and circumferential strain (myocardial shortening) from short-axis views; transverse (myocardial thickening) and longitudinal strains (myocardial shortening) assessed from apical views. Furthermore, speckle tracking analysis allows a complete characterization of LV rotation (occurrence, direction and velocity) during cardiac cycle[32].
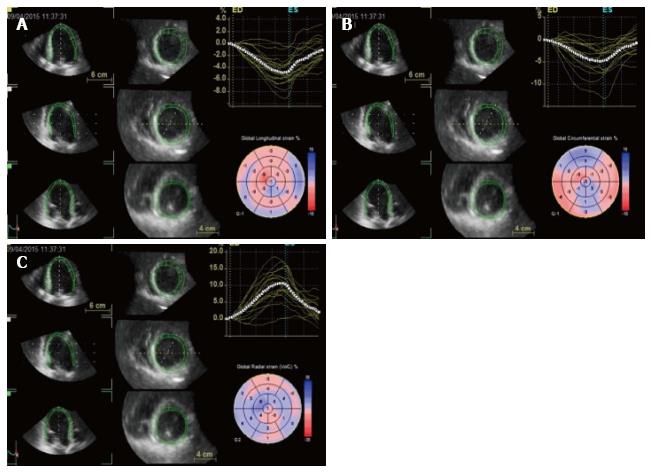
Longitudinal strain describes the systolic myocardial fibers shortening from the base to the apex. This deformation is expressed in negative trend curves, obtained analysing the myocardial shortening in apical 4-chamber, 2-chamber and long axis view (Figure 3). Both regional, so for each of the 17 LV myocardial segments, and global values are computed. Global longitudinal strain value has been shown to be a quantitative index of LV systolic performance[33]. Longitudinal strain can also be applied to LA[34] and right ventricle (RV) analysis strain[35], respectively assessing the peak atrial longitudinal strain and RV longitudinal strain values.
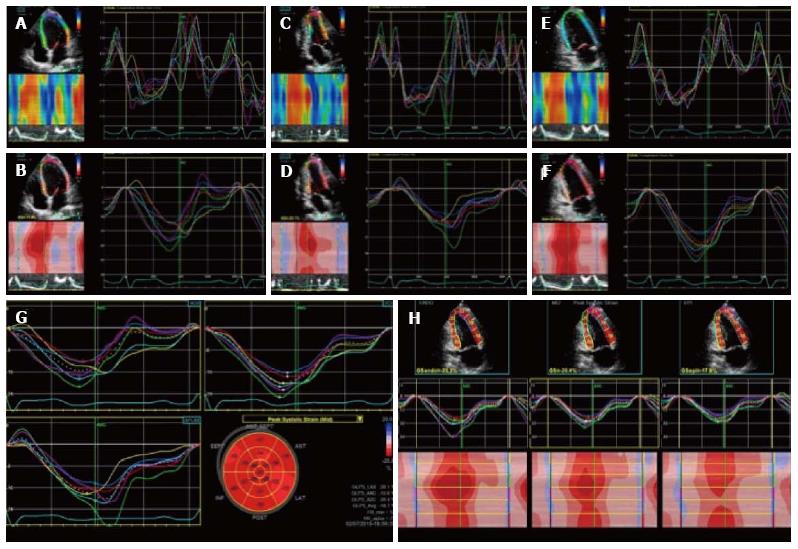
Figure 3 Two dimensional longitudinal strain.
2D longitudinal strain rate (A, C, E) and strain (B, D, F) analysis. Longitudinal strain is obtained from the 3 LV apical views (4C view, 2C view and 3C view). Strain values are then displayed in a bulls eye reconstruction (G). Longitudinal strain values for endocardium, mesocardium and epicardium can also be obtained (H). LV: Left ventricle; 2D: 2 dimensional.
Radial strain describes myocardial deformation directed radially towards the centre of LV cavity, represented by systolic thickening and diastolic thinning (Figure 4). Therefore, during the systolic phase radial strain curve will have positive values. Radial strain can be obtained through the analysis of parasternal short axis view, both from the basal and the apical cut[36].
Figure 4 Radial strain analysis with 2 dimensional speckle tracking.
Synchronous strain pattern in a normal patient. During systole, radial strain values are represented by positive curves.
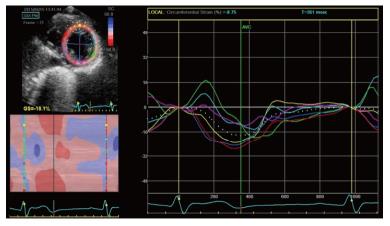
Circumferential strain is also obtained from speckle tracking analysis of the parasternal short axis view[37]. It represents the LV myocardial systolic shortening along its circumference and is expressed by systolic negative curves (Figure 5). A global circumferential strain value can also be calculated.
Figure 5 Two dimensional circumferential strain in a normal patient.
