Published online Feb 26, 2016. doi: 10.4330/wjc.v8.i2.132
Peer-review started: July 17, 2015
First decision: August 16, 2015
Revised: August 23, 2015
Accepted: December 4, 2015
Article in press: December 8, 2015
Published online: February 26, 2016
Processing time: 220 Days and 11.5 Hours
Non-ischemic cardiomyopathies include a wide spectrum of disease states afflicting the heart, whether a primary process or secondary to a systemic condition. Cardiac magnetic resonance imaging (CMR) has established itself as an important imaging modality in the evaluation of non-ischemic cardiomyopathies. CMR is useful in the diagnosis of cardiomyopathy, quantification of ventricular function, establishing etiology, determining prognosis and risk stratification. Technical advances and extensive research over the last decade have resulted in the accumulation of a tremendous amount of data with regards to the utility of CMR in these cardiomyopathies. In this article, we review CMR findings of various non-ischemic cardiomyopathies and focus on current literature investigating the clinical impact of CMR on risk stratification, treatment, and prognosis.
Core tip: Cardiac magnetic resonance imaging (CMR) has established itself as a vital modality in the evaluation of numerous aspects of non-ischemic cardiomyopathies, ranging from establishing a diagnosis to detailed analysis of cardiac function. Lately, increasing data has become available regarding the clinical utility of CMR in the evaluation of these patients, although few articles have consolidated this these findings regarding CMR’s impact in these pathologies. This review will summarize current literature investigating the clinical impact of CMR on risk stratification, treatment, and prognosis in the setting of non-ischemic cardiomyopathies.
- Citation: Kalisz K, Rajiah P. Impact of cardiac magnetic resonance imaging in non-ischemic cardiomyopathies. World J Cardiol 2016; 8(2): 132-145
- URL: https://www.wjgnet.com/1949-8462/full/v8/i2/132.htm
- DOI: https://dx.doi.org/10.4330/wjc.v8.i2.132
Non-ischemic cardiomyopathies (NICM) include a wide spectrum of disease states afflicting the heart, whether a primary process or secondary to a systemic condition[1,2]. Several imaging modalities are used in the evaluation of NICM, particularly echocardiography, nuclear medicine, and cardiac catheterization. Cardiac magnetic resonance imaging (CMR) has established itself as an important modality in the evaluation of cardiomyopathies. The last decade has seen tremendous technological advances in CMR, both in software and hardware[3]. CMR offers a number of advantages that makes it an ideal imaging modality in a number of clinical settings. CMR allows for the non-operator dependent acquisition of high spatial and temporal resolution images in any desired imaging plane and regardless of patient-specific factors such size and body composition. With these high resolution images, accurate assessments of various chamber and vessels functional parameters can be made. Additionally, CMR is free of ionizing radiation, which makes it an ideal modality for evaluation of young patients, and those who may require frequent or regular follow-up assessments.
The increased use of CMR has resulted in accumulation of a tremendous amount of data on the utility of CMR in the clinical management of these patients. CMR is moving from simply an initial diagnostic tool to one whose findings can also have for significant clinical impact, including those on therapy response, risk stratification, and prognosis determination.
In this article, we review CMR findings of various non-ischemic cardiomyopathies and focus on current literature investigating the clinical impact of CMR on risk stratification, treatment, and prognosis.
In a patient with NICM, several dedicated CMR sequences are used as a part of the magnetic resonance imaging (MRI) protocol. Steady-state free precession (SSFP) is the most commonly used sequence, which helps in evaluating ventricular morphology and function. In addition, ventricular function can also be quantified by drawing endocardial and epicardial contours. Velocity-encoded phase contrast MR images enable flow and velocity quantification in vascular and valvular structures. Multi-echo gradient echo images are used for detecting and quantifying myocardial iron. T2-weighted images are useful in detection of myocardial edema, seen in acute myocardial infarction or myocarditis. T2-mapping is a more accurate technique of quantifying the myocardial fluid. Dynamic first-pass perfusion images are utilized for evaluation of perfusion defects or microvacular dysfunction. Delayed-enhancement images show scar and fibrosis, seen as different patterns of late gadolinium enhancement (LGE), which is useful in the characterization of cardiomyopathies. T1-mapping techniques can quantify the T1 values of myocardium, either before (native) or after administration of contrast and can measure extracellular volume (ECV), which is a biomarker of fibrosis. MR angiography is useful in evaluation of vascular anatomy. 3D-whole heart navigator gated SSFP sequence is useful for evaluation of coronary artery anatomy as well as vascular anatomy without administration of contrast.
A summary of main diagnostic CMR findings as well as the commonly evaluated CMR parameters and their clinical implications, discussed in greater in the following sections, are included in Table 1.
| Cardiomyopathy | Key diagnostic CMR findings | Prognostic CMR parameters | Clinical outcomes evaluated |
| Iron overload cardiomyopathy | Myocardial T2* < 20 ms | Myocardial T2* | Adverse cardiac events, sudden cardiac death, treatment monitoring |
| Idiopathic dilated cardiomyopathy | LV dilatation, global systolic dysfunction, mid-myocardial septal LGE | LGE, longitudinal myocardial strain | Adverse cardiac events, transplant status, sudden cardiac death, treatment monitoring |
| Hypertrophic cardiomyopathy | Asymmetric septal hypertrophy, patchy LGE (RV insertion points), mitral valve systolic anterior motion | LGE | Adverse cardiac events, sudden cardiac death |
| Sarcoidosis | Mid-myocardial or sub-epicardial LGE with (acute) or without (chronic) edema | LGE | Adverse cardiac events, treatment monitoring |
| Myocarditis | Myocardial edema, high T2 in T2 mapping, early gadolinium enhancement, mid-myocardial or subepicardial distribution LGE | LGE | Adverse cardiac events, sudden cardiac death, cardiac function recovery |
| Amyloidosis | Diffuse subendocardial-transmural enhancement, early myocardial nulling on T1 mapping | LGE, ECV estimation, T2 ratio | Mortality, disease subtype differentiation |
| Left ventricular non-compaction | Non-compacted to compacted myocardium ratio (end diastole) > 2.3 | Non-compacted to compacted thickness ratio, LGE | Functional status, adverse cardiac events, sudden cardiac death |
| Arrhythmogenic right ventricular dysplasia | Major wall motion abnormality, low ejection fraction, dilated RV (major criteria) | RV and LV abnormalities, LGE | Adverse cardiac events, sudden cardiac death, treatment planning |
| Takotsubo cardiomyopathy | Reduced global systolic function, abnormal apical wall motion with normal/hyperkinetic basal segments | Type of segmental involvement, LGE | Cardiac dysfunction severity and recovery |
| Fabry disease | Concentric LV thickening, basal inferolateral segment mid myocardial-subepicardial LGE | LGE, T1 mapping | Adverse cardiac events, sudden cardiac death, treatment monitoring |
| Muscular dystrophy | Ventricular dilation, systolic dysfunction, mid myocardial-subepicardial LGE | LGE, T1 mapping, ECV estimation, myocardial strain | Adverse cardiac events |
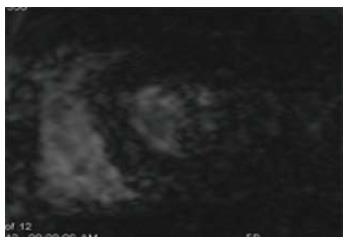
Myocardial iron deposition is shown on gradient-echo images, with lower signal at higher Echo time (TE) values (Figure 1). Utilizing gradient echo images at different TE levels (Multi-echo GRE), the absolute myocardial T2* can be measured and this has shown to be a more reliable indicator of true myocardial iron content as compared to serum ferritin levels or liver iron[4,5]. Myocardial T2* < 20 ms is considered to be significant iron deposition and < 10 ms is considered to be advanced iron deposition.
Myocardial T2* values have also been shown to detect myocardial changes of iron overload, significantly earlier than changes in left ventricular ejection fraction (LVEF)[4]. Myocardial T2* has been shown to be a strong independent predictor of adverse clinical outcomes such as development of heart failure, arrhythmias, and sudden cardiac death. A study by Anderson et al[4] showed that patients with a with T2* < 20 ms were at significantly increased risk for arrhythmias, and this risk was also shown to be increased further at lower T2* levels. T2* value < 10 ms had a substantially higher risk of developing heart failure at the time of follow-up with risk increasing further for patients with T2* < 6 ms. As with the level of myocardial iron content, these outcomes predictors did not correlate with parameters such as serum ferritin or liver iron content. Similar findings were also seen in data from Patton et al[5], which also included sudden cardiac death as a part of their composite outcome. Data from this study also demonstrated worsening outcomes measured at lower T2* levels, leading them to propose a three-tiered risk stratification model based on T2* values - low risk: T2* > 20 ms; intermediate risk: T2* between 10 ms and 20 ms; and higher risk: T2* < 10 ms.
In addition to predicting outcomes, CMR has also shown to be an invaluable tool in the monitoring of treatment response to chelation therapies, which comprises a crucial element of the treatment of iron-overload cardiomyopathy. Multiple published studies have shown improvements in T2*[6-13] and LVEF[6-11] when evaluating treatment responses to several different chelating agents over variable treatment durations. The longest studied follow-up time was performed by Ambati et al[11], which demonstrated continued improvement in both T2* and LVEF extending to five years after treatment initiation. Although most studies evaluating cardiac response of chelation therapies have focused on objective parameters such as T2* and LVEF, Pennell et al[14] demonstrated that improvements in myocardial T2* and LVEF were also associated with significantly reduced risk of developing heart failure. It should be noted that this observed risk reduction was seen in the setting of only minimally improved LVEF, suggesting that, in the setting the chelation treatment of iron overload cardiomyopathy, conventional functional parameters such as LVEF may underestimate the clinical impact of therapies.
Given the evidence for the use of CMR in the diagnosis, risk stratification, and treatment monitoring in iron overload cardiomyopathy, CMR is recognized in the most current American Heart Association (AHA) Consensus Statement[15] as a critical tool in the diagnosis and clinical management of patients with iron overload cardiomyopathy. Additionally, the widespread adoption of CMR in management of these patients has correlated with the reduction in mortality from cardiac iron overload in patients in the United Kingdom[16,17], which has been largely attributed to clinical guidance by CMR findings in these patients. For example, Modell et al[16] showed that the death rate from iron overload between 2000 and 2003 was 2.3 per 1000 patients, significantly decreased from 7.9 per 1000 prior to the initiation of CMR screening in thalassemia patients, Additionally, Chouliaras et al[17] estimated that the risk of cardiac death before CMR screening of United Kingdom thalassemia patients was 82% higher compared to the risk observed after CMR screening.
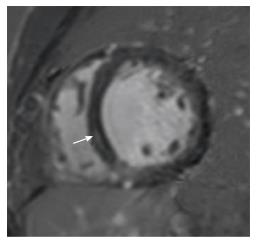
Idiopathic dilated cardiomyopathy is characterized by dilation of the left ventricular left ventricle (LV) with global systolic dysfunction. A linear mid-myocardial pattern of LGE in the septum (Figure 2) has been reported in these patients[18], due to presence of fibrosis. A study by McCrohon et al[19] showed that in a population with dilated cardiomyopathy, this linear mid-myocardial pattern was seen in 28% of patients, with no particular enhancement in 59% of patient. In 13% of these patients, a subendocardial pattern was seen in spite of normal coronary arteries in catheterization[19].
Buss et al[20] demonstrated the association of various strain parameters with cardiac outcomes including cardiac death and transplantation. In their analysis, longitudinal strain was shown to be a superior predictor of outcome compared to not only conventional parameters such as LVEF and New York Heart Association functional class, but the presence of LGE as well. Additionally, preserved longitudinal strain was associated with better outcomes, even in the presence of LGE or depressed LVEF[20].