It represents left ventricle myocardial fiber shortening along the circular perimeter on a short-axis view and during systole, it is represented by synchronous negative curves.
Finally LV twisting[37], a fundamental component of LV systolic contraction, can be can be studied with speckle tracking analysis in terms of systolic reciprocal rotation of LV base and apex. LV twisting is computed as the difference between the mean rotation of the basal and the apical levels respectively, that can be the normalized for the apex-to-base distance, obtaining a “LV torsion” value[38].
Speckle tracking allows an early identification of global and segmental myocardial dysfunction, analysing the percentage of myocardial deformation that reflects the changes occurring in myocardial ultrastructure. Therefore lots of potential clinical application of this technique can be proposed, including the possibility to detect LV subclinical systolic impairment, if an alteration of longitudinal strain is discovered, for example in the setting of diabetes, coronary artery disease or valvulopathies. Several authors contributed to confirm this clinical application. Choi et al[39] studied asymptomatic patients without wall abnormalities and found that the presence of lower longitudinal strain values was a strong predictor of stable ischemic disease. Recently Voight et al[40] identified post systolic motion, after aortic valve closure, as a significant quantitative marker of the ischaemic myocardium. Further, it has been showed that with New York Heart Association (NYHA) functional class worsening from I to IV, progressively lower longitudinal strain values are observed in HF patients; in addition, in NYHA class III and IV, a systolic impairment of LV circumferential and radial strain become evident[41,42]. Stanton et al[43] studied HF patients with low EF and found global circumferential strain to be a strong predictor of cardiovascular adverse events. Furthermore, global longitudinal strain was also found to be a stronger predictor of outcome than EF in Mele et al[44] study. In low EF patients with indication to CRT, strain parameters have recently been shown to identify CRT responders with good reproducibility and accuracy[45]. In particular, dyssynchrony analysis by radial strain has been shown to effectively predict CRT efficacy[46,47].
Three-dimensional speckle tracking echocardiography (3D-STE) is the newest tool among deformation imaging and dyssynchrony analysis[48,49]. Differently from 2D speckle tracking, that analyses only a single plane and may oversimplify the complexities of LV mechanics, 3D speckle tracking takes advantage of pyramidal and strain data that include the whole LV, acquired with a matrix arrays transducer, therefore tracking speckles moving through a 3D space (Figure 6).
Figure 6 Three dimensional speckle tracking: data sets are displayed in different cross-sections including standard three short-axis views and apical four- and two-chamber views.
The software automatically divides data into 16 standard segments to generate corresponding time–strain curves. Wall motion parameters are simultaneously displayed in particular, the 3 orthogonal strain values (longitudinal strain in A, circumferential strain in B and radial strain in C).
Acquisition of a full-volume dataset requires smaller wedge-shaped sub-volumes from at least four consecutive heart beats (asking the patient to hold the breath), automatically combined in a single larger pyramidal volume. 3D datasets are displayed in different cross-sections including standard three short-axis views and apical four- and two-chamber views that could be modified interactively. Regions of interest are placed on the endocardium and epicardium from apical views, and the software automatically divides data into 16 standard segments to generate corresponding time-strain curves[50]. 3D-STE offers the advantage of simultaneously calculating radial, circumferential and longitudinal strain values in the whole LV myocardium. Furthermore, 3D-STE could help in selection of patients who may be CRT responders as it offers an accurate mechanical dyssynchrony map, and potentially it could guide the electrophysiologists during CRT implantation, precisely localizing the site of latest myocardial activation.
ROLE OF DEFORMATION IMAGING IN MITRAL VALVE DISEASE
STE in patients with mitral valve disease has been usually performed for LV and LA functional assessment. Asymptomatic patients with severe organic MR might develop latent LV systolic impairment even if EF appears to be normal. In asymptomatic patients affected by severe MR, preoperative evidence of LV dysfunction is associated with post-operative lower long term survival and worsening of systolic function. In fact, these patients usually have lower post-operative EF, higher incidence of heart failure and mortality, as compared to patients with severe MR without LV impairment before surgery[51]. Agricola et al[52] studied patients with MR and normal EF using TDI of the mitral annulus. They found out that longitudinal function can be altered despite normal EF and that systolic TDI value can predict post-operative LV impairment. Thus TDI has been proposed as a simple, available and immediate method to early recognize LV dysfunction due to volume overload in patients with significant MR. More recently, Lancellotti et al[53] underlined that limited exercise increase of global longitudinal strain in patients with degenerative MR, candidates to cardiac surgery, predicted post-operative LV dysfunction development.
Moonen et al[54] studied patients with MR and matched healthy controls using longitudinal strain analysis with 2D speckle tracking, both at rest and during exercise. At rest global longitudinal strain was significantly lower in MR patients. During exercise, a lower increase of this value was observed in MR patients compared to control group. In addition, up a small increase of global longitudinal strain at peak exercise was shown to be a predictor LV dysfunction during follow up[54].