Several published studies have shown the presence of LGE in these patients to be a significant risk factor for the development of arrhythmic events, including sudden cardiac death[18,21-24]. A pair of studies[18,24] have shown specifically the presence of mid-wall fibrosis to be associated with increased risk of adverse cardiac events and sudden death[18,24]. Furthermore, a study by Perazzolo Marra et al[21] demonstrated that the presence of LGE was a superior predictor to traditional parameters including depressed LVEF (less than 35%) in predicting arrhythmic events and sudden cardiac events. The presence of LGE has also been shown to be a useful predictor of adverse cardiac events in cohorts of asymptomatic and minimally symptomatic patients[25].
Prospective data is limited regarding the impact on screening dilated cardiomyopathy patients on management or treatment outcomes. However, in an analysis by Gulati et al[24], assuming a 15% threshold for sudden cardiac death risk for implantable cardioverter defibrillator (ICD) implantation, the addition of LGE to their risk assessment model would have resulted in nearly 19% of studied patients would have undergone ICD implantation, and 11% would have avoided ICD implantation. Although long-term clinical outcome data is lacking, this suggests that measurement of LGE at CMR may be an effective way to guide ICD therapies in these patients.
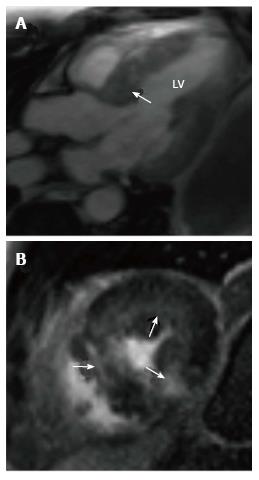
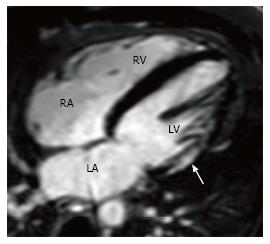
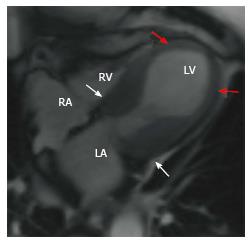
Hypertrophic cardiomyopathy (HCM) is a genetic disorder with a heterogeneous phenotypic expression. MRI can diagnose HCM and also characterize the morphology. The most common morphological type is asymmetric septal hypertrophy (ASH), and other forms include apical, mid-ventricular, concentric, spiral and mass-like forms. In ASH, there is hypertrophy of the basal septum (Figure 3A). MRI can detect and quantify LVOT flow obstruction and the flow velocity/gradient. Systolic anterior motion of the mitral valve and mitral regurgitation can also be detected and quantified. MRI is also useful in detection of papillary muscle abnormalities such as anomalous insertion, double bifid morphology, anteroapical displacement and hypermobile papillary muscles, which can cause obstruction without significant myocardial hypertrophy. Delayed enhancement is seen in 60% of patients[26] with HCM due to interstitial fibrosis, microfibrillar disarray or microvascular obstruction. This is typically seen in a mid-myocardial, patchy pattern at the RV insertion points, but is also seen in the rest of the hypertrophied (Figure 3B) and non-hypertrophied myocardium.
The presence of LGE at CMR plays an important role in risk stratification and estimating prognosis in HCM. Several studies have demonstrated the independent predictive ability of the presence of LGE for cardiac outcomes including worsening heart failure symptoms, ventricular arrhythmias, ICD discharge, and sudden cardiac death[27-30]. Furthermore, the absence of LGE has shown to have useful negative predictive value in that the absence of LGE was associated with a lower, but not absent, risk for adverse cardiac outcomes[31]. However, unlike in dilated cardiomyopathy, several larger studies in HCM patients have noted that the extent of LGE, rather than its presence alone, is a significant predictor of adverse cardiac outcomes[31-34]. This observation may be in part due these larger studies being better powered to evaluate the full range of adverse outcomes. For example, a study by Ismail et al[35], the largest published to date evaluating CMR findings and clinical outcomes in over four hundred patients, demonstrated that only the extent of myocardial LGE was a strong predictor of cardiac events and mortality. However, contrary to other studies, LGE was not shown to be the strongest predictor (behind LVEF) of adverse events in this patient cohort.
To date, limited studies are available regarding the use of CMR in monitoring of treatment for hypertrophic HCM, whether pharmacologic, minimally invasive, or surgical. A study by Yuan et al[36] demonstrated the utility of CMR in characterizing the infarct size from septal ablations as well as decreased LV mass followed up to one year, although clinical outcome data was not included.
Although CMR remains an important modality in the diagnosis of hypertrophic cardiomyopathy, particularly in the setting of equivocal echocardiogram findings, it is yet to be formally recommended for all patients[37,38]. According to the most recent consensus AHA guidelines from 2011[37], the use of LGE with CMR for risk stratification received at a class IIa recommendation and may be considered when risk stratification with conventional risk factors (i.e., prior history of ventricular arrhythmias, family history of sudden cardiac death, and personal history of syncopal episode) are inconclusive.
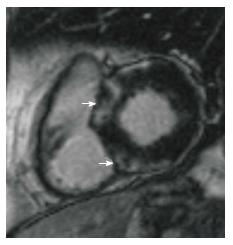
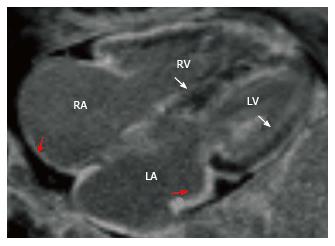
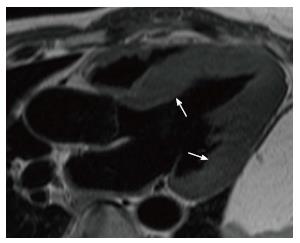
Cardiac sarcoidosis is characterized by the presence of nectrotizing granulomas in the myocardium. In the acute phase, myocardial thickening and edema may be seen. LGE is seen in a mid-myocardial (Figure 4) or sub-epicardial distribution. In chronic phase, wall thickening and LGE is seen, but edema is absent. In burnt out sarcoidosis, transmural enhancement may be seen[39].