MR generally progresses insidiously. Patients can be asymptomatic for a long time and, as the heart compensate to the regurgitant volume with LA enlargement, interpretation of LV EF can be challenging in presence of significant MR. Later, chronic volume overload will progressive LV dysfunction, subsequently worsening outcome.
Left atrial remodeling and dilation is associated with myocardiocyte hypertrophy and interstitial fibrosis, bringing with it the risk of atrial fibrillation (AF)[55]. Furthermore, the presence of LA remodeling predicts cardiovascular events, in particular stroke, death and heart failure. Transthoracic echocardiography permits only the evaluation of LA dimensions LA that has prognostic implication[56]. However the study of regional LA function may add information about atrial electromechanical remodeling, being useful for prognostic stratification, AF risk and management[55].
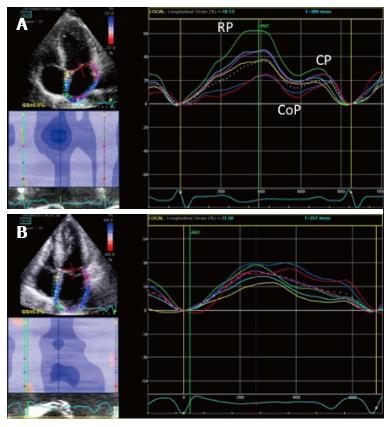
LA longitudinal deformation dynamics can be assessed by speckle tracking analysis. Peak atrial longitudinal strain allows the quantification of the reservoir phase of LA, that depends on atrial compliance. In fact, during this phase, LA longitudinal strain increases, reaching a peak at the end of LA filling, just before mitral valve opening (Figure 7). Cameli et al[57] demonstrated an inverse correlation between global peak longitudinal strain (PALS) and MR degree, as lower values of PALS were observed in patients with moderate and severe MR, compared to patients with mild MR. In this study, LA myocardial reservoir function impairment was associated with an higher incidence of paroxysmal AF.
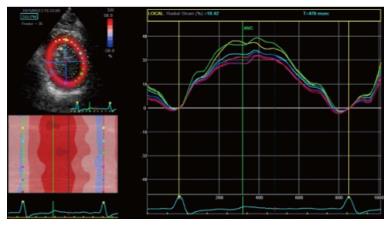
Figure 7 Two dimensional longitudinal atrial strain.
A: PALS in a normal patient. Triphasic strain pattern is evident: Reservoire phase (RP), conduit (CoP) and contractile phase (CP); B: Reduced PALS in a patient with a large MV flail and severe MR, without triphasic strain pattern. PALS: Peak atrial longitudinal strain; MV: Mitral valve; MR: Mitral regurgitation.
DEFORMATION IMAGING IN THE EVALUATION OF MECHANICAL DYSSYNCHRONY
Mechanical dyssynchrony can be assessed using different imaging modalities: Conventional M-Mode, Doppler echocardiography, tissue Doppler imaging (TDI) and newer modalities such as strain rate imaging (SRI) and 3D STE.
Echocardiographic evaluation of mechanical dyssynchrony has been of great interest for the identification of potential responders to CRT. Even though the largest body of publications on LV dyssynchrony and CRT response prediction is based on TDI[58,59]. However, in the PROSPECT (Predictors of Responders to Cardiac Resynchronization Therapy) trial time to peak time-to-peak dyssynchrony evaluation did not have enough predictive value to replace standard selection criteria for resynchronization therapy[60]. Also pulsed-Doppler evaluation of interventricular dyssynchrony may predict the response to CRT, but more solid evidence supports intraventricular dyssynchrony assessment by speckle tracking as a mean to identify CRT responder.
LV dyssynchrony study by speckle tracking was firstly proposed by Suffoletto et al[61]. In this study radial strain dyssynchrony analysis was performed in a cohort of 50 HF patients with standard indications to CRT. The authors found the presence of baseline significant radial dyssynchrony to be associated with a significant increase in EF at 5 to 8 mo after CRT. Furthermore a greater increase in EF was observed in patients with lead position concordant to the latest site of activation identified at the radial strain study, compared to patients with discordant lead position.
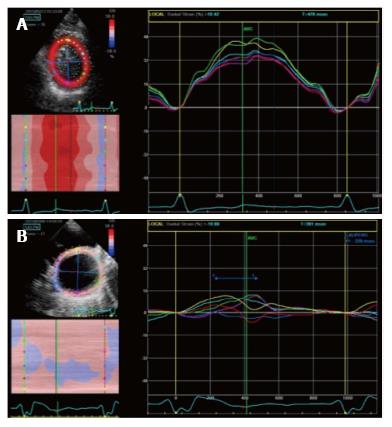
Gorcsan et al[62] studied 176 HF patients candidates to CRT with both 12-site TDI time to peak dyssynchrony analysis and radial strain. They found that 95% of patients with significant dyssynchrony both at TDI (> 60 msec) and radial strain (> 130 msec) studies showed an EF improvement, while only the 21% of patients without dyssynchrony at both tests had an EF response[62]. Based on several trials, actually a value of antero-septal to posterior wall peak delay > 130 msec is considered indicative of significant radial dyssynchrony (Figure 8). Bank et al[63] in the PROMISE-CRT trial studied HF patients with radial strain analysis and concluded that the presence of radial dyssynchrony predicted reverse remodeling after CRT. However the sample was small and so placebo effect could not be overcome. Gorcsan et al[64] then studied 197 candidates to CRT with radial strain, considering a delay > 130 msec significant for radial dyssynchrony. They found that patients with significant radial dyssynchrony before CRT had a lower incidence of adverse events [heart transplant, need for a LV assistance device (LVAD), death] at 4-year follow up, compared to patients without baseline dyssynchrony.
Figure 8 Radial strain analysis with 2 dimensional speckle tracking.
A: Synchronous strain pattern in a normal patients; B: Significant intraventricular dyssynchrony in a patient with dilated cardiomyopathy. A significant delay (≥ 130 msec) between anteroseptal peak strain (yellow segment) and posterior peak strain (pink segment) is evident.
Most recently, in the STAR trial by Tanaka et al[65], baseline dyssynchrony assessed with both radial and transverse strain was found to be a predictor of response to CRT, in terms of EF improvement and better long term survival, as only 11%-13% of these patients died or underwent heart transplant or LVAD implantation. Patients without either radial or transverse dyssynchrony prior to CRT had a worse prognosis, as in 50% of these cases an unfavourable event occurred. On the other hand, in one third of patients responders to CRT, both longitudinal and circumferential strain failed to detect significant dyssynchrony. Thus, the authors concluded that radial and transverse strain are the most reliable methods to assess dyssynchrony and predict response to CRT. Furthermore, radial strain dyssynchrony evaluation permitted the identification of the most delayed site of LV activation. Ypenburg et al[66] studied 244 patients before CRT with 2D radial strain analysis and found that the latest site of LV activation was most frequently represented by the posterior (36%) and the lateral segments (33%). They also evidenced that if the LV lead position was concordant to the identified latest site of activation, better echocardiographic response and long term outcome could be expected after CRT.
As for longitudinal dyssynchrony, Lim et al[67] studied 100 HF patients before CRT using longitudinal strain and derived a strain delay index, that resulted to be a marker of dyssynchrony and viability or scar. They reported a strain delay index > 25% to be consistently associated with LV reverse remodeling after CRT.
Shi et al[68] studied 53 HF patients with 2D speckle tracking obtaining standard deviation of time to PALS in 12 LV segments (Tstrain-SD) and standard deviation of time to the end of longitudinal systolic strain rate in six basal LV segments (Tsr-SD). No significant difference was observed in baseline Tsr-SD, and Tstrain-SD between non-responders and responders to CRT. However, the Tsr-SD was significantly higher in responders than non-responders. Ma et al[69] found that both global and regional longitudinal strain can be predictors of long-term response to CRT in patient with ischemic cardiomyopathy. Further, baseline longitudinal strain values in the site of LV leads were consistently higher in responder patients. Finally, also baseline global longitudinal strain was found to be higher in CRT responder patients than in non-responders, with a global longitudinal strain of -13% predicting response to CRT, thus suggesting that patients with better global LV function had less scar tissue and are likely to benefit more significantly from CRT. Becker et al[70] applied circumferential strain analysis to HF patients before CRT, showing that dyssynchrony assessed by circumferential strain did not differ between CRT responders and non-responders. Of note, this trial was more focused on the effect of LV lead position on CRT response than on the role of dyssynchrony. Delgado et al[71] compared circumferential, longitudinal and radial strain and found that only dyssynchrony assessed with radial strain was able to predict CRT responders in a study group of 161 patients, while circumferential and longitudinal strain were not. As a consequence of these several trials, we can assume that LV dyssynchrony analysed with radial strain is more informative than longitudinal or circumferential strain analysis and can predict CRT responders. Furthermore, also magnetic resonance imaging studies confirmed that radial dynamics analysis can be more sensitive in identifying the presence of dyssynchrony, compared to longitudinal myocardial deformation study[72,73]. However higher global longitudinal strain and regional longitudinal strain at the site of LV lead positioning can also predict the response to CRT[74]. The dyssynchrony strain pattern evidenced by 2D speckle tracking echo is also very important to predict the response to CRT. In patients with heart failure, the presence of intraventricular dyssynchrony is usually evidenced by left bundle branch block (LBBB) at EKG. In presence of a true LBBB or right ventricular pacing, the contraction pattern is characterized by early contraction in early activated walls (septum) and pre-stretch followed by late contraction in late activated walls (lateral wall). The strain-pattern can reflect a complete LBBB in the so called “classical” pattern, that is defined by three components: (1) early activation of at least one basal or midventricular segment in the septal or anteroseptal wall and early stretching in at least one basal or midventricular segment in the opposite wall; (2) early peak contraction not exceeding 70% of the ejection phase; and (3) early stretching wall showing peak contraction after aortic valve closure. Patients who do not fulfil all these three criteria are considered having a heterogeneous strain-pattern. Risum et al[74] showed that contraction patterns reflective of a consistently delayed LV activation are predictive of response. The presence of a “classic” strain pattern strongly predicted the CRT efficacy, while other patients with wall motion patterns inconsistent with a LV activation delay were less likely to benefit. Importantly, the presence of a classical pattern significantly added to other predictors of response (etiology and QRS > 150 ms) further emphasizing a valuable role for pre-implantation assessment of mechanical dyssynchrony by speckle tracking approach.