The presence of LGE in sarcoidosis has been shown to be associated with adverse outcomes[40-42]. For example, Greulich et al[40] demonstrated that the presence of LGE as the strongest independent predictor death as well as other adverse events such as aborted sudden death, appropriate ICD discharge, and ventricular arrhythmias. The presence of LGE was also shown to be a stronger predictor of adverse outcomes relative to other functional and clinical parameters such as LVEF and clinical symptoms at presentation. Additionally, no included patients without LGE in this study died at the time of follow-up suggesting the potential high negative predictability of LGE in this patient population.
CMR has also been shown in several small studies to be effective in monitoring cardiac improvement in response to steroid therapy[42-44]. Overall, steroid therapy has been shown to be associated with not only improved functional parameters such as LVEF and LV end diastolic volume (EDV) index, but also decrease in LGE. However, data from Ise et al[42] suggest that CMR response to steroid therapy may depend on the extent of LGE upon treatment initiation. In the studied population, treated patients with a lower amount of LGE had significantly decreased LVEF and LV EDV after treatment. However, patients with more severe disease as indicated disease as evidenced by a larger extent of LGE were noted to not only have no significant change in LVEF or LV EDV, but also had worse clinical outcomes.
Similar to the assessment of dilated cardiomyopathy, current appropriate use guidelines from the AHA[3,45] still do not specifically recommend CMR exclusively for the purposes of risk stratification or prognostication with its use reserved for diagnosis and differentiation from other cardiomyopathies as well as functional assessment.
Acute myocarditis seen in MRI as high signal in T2-weighted images and elevated values in T2 mapping due to myocardial edema, early gadolinium enhancement and LGE in a mid-myocardial or subepicardial distribution (Figure 5). Different patterns of enhancement have been described based on the etiological agent. Parvovirus B19 infection often involves the basal inferolateral segment, in a mid-myocardial/subepicardial pattern and usually recovers without lasting damage, whereas human herpesvirus-6 more commonly involves the septum, in a linear mid-myocardial pattern and rapidly progresses to heart failure[46].
As in other cardiomyoapthies, the presence and persistence of LGE in the setting the myocarditis reflects the presence of irreversible myocardial injury[47]. The presence, amount, and distribution of LGE at the time of diagnosis has shown to have important implication in cardiac functional parameters at follow-up after recovery from acute illness. For example, Mahrholdt et al[46] showed total amount of LGE (%LGE) was a significant independent predictor of impaired ventricular function and ventricular dilatation at follow-up. Additionally, the presence of LGE in the ventricular septum was shown to be the strongest CMR predictor for chronic ventricular dysfunction as well as ventricular dilatation.
CMR has shown promise in predicting clinical outcomes and adverse events in patients with myocarditis. Schumm et al[48] demonstrated that in the setting of suspected myocarditis, patients with abnormal CMR (defined at abnormalities in either LVEF, LV volume, or presence of LGE) had significantly more major adverse cardiac events including cardiac death, sudden cardiac death, ICD discharge, and aborted SCD. Additionally, no patients with a normal CMR suffered death or any major adverse cardiac events, suggesting a much more favorable recovery and long term course in patients with normal CMR findings. Similar to the other aforementioned non-ischemic cardiomyopathies, the presence of LGE on the diagnostic CMR was associated with increased of all-cause and cardiac mortality, independent of clinical presentation at diagnosis[49]. The absence of LGE was also associated with a more favorable clinical outcome with no sudden cardiac death events seen at a median follow-up of nearly five years in the study population.
Although typically regarded clinically as an acute, self-limiting illness[50], abnormal CMR findings may persist after the resolution of the acute phase of illness. Specifically, several studies have followed the presence of CMR abnormalities in various groups of myocarditis over their clinical course[46,51,52]. Specifically, LGE has been shown in anywhere between 24%-40% at the time of follow-up, with the relatively wide range of values likely reflective of heterogeneity of the studied patient populations[51].
Additionally, Wagner et al[53] showed in a small cohort of patients that the presence of CMR inflammatory markers at four weeks post-diagnosis was associated with poorer long-term LVEF and symptom score. Thus, given the impact of CMR findings at initial diagnosis on long-term cardiac functional parameters and clinical outcomes as well as potential prognostic implication of persistent abnormal CMR findings, a follow-up CMR exam at least 4 wk after the onset of disease can be considered to differentiate uncomplicated involvement of the myocardium in a systemic viral illness from a more complicated, persistent course[47].
Cardiac amyloidosis is characterized by diffuse subendocardial to transmural enhancement of not only the left ventricle, but also the right ventricle, interatrial septa and atrial walls (Figure 6). The T1 kinetics are altered, with the myocardium nulling before the blood pool (normal - the myocardium always nulls after the blood pull). The blood pool also appears darker on cardiac amyloidosis, due to high ECV and rapid redistribution of gadolinium from the blood pool. There is also concentric myocardial thickening, along with thickening of the interatrial septa and atrial walls.
Unlike many other non-ischemic cardiomyopathies, the use of LGE in risk stratification and evaluation of prognosis has seen mixed results. While several studies[54,55] have shown a significant association between the presence of LGE in cardiac amyloidosis patients after adjustment for other clinical parameters, data in other studies have not shown this trend. For example, Migrino et al[56] demonstrated a significantly higher one-year mortality rate for those patients with LGE, although LGE failed to remain predictive of mortality when observation carried out to five years. However, instead of presence or absence of LGE in amyloidosis patients, gadolinium kinetics may prove to be more useful in assessing prognosis. In a study by Maceira et al[57], presence of LGE in itself was not predictive of mortality; however, post-gadolinium intra-myocardial T1 difference between the subepicardial and subendocardial greater than 23 ms was instead shown to predict mortality with 85% accuracy. Lastly, as a modification of the more conventional CMR LGE analysis, White et al[58] showed that the presence of diffuse hyperenhancement by a visual T1 assessment is not only able to identify patients with cardiac involvement among patients with high clinical suspicion, but is also a strong predictor of mortality.
Additionally, the emerging techniques of T1 mapping and ECV estimation have shown promise in correlating with cardiac function and risk stratification[59,60]. For example, ECV measured at contrast equilibrium greater than 0.45 and pre-contrast T1 > 1044 ms have shown to be predictors of mortality. ECV was also shown to be predictive of mortality even when corrected for markers of ventricular function and serum proBNP values[59]. Furthermore, T2-weigted imaging has also shown prognostic implication in cardiac amyloidosis in that low T2 signal (i.e., T2 ratio < 1.5) at triple-inverted fast spin echo imaging was associated with decreased survival[61].
In addition to its role in identifying cardiac involvement in amyloidosis, CMR has also shown promise in differentiating among subtypes of cardiac amyloidosis, namely between light chain amyloid (AL) and transthyretin-related amyloidosis (ATTR) based on parameters such as LV mass as well as location and extent of LGE. Distinguishing among cardiac amyloidosis subtypes is of critical importance given the marked difference of treatment strategies[62]. Additionally, cardiac amyloidosis subtype also impacts prognosis, with survival worse in AL as compared to ATTR subtype[62].
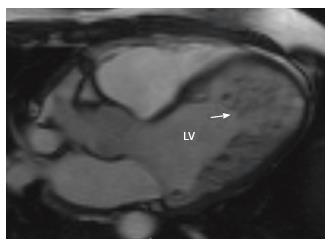
LV non-compaction is caused by persistence of embryonal sinusoids, resulting in an exaggerated presence of non-compacted myocardium compared to compacted myocardium. On MRI, a ratio of > 2.3 between non-compacted and compacted myocardium in end-diastole is considered diagnostic of non-compaction (Figure 7)[63]. Thrombosis, arrhythmia and LV dysfunction are complications.
The degree of LV non-compaction assessed at CMR has shown to correlate with not only cardiac function but risk assessment as well[64,65]. For example, Ashrith et al[64] showed that patients with a maximum non-compacted to compacted thickness ratio less than three were shown to have significantly greater improvement in NYHA functional class at follow up than those with ratio greater than three. Additionally, in patients with reduced LVEF, change in LVEF at follow up was also shown inversely correlated with non-compaction-compaction ratio. Furthermore, data from Stacey et al[65] suggest that measurement of non-compaction to compacted ratio measured at end-systole had a higher calculated higher odds ratio for combined cardiovascular events, including death than calculated at end-diastole.
Assessment of late gadolinium enhancement, both trabecular and myocardial, has also shown value in the clinical assessment of LV non-compaction[64,66-68]. The degree of trabecular LGE has shown to be an independent predictor of LVEF as well as correlate with severity of clinical stage of disease[66]. Additionally, both the presence and extent of myocardial LGE were shown to be significantly related to symptomatic status and electrocardiographic abnormalities as well as a significant predictor of LVEF, suggesting non-compaction as a marker of an underlying diffuse cardiomyopathy[67].
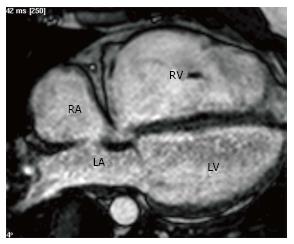
Arrhythmogenic right ventricular dysplasia/cardiomyopathy (ARVD/C) is characterized by fibrofatty replacement of the right ventricular myocardium. The diagnosis is based on Task Force criteria. On MRI, the presence of a major wall motion abnormality (aneurysm, akinesis, dyskinesis, asynchronous contraction) along with either low ejection fraction (EF) (< 40%) or dilated RV (EDVi > 110 mL/m2 in men, > 100 mL/m2 in women) is considered a major criteria (Figure 8). Major wall motion abnormality along with low EF (40%-45%) or dilated RV (EDVi 100-110 mL/m2 in men, 90-100 mL/m2 in women) is considered minor criteria. Other criteria include family history, tissue characterization, repolarization, depoloraization and arrhythmia. Two major or one major and two minor or four minor criteria are required for a diagnosis of ARVD. Fat may be seen in the RV myocardium, but this is not critical for diagnosis. LGE may be seen in the RV free wall. Furthermore, if myocardial biopsy is warranted to help confirm the diagnosis of ARVD, CMR findings can be used to help select an appropriate target for biopsy[69].
In the setting of clinically diagnosed ARVD, the presence of abnormalities at CMR has been shown to be associated with adverse cardiac outcomes[70-72]. Patients with right ventricular abnormalities at CMR experienced higher rates of cardiac death, ICD discharge, and ventricular arrhythmias. Furthermore, the presence of multiple abnormalities at CMR was shown to carry a higher clinical risk, while a normal CMR in patients meeting clinical criteria for ARVC was associated with a significantly better prognosis[70].
Although LGE assessment in the right ventricle can be somewhat limited as compared to that within the left ventricle[73], LGE has shown to be useful in risk stratification in ARVD patients. In patients meeting diagnostic criteria for ARVD, up to 88% of patients demonstrated areas of LGE at CMR[74]. The presence of LGE has also shown to play a role in ARVD risk assessment with right ventricular LGE predicting the induction of ventricular tachycardia at electrophysiological testing[75].
Despite the emphasis placed on right ventricular findings, LV changes are also frequently seen in the setting of ARVD with CMR allowing assessment of LV changes not seen at other modalities[76]. Additionally, LV changes may also be more pronounced than those seen within the right ventricle (“left-dominant” disease). LV involvement at CMR was associated with a higher prevalence of ventricular arrhythmias, even in the setting of normal right ventricular size and function[76,77].
Lastly, CMR is an emerging as a tool in guiding ablation therapies in ARVD patients. For example, in a recent study by Wijnmaalen et al[78] CMR has been proposed as a useful adjunct in combination with voltage mapping in guidance of techniques in providing a potential roadmap for myocardial ablation. Specifically, CMR was shown to identify areas of non-transmural scar and infarct grey zones not detected by traditional voltage mapping.