Also apical transverse motion (ATM), to quantify “apical rocking”, has been introduced as a new and integrative parameter for for LV dyssynchrony assessment and as a promising predictor of CRT efficacy[75]. ATM was proposed by Voigt et al[76], who suggested that ATM integrated information about temporal and regional inhomogeneities of LV function and exhibited a significant correlation with the difference between tissue Doppler-derived average strains of the septal and lateral wall. Gürel et al[77] showed that ATM is closely associated with radial dyssynchrony assessed by 2D speckle tracking analysis. Of note, a cut-off value of 2.5 mm for ATM loop could clearly differentiate between patients with and without radial dyssynchrony[77].
Previous studies showed that LV rotational mechanics are altered in patients with advanced HF and prolonged QRS[78]. In those with significant dyssynchrony, not only torsion is reduced but the basal and apical rotation sometimes follows the same direction of rotation[79]. Further, Sade et al[80] showed that LV altered rotational mechanics can be restored by resynchronization therapy. These authors then suggested the use of LV rotational parameters (LV torsion and twist)[80] for predicting CRT responders.
Potential important technical challenges encountered with 2D STE include interpretation of biphasic or multiple peaks in one segment at strain analysis. Seo et al[81] suggested to consider the earliest peak of the segmental strain curve, when more than one peak is evident, as it appears to be the most predictive of response to CRT. Another important limitation of strain analysis has to be faced in presence of akinetic or scar regions, as it could be difficult to get reliable strain curves of these segments. However, LV dyssynchrony is also a 3D phenomenon. Therefore 3D speckle tracking dyssynchrony analysis has been recently introduced and validated. Valuable studies in several studies, 3D-echocardiography has been used to study LV dyssynchrony, assessing volumetric changes in endocardial movement and regional blood displacement[82,83]. Tanaka et al[84] enrolled 54 candidates to CRT and used a 3D speckle tracking system to assess LV dyssynchrony. Radial dyssynchrony was analysed using a 16-segments scheme, expressed as maximal opposing wall delay in time-to-peak strain and standard deviation of time-to-peak strain. The authors found that both parameters significantly correlated with 2D radial strain antero-septal to posterior delay. Furthermore, 3D speckle tracking offers the advantage of identifying the most delayed myocardial region in 3D, in contrast to 2D speckle tracking and TDI analysis.
In fact radial strain 3D speckle tracking analysis offers a complete 3D mechanical activation map with the color-code 3D cine-loop map that provide an immediate visual assessment of the latest activation site among the 16 segments. In another study, Tanaka et al[85] used 3D speckle tracking to study 57 HF patients with prolonged QRS due to either LBBB (group 1) or RV pacing (group 2) with a need to undergo CRT upgrading. They found that the site of earliest mechanical activation was consistently different between the 2 groups (apex 6% in group 1 vs 28% in group 2 respectively), but the most delayed site of activation was similar in the both kind of LV conduction delay. These data supported the use of resynchronization therapy in patients with low EF and a need for pacing.
According to these data, we can conclude that deformation imaging can help to define the presence of intraventricular dyssynchrony better than TDI. Radial speckle tracking and 3D strain analysis appear to be the best method to quantify intraventricular delay and to predict CRT responders.
DEFORMATION IMAGING IN PATIENTS WITH MECHANICAL DYSSYNCHRONY AND FMR
LV dyssynchrony also plays a role in the pathophysiology of FMR. In fact it has been described that intraventricular mechanical dyssynchrony is an important contributor to functional MR[86,87].
Liang et al[11] enrolled patients with EF < 50% and at least mild MR, using TDI to assess global systolic dyssynchrony (maximal difference in time to peak systolic velocity among the 12 LV segments) and regional dyssynchrony (delay between anterolateral and posteromedial papillary muscles insertion regions). These authors concluded that only global dyssynchrony could be considered a FMR determinant, with an incremental value to valve remodeling parameters (tenting area) that play the main role in FMR pathophysiology.
Soyama et al[21] analysed 32 patients with non-ischaemic DCM using TDI derived strain of the papillary muscles; they found FMR to be more frequent in patients with a significant delayed activation of the papillary muscles adjacent LV segments, and concluded that regional dyssynchrony and LV sphericity were independent predictors of FMR.
Agricola et al[88] evaluated 74 patients with chronic LV dysfunction (53% ischemic patients) with varying degrees of MR and suggested that systolic tenting was the main determinant of FMR, as a consequence of global and regional LV remodeling. The authors reported that regional dyssynchrony was independently associated with MR severity, with a minor influence, only in patients with DCM and not in those with ischaemic cardiomyopathy. They also found that the QRS duration had no effect on the severity of FMR. Of note, in this study intraventricular dyssynchrony was expressed as the SD of time-to-peak systolic velocity of 8 (not 12) LV segments. Donal et al[22], using regional strain analysis in 87 patients with DCM, demonstrated that the degree of FMR was determined by mitral orifice morphology, LV features, especially longitudinal contractility (strain of LV mid-lateral wall) and dyssynchrony defined as the delay between the septal and lateral mid-portion strain divided by RR squared root. In addition the authors found that MR was not correlated with interventricular mechanical delay. Also Sardari et al[89] demonstrated that the severity of MR was not correlated with the QRS duration nor with the echocardiographic interventricular dyssynchrony indices in the patients with ischemic or DCM. Moreover, in this study also intraventricular dyssynchrony was not correlated with MR severity. However in this study only Doppler imaging was applied to evaluate LV synchronicity, as neither strain nor time to peak systolic strain analysis were performed.
As for ischemic cardiomyopathy, inferior wall myocardial infarction is known to be associated with more severe MR degree, while anterior myocardial infarction should theoretically be characterized by a higher dyssynchrony index, due to larger infarct dimensions. Patients with anterior acute myocardial infarction (AMI), but not inferior AMI have worse prognosis, and either a larger dyssynchrony index or increased MR severity determine LV remodeling and outcome. Hung et al[90] found that both global and regional dyssynchrony in patients with anterior MI were independently associated with FMR degree. Dyssynchronized myocardial segments were assessed by 3D echo showing an independent impact on FMR grade in a narrow QRS population.
The dyssynchronous contraction of LV papillary muscles is a leading cause of FMR in HF patients, as inferior, posterior and lateral regions are usually identified as the most delayed sites and papillary muscles are regularly located adjacent to lateral and inferior walls. As the majority of the studies regarding papillary muscles dyssynchrony reported different cut off values, the optimal delay for cardia resynchronization has not been established yet. Thus, there is the need for defined cut off values in order to clearly identify the presence of papillary muscles dyssynchrony before CRT in patients with FMR. Tigen et al[91] reported that papillary muscle dyssynchrony with > 60 msec delay (assessed by TDI-derived longitudinal strain) was able to predict a regurgitant volume > 20 mL in DCM patients. Kjordybach et al[92] studied 31 patients with EF lower than 35% of both ischemic and non-ischaemic aetiology and evaluated papillary dyssynchrony by TDI derived time to peak strain. They showed that papillary muscles dyssynchrony was associated with the deformation of mitral apparatus (tenting area), but the haemodynamic consequences of MR (in particular left atrial area) could be better characterized by papillary dyssynchrony only in DCM. Ypenburg et al[13] and Goland et al[93] both assessed dyssynchrony at the papillary muscles insertion sites using radial strain analysis. They reported that MR improvement after CRT was significantly more frequent in patients with baseline dyssynchrony. In 2010, Tigen et al[94] firstly investigated both papillary muscles with 2D speckle tracking from the longitudinal axis in patients with DCM. They found that FMR was significantly correlated with intraventricular dyssynchrony and mitral valve remodeling parameters.
In addition, in this study significant papillary muscles dyssynchrony was found to be the only independent predictor of more than moderate MR. The proposed cut-off value for papillary muscles dyssynchrony (30 ms) predicted a mitral regurgitant volume > 20 mL or EROA > 0.20 cm2 with high sensitivity and specificity.
STE has shown a fundamental role of intraventricular dyssynchrony in determining FMR especially in DCM, rather than in ischemic cardiomyopathy, in which MR severity seems to be more related to mitral valve deformation indexes. Finally the assessment of papillary muscle dyssynchrony can help to identify optimal candidates to CRT, especially among patients with DCM-associated FMR.