Stress-induced cardiomyopathy is classically seen on MRI as decreased global systolic function and abnormal wall motion of the apical segments with normal/hyperkinetic basal segments (Figure 9). There may be myocardial edema, but LGE is not typically seen. Variants include a reverse Takotsubo cardiomyopathy, with akinesis of the basal segments and hyperkinesis of the apical segments. These functional abnormalities are transient and recover with treatment of cardiac failure.
Takotsubo variants can readily be distinguished at CMR[79]. Accurate characterization of the particular segmental involvement is important as certain variants, namely typical and mid-ventricular types, have been associated worse worsened LV function[80]. Furthermore, CMR can readily detect associated valvular complications such as mitral regurgitation, which can complicate certain takotsubo subtypes[79]. Additionally, CMR can more easily detect right ventricular involvement, which can be seen in approximately one-third of cases[79]. Detection of right ventricular involvement, if present, has been associated with longer hospitalization and worse LV function[81].
While not a prominent feature in Takotsubo cardiomyopathy, LGE can be present to varying degrees, as shown in several small studies[82-85]. However, its implications for adverse events and recovery are mixed. For example, a pair of studies[82,83] showed that the presence of LGE on CMR performed in the acute or subacute phase (i.e., within one week of presentation) was associated with increased risk of cardiogenic shock, longer duration for ECG normalization, and longer duration of wall motion abnormality recovery. Conversely, multiple studies[84,85] have shown no association with worsened LVEF or development of adverse outcomes as compared to patients without LGE.
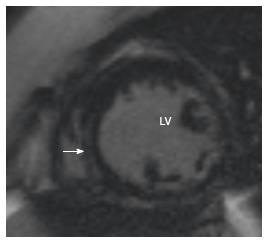
Fabry’s disease is seen on MRI as concentric LV thickening (Figure 10), which is not infrequently confused with HCM. There may be mid myocardial or subepicardial pattern of LGE, typically in the basal inferolateral segment[86].
The presence of LGE in Fabry’s patients has shown to be associated with development of ventricular arrhythmias as well as sudden cardiac death[87]. However, a patient’s annual increase in fibrosis as determined of LGE findings, rather than presence or absence of LGE, was the only independent predictor ventricular arrhythmias. Additionally, CMR findings of fibrosis were found to poorly correlate with blood serum markers of fibrosis[87]. T1 mapping techniques have also been applied to the characterization of Fabry’s cardiomyopathy. Prior to the onset of LV hypertrophy, reduction in T1 values was associated with reduced longitudinal strain as well as early diastolic dysfunction, suggesting that T1 mapping may be useful in detecting early systolic and diastolic dysfunction before onset of cardiac structural abnormalities[88].
CMR has also been used in monitoring cardiac treatment response to enzyme replacement therapies[87,89-91]. While no significant changes in LVEF were seen at follow-up, reductions in LV mass at CMR with corresponding improvement in symptoms were noted[89-91]. Furthermore, a study by Krämer et al[87] showed that of a limited number of patients who underwent enzyme replacement therapy, LGE actually progressed despite therapy suggesting that patients undergoing treatment are still prone to developing worsening fibrosis. However, no clinical outcomes at follow-up were noted for these patients.
On MRI, muscular dystrophy may present with ventricular dilation, systolic dysfunction and mid myocardial/subepicardial pattern of LGE (Figure 11).
The significance of the presence of LGE with arrhythmic events has shown mixed results. While a pair of studies[92,93] have demonstrated significant association between LGE and the development of arrhythmias, Tandon et al[94] demonstrated no significant increased risk in arrhythmia seen in patients with at least one LGE-positive segments. Additionally, in the same study, greater number of LGE positive cardiac segments was predictive of decreases in LVEF, while decreases in LVEF were not seen at follow-up in patients without LGE. T1 mapping and ECV estimation have also been evaluated in muscular dystrophy. Calculated global ECV have been shown to correlate to LVEF and to the number of LGE-positive segments with global ECV significantly associated with occurrence of arrhythmic events[93]. Lastly, myocardial strain analysis has also been applied in this patient population with several studies[95,96] demonstrating that changes in myocardial strain precede changes in LVEF. However, data regarding association with clinical outcomes is lacking.
Limited data is available regarding CMR changes in response to steroid therapy. In a single study[94], longer steroid treatment durations were associated with lower age-related increases in LGE-positive segments, although its impact on clinical outcome is unknown.
Although CMR has been shown to be a powerful tool in diagnosis and clinical assessment and offers a number of distinct advantages over other modalities, certain limitations and challenges are still present. Specific areas in which data is still lacking or contradictory for particular clinical outcomes was discussed in greater detail in the preceding sections. As a whole, although data on the utility of CMR has grown substantially, formal recommendations regarding the specific use of CMR in various clinical settings is lacking for most non-ischemic cardiomyopathies, which may limit its utilization. Furthermore, various technical and logistical aspects of CMR may also limit its usefulness. General contraindications to MRI such as the presence of metallic devices, particularly pacemakers and implantable defibrillators, may limit the usefulness in some cardiac patients. Furthermore, due to the risk of nephrogenic systemic fibrosis, the use gadolinium-based contrast agents, and therefore the assessment of LGE, is limited in patients with renal disease. Lastly, other factors such as the lack of widespread availability and intensive post-processing may further limit the use of CMR in some settings.
MRI is a valuable tool in the evaluation of non-ischemic cardiomyopathies, not only in the diagnosis, but also in risk stratification and prognostic determination. The results of several large scale studies show that there is a good correlation between MRI findings and clinical outcomes, which demonstrate the impact of cardiac MRI on the management of these patients.