THERAPEUTIC CONSIDERATIONS
Medical therapy
The currently accepted optimal pharmacological therapy for HF embraces ACE-inhibitors, diuretics, aldosterone antagonists and beta-blockers[95], and its beneficial effects on HF symptoms in subjects with FMR and LV dysfunction may be remarkable. This combination therapy acts on both neurohormonal activation and the underlying maladaptive pathways, leading to a favourable myocardial remodeling. Several combinations of the above-mentioned drugs are commonly used aiming at reducing the severity of MR and reversing or at least delaying the LV remodeling progression. Afterload-reducing drugs, i.e., ACE-inhibitors, decrease MR regurgitant volume and increase forward output by reducing the pressure gradient between LV and LA. Vasodilators decrease MR regurgitant volume through a systolic unloading on the EROA. Likewise a reduction in MR might be achieved with preload reduction agents, i.e., diuretics, through LV unloading and accordingly a decrease in leaflet tethering. The administration of ACE-inhibitors and beta-blockers is an independent predictor of better long-term survival in subjects with ischemic MR and LV dysfunction since they reduce the progression of LV remodeling and prevent sudden death. Beta-blocker therapy in HF patients reduces all cause mortality, cardiovascular mortality and mortality due to LV systolic dysfunction and sudden death by roughly 31%-39%[96]. In addition, it has been demonstrated that a combined therapy of carvedilol plus ACE-inhibitors decreases FMR by reducing LV dilation[97].
Indications for intervention
FMR surgery is indicated in patients with severe MR and LVEF > 30% undergoing coronary artery bypass grafting (CABG) (recommendation class I, level of evidence C)[98]. It should be considered in patients with moderate MR undergoing CABG (IIa, C) and in symptomatic patients with severe MR, LVEF < 30%, option for revascularization and evidence of myocardial viability (IIa, C). Furthermore FMR surgery may be considered in patients with severe MR and LVEF > 30% with persisting symptoms despite optimal medical management and with low comorbidity, when revascularization is not indicated (IIb, C). In the other patients, optimal medical treatment and extended HF treatment is currently the best option.
Percutaneous mitral valve repair is feasible at low procedural risk in patients with secondary MR and may provide short-term improvement in functional condition and LV function. The percutaneous MitraClip procedure may be considered in patients with symptomatic severe secondary MR despite optimal medical therapy who fulfil the echocardiographic criteria of eligibility, are judged at high surgical risk by a team of cardiologists and cardiac surgeons, and who have a life expectancy greater than one year (IIb, C). A recent metanalysis showed that MitraClip represents an efficacious strategy for patients with HF and severe MR, improving functional class and cardiac remodeling[99].
The management of moderate ischaemic MR in patients undergoing CABG is still unclear. In this circumstance, valve repair is preferable. In patients with low EF, mitral valve surgery should be considered if there is evidence of myocardial viability and if comorbidity is low. Exercise echocardiography should be considered in patients capable of exercising, since exercise-induced dyspnoea and a substantial increase in MR severity and systolic pulmonary artery pressure support mitral surgery in addition to myocardial revascularization.
CRT in patients with FMR
It has been widely demonstrated that CRT decreases mortality and hospitalization rate, improving cardiac function and structure in symptomatic chronic HF patients managed with optimal medical treatment[100], who present severely depressed LVEF (≤ 35%) and complete LBBB (class I recommendation, level of evidence A). In these patients, CRT is superior either to optimal medical therapy or to ICD alone. Efficacy tends to be lower in patients with NYHA class I and IV and in case of non-LBBB morphology with QRS duration < 150 ms. Therefore, in HF patients without LBBB and QRS ≥ 150 ms or LBBB and QRS duration 120-149 ms, CRT is still recommended but considered class IIa or IIb indication[101].
However the improvement in HF symptoms and survival profile after CRT is proportionate to the extent of improvement in LV systolic function. CRT reduces MR severity in patients with chronic HF and FMR. As showed by Upadhyay et al[102], reduction in MR after CRT is considerably related to lesser HF hospitalization and improved survival. In this study baseline MR degree and longer surface QRS to LV lead time were significant predictors of MR change. Furthermore mitral valve was less remodelled in patients with evidence of MR reduction after CRT. Indeed these patients exhibited a lower tenting area and coaptation height than those with stable or worsening MR, suggesting that ventricular geometry improvement could be a mechanism for MR change.
CRT is then responsible for immediate and late reduction in FMR contrasting its pathophysiologic determinants by reducing or virtually eliminating LV dyssynchrony through different mechanisms: (1) increasing “closing forces” (global synchronization); (2) reducing “tethering forces” (local synchronization); (3) reshaping annular geometry and function (local synchronization); and (4) correcting diastolic MR [atrio-ventricular (AV) synchronization].