P- Reviewer: Landesberg G, Paraskevas KI, Skobel E S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ
| 1. | Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, Moss AJ, Seidman CE, Young JB. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113:1807-1816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2284] [Cited by in RCA: 2242] [Article Influence: 118.0] [Reference Citation Analysis (0)] |
| 2. | Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F, Charron P, Dubourg O, Kühl U, Maisch B, McKenna WJ. Classification of the cardiomyopathies: a position statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2008;29:270-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1766] [Cited by in RCA: 1892] [Article Influence: 105.1] [Reference Citation Analysis (0)] |
| 3. | Hundley WG, Bluemke DA, Finn JP, Flamm SD, Fogel MA, Friedrich MG, Ho VB, Jerosch-Herold M, Kramer CM, Manning WJ. ACCF/ACR/AHA/NASCI/SCMR 2010 expert consensus document on cardiovascular magnetic resonance: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. J Am Coll Cardiol. 2010;55:2614-2662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 531] [Cited by in RCA: 474] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 4. | Anderson LJ, Holden S, Davis B, Prescott E, Charrier CC, Bunce NH, Firmin DN, Wonke B, Porter J, Walker JM. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J. 2001;22:2171-2179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1169] [Cited by in RCA: 1212] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 5. | Patton N, Brown G, Leung M, Bavishi K, Taylor J, Lloyd J, Lee SH, Tay L, Worthley S. Observational study of iron overload as assessed by magnetic resonance imaging in an adult population of transfusion-dependent patients with beta thalassaemia: significant association between low cardiac T2* & lt; 10 ms and cardiac events. Intern Med J. 2010;40:419-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Anderson LJ, Westwood MA, Holden S, Davis B, Prescott E, Wonke B, Porter JB, Walker JM, Pennell DJ. Myocardial iron clearance during reversal of siderotic cardiomyopathy with intravenous desferrioxamine: a prospective study using T2* cardiovascular magnetic resonance. Br J Haematol. 2004;127:348-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 308] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 7. | Pennell DJ, Berdoukas V, Karagiorga M, Ladis V, Piga A, Aessopos A, Gotsis ED, Tanner MA, Smith GC, Westwood MA. Randomized controlled trial of deferiprone or deferoxamine in beta-thalassemia major patients with asymptomatic myocardial siderosis. Blood. 2006;107:3738-3744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 350] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 8. | Anderson LJ, Wonke B, Prescott E, Holden S, Walker JM, Pennell DJ. Comparison of effects of oral deferiprone and subcutaneous desferrioxamine on myocardial iron concentrations and ventricular function in beta-thalassaemia. Lancet. 2002;360:516-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 307] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 9. | Tanner MA, Galanello R, Dessi C, Smith GC, Westwood MA, Agus A, Roughton M, Assomull R, Nair SV, Walker JM. A randomized, placebo-controlled, double-blind trial of the effect of combined therapy with deferoxamine and deferiprone on myocardial iron in thalassemia major using cardiovascular magnetic resonance. Circulation. 2007;115:1876-1884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 357] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 10. | Tanner MA, Galanello R, Dessi C, Smith GC, Westwood MA, Agus A, Pibiri M, Nair SV, Walker JM, Pennell DJ. Combined chelation therapy in thalassemia major for the treatment of severe myocardial siderosis with left ventricular dysfunction. J Cardiovasc Magn Reson. 2008;10:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 175] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 11. | Ambati SR, Randolph RE, Mennitt K, Kleinert DA, Weinsaft JW, Giardina PJ. Longitudinal monitoring of cardiac siderosis using cardiovascular magnetic resonance T2* in patients with thalassemia major on various chelation regimens: a 6-year study. Am J Hematol. 2013;88:652-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Pennell DJ, Porter JB, Piga A, Lai Y, El-Beshlawy A, Belhoul KM, Elalfy M, Yesilipek A, Kilinç Y, Lawniczek T. A 1-year randomized controlled trial of deferasirox vs deferoxamine for myocardial iron removal in β-thalassemia major (CORDELIA). Blood. 2014;123:1447-1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 95] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 13. | Pennell DJ, Porter JB, Cappellini MD, Chan LL, El-Beshlawy A, Aydinok Y, Ibrahim H, Li CK, Viprakasit V, Elalfy MS. Deferasirox for up to 3 years leads to continued improvement of myocardial T2* in patients with β-thalassemia major. Haematologica. 2012;97:842-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 14. | Pennell DJ, Carpenter JP, Roughton M, Cabantchik Z. On improvement in ejection fraction with iron chelation in thalassemia major and the risk of future heart failure. J Cardiovasc Magn Reson. 2011;13:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Pennell DJ, Udelson JE, Arai AE, Bozkurt B, Cohen AR, Galanello R, Hoffman TM, Kiernan MS, Lerakis S, Piga A. Cardiovascular function and treatment in β-thalassemia major: a consensus statement from the American Heart Association. Circulation. 2013;128:281-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 290] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 16. | Modell B, Khan M, Darlison M, Westwood MA, Ingram D, Pennell DJ. Improved survival of thalassaemia major in the UK and relation to T2* cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2008;10:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 391] [Cited by in RCA: 426] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 17. | Chouliaras G, Berdoukas V, Ladis V, Kattamis A, Chatziliami A, Fragodimitri C, Karabatsos F, Youssef J, Karagiorga-Lagana M. Impact of magnetic resonance imaging on cardiac mortality in thalassemia major. J Magn Reson Imaging. 2011;34:56-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Assomull RG, Prasad SK, Lyne J, Smith G, Burman ED, Khan M, Sheppard MN, Poole-Wilson PA, Pennell DJ. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol. 2006;48:1977-1985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 845] [Cited by in RCA: 856] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 19. | McCrohon JA, Moon JC, Prasad SK, McKenna WJ, Lorenz CH, Coats AJ, Pennell DJ. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance. Circulation. 