As for global synchronization, CRT can restore AV and LV synchrony, increasing global LV contraction efficiency and therefore MV coaptation forces. In fact CRT generates a higher pressure-gradient through MV with a consequent rise in trans-mitral closing forces counteracting the tethering forces. Breithardt et al[17] studied 24 HF patients with LBBB and FMR after CRT implantation and confirmed that FMR reduction is directly related to the increased closing force (expressed as LV dP/dt max) that aid mitral valve closure. In addition CRT reduces FMR not only by increasing closing forces but also through “local” synchronization[36]. It was noticed that in CRT responders with FMR reduction, resynchronization was induced at the level of basal and mid-LV segments. At the multivariate analysis mid-LV segments synchrony was the most significant predictor of FMR reduction, suggesting that a more “local” synchronous contraction involving the segments adjacent to papillary muscles could determine FMR improvement. Kanzaki’s et al[38] firstly correlated the immediate reduction in MR after CRT with a more synchronized mechanical activation of papillary muscle insertion points. Further, Goland et al[93], using through 2D Speckle Tracking Radial Strain (2D-RS), showed that a significant delay of time-to-peak 2D-RS in the mid-posterior and inferior segments prior to CRT, along with preserved radial strain in the posterior and inferior segments, were strong predictors of FMR improvement after CRT. Most recently three echocardiographic aspects have been independently associated with FMR change after CRT[103]: Antero-septal to posterior wall radial strain dyssynchrony > 200 ms, non-severe LV dilatation (LV end-systolic diameter index < 29 mm/m2), absence of scar at papillary muscle insertion sites. In the same study the importance of myocardial viability in predicting FMR response was stressed, because CRT can be effective only when responsive viable segments are present. Sénéchal et al[104] evaluated the presence of viability using dobutamine-stress echocardiography before CRT and confirmed that local viability was able to predict acute response to CRT with a proper sensitivity, making local viability an essential precondition for response to CRT. CRT is also thought to improve contraction of the posterior mitral annulus, coordinating the contraction of the segments at the base of the LV. However data are discordant as some studies demonstrate no immediate changes in mitral annular dimensions after CRT and other studies[14] show that annular contraction is correlated to FMR reduction after CRT, although as a minor determinant.
Interestingly all these described effects are pacing dependent as the interruption of CRT causes an immediate recurrence of MR.
According to the timing of response to CRT, there is clear distinction between two phases of MR reduction: (1) immediate MR reduction, occurring suddenly after CRT implantation; and (2) long-term MR reduction, occurring from weeks to months after CRT.
MR may show an immediate improvement after CRT, but the underlying mechanism is not completely clear. It is probably more likely to occur when LV dyssynchrony is mainly related to papillary muscles dyssynchrony. Ypenburg et al[105] showed that CRT may lead to an acute reduction in MR in LV dyssynchrony involving the posterior papillary muscle, as opposed to a late response when the lateral wall is involved. Long-term reduction is the consequence of LV reverse remodeling. In addition, CRT can be associated with acute decrease in resting MR but not in exercise-induced MR. In fact, after CRT only late reversed LV remodeling, restoring mitral apparatus geometry, is associated with a reduction in both resting and exercise-induced MR[106].
Between the two phases of FMR reduction, immediate MR reduction is the major determinant of favourable response to CRT, as it contributes to the acute reduction of volume overload, determining a rapid reverse remodeling. Therefore, immediate MR reduction is a major prognostic determinant after CRT[107].
Surgery
The ideal surgical strategy for the management of ischemic MR is still debated. Peri-operative mortality is higher compared to primary MR, and the long-term prognosis is worse mainly due to the more severe associated comorbidities. Moreover, there is a significant persistence and recurrence rate of MR after valve repair, as McGee demonstrated on a cohort of 585 patients with FMR[108], as well as the absence of prognostic evidences. Severe ischaemic MR is not usually improved by sole revascularization. At the same time the impact of valve surgery on survival remains uncertain because randomized trials are missing and the available observational studies do not draw definite conclusions because of study limitations[109]. With regard to prognosis, most studies failed to demonstrate improved long-term clinical outcome following surgical correction of secondary MR[110,111]. Fattouch et al[112] compared CABG vs CABG plus valve repair in patients with moderate ischemic MR, showing that the addition of MR repair improved functional class, EF, pulmonary artery pressure and LV diameter in the short-term. The study though was not designed to analyse the effect on survival of the addition of valve repair to CABG. When surgery is indicated, valve repair using undersized rigid ring annuloplasty is the first option, offering a low operative risk although it is associated with a significant rate of MR recurrence[113]. Preoperative predictors of recurrent secondary MR after undersized annuloplasty, associated with a worse prognosis, are left ventricular end diastolic diameter, posterior mitral leaflet angle, distal anterior mitral leaflet angle, systolic tenting area, coaptation distance, end-systolic inter-papillary muscle distance, and systolic sphericity index[114]. A meta-analysis of retrospective studies by Vassileva et al[115] suggested better short-term and long-term survival following valve repair compared to its replacement. A recent study on 251 patients with severe ischemic mitral regurgitation randomized to either mitral-valve repair or chordal-sparing replacement revealed no significant difference in LV reverse remodeling or survival at 12 mo; replacement provided a more durable correction of mitral regurgitation, but there was no significant difference in clinical outcomes between-group[116].