2003;108:54-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 855] [Cited by in RCA: 813] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 20. | Buss SJ, Breuninger K, Lehrke S, Voss A, Galuschky C, Lossnitzer D, Andre F, Ehlermann P, Franke J, Taeger T. Assessment of myocardial deformation with cardiac magnetic resonance strain imaging improves risk stratification in patients with dilated cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2015;16:307-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 213] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 21. | Perazzolo Marra M, De Lazzari M, Zorzi A, Migliore F, Zilio F, Calore C, Vettor G, Tona F, Tarantini G, Cacciavillani L. Impact of the presence and amount of myocardial fibrosis by cardiac magnetic resonance on arrhythmic outcome and sudden cardiac death in nonischemic dilated cardiomyopathy. Heart Rhythm. 2014;11:856-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 137] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 22. | Wu KC, Weiss RG, Thiemann DR, Kitagawa K, Schmidt A, Dalal D, Lai S, Bluemke DA, Gerstenblith G, Marbán E. Late gadolinium enhancement by cardiovascular magnetic resonance heralds an adverse prognosis in nonischemic cardiomyopathy. J Am Coll Cardiol. 2008;51:2414-2421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 495] [Cited by in RCA: 458] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 23. | Lehrke S, Lossnitzer D, Schöb M, Steen H, Merten C, Kemmling H, Pribe R, Ehlermann P, Zugck C, Korosoglou G. Use of cardiovascular magnetic resonance for risk stratification in chronic heart failure: prognostic value of late gadolinium enhancement in patients with non-ischaemic dilated cardiomyopathy. Heart. 2011;97:727-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 178] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 24. | Gulati A, Jabbour A, Ismail TF, Guha K, Khwaja J, Raza S, Morarji K, Brown TD, Ismail NA, Dweck MR. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA. 2013;309:896-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 775] [Cited by in RCA: 887] [Article Influence: 73.9] [Reference Citation Analysis (0)] |
| 25. | Masci PG, Barison A, Aquaro GD, Pingitore A, Mariotti R, Balbarini A, Passino C, Lombardi M, Emdin M. Myocardial delayed enhancement in paucisymptomatic nonischemic dilated cardiomyopathy. Int J Cardiol. 2012;157:43-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Green JJ, Berger JS, Kramer CM, Salerno M. Prognostic value of late gadolinium enhancement in clinical outcomes for hypertrophic cardiomyopathy. JACC Cardiovasc Imaging. 2012;5:370-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 331] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 27. | O'Hanlon R, Grasso A, Roughton M, Moon JC, Clark S, Wage R, Webb J, Kulkarni M, Dawson D, Sulaibeekh L. Prognostic significance of myocardial fibrosis in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010;56:867-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 589] [Cited by in RCA: 625] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 28. | Bruder O, Wagner A, Jensen CJ, Schneider S, Ong P, Kispert EM, Nassenstein K, Schlosser T, Sabin GV, Sechtem U. Myocardial scar visualized by cardiovascular magnetic resonance imaging predicts major adverse events in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010;56:875-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 446] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 29. | Rubinshtein R, Glockner JF, Ommen SR, Araoz PA, Ackerman MJ, Sorajja P, Bos JM, Tajik AJ, Valeti US, Nishimura RA, Gersh BJ. Characteristics and clinical significance of late gadolinium enhancement by contrast-enhanced magnetic resonance imaging in patients with hypertrophic cardiomyopathy. Circ Heart Fail. 2010;3:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 306] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 30. | Adabag AS, Maron BJ, Appelbaum E, Harrigan CJ, Buros JL, Gibson CM, Lesser JR, Hanna CA, Udelson JE, Manning WJ. Occurrence and frequency of arrhythmias in hypertrophic cardiomyopathy in relation to delayed enhancement on cardiovascular magnetic resonance. J Am Coll Cardiol. 2008;51:1369-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 499] [Cited by in RCA: 465] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 31. | Chan RH, Maron BJ, Olivotto I, Pencina MJ, Assenza GE, Haas T, Lesser JR, Gruner C, Crean AM, Rakowski H. Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation. 2014;130:484-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 755] [Article Influence: 68.6] [Reference Citation Analysis (1)] |
| 32. | Kwon DH, Setser RM, Popović ZB, Thamilarasan M, Sola S, Schoenhagen P, Garcia MJ, Flamm SD, Lever HM, Desai MY. Association of myocardial fibrosis, electrocardiography and ventricular tachyarrhythmia in hypertrophic cardiomyopathy: a delayed contrast enhanced MRI study. Int J Cardiovasc Imaging. 2008;24:617-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 33. | Fluechter S, Kuschyk J, Wolpert C, Doesch C, Veltmann C, Haghi D, Schoenberg SO, Sueselbeck T, Germans T, Streitner F. Extent of late gadolinium enhancement detected by cardiovascular magnetic resonance correlates with the inducibility of ventricular tachyarrhythmia in hypertrophic cardiomyopathy. J Cardiovasc Magn Reson. 2010;12:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 34. | Aquaro GD, Masci P, Formisano F, Barison A, Strata E, Pingitore A, Positano V, Spirito P, Lombardi M. Usefulness of delayed enhancement by magnetic resonance imaging in hypertrophic cardiomyopathy as a marker of disease and its severity. Am J Cardiol. 2010;105:392-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Ismail TF, Jabbour A, Gulati A, Mallorie A, Raza S, Cowling TE, Das B, Khwaja J, Alpendurada FD, Wage R. Role of late gadolinium enhancement cardiovascular magnetic resonance in the risk stratification of hypertrophic cardiomyopathy. Heart. 2014;100:1851-1858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 142] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 36. | Yuan J, Qiao S, Zhang Y, You S, Duan F, Hu F, Yang W. Follow-up by cardiac magnetic resonance imaging in patients with hypertrophic cardiomyopathy who underwent percutaneous ventricular septal ablation. Am J Cardiol. 2010;106:1487-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 37. | Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, Naidu SS, Nishimura RA, Ommen SR, Rakowski H. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;58:2703-2738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 208] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 38. | Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, Hagege AA, Lafont A, Limongelli G, Mahrholdt H. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2733-2779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2292] [Cited by in RCA: 3068] [Article Influence: 278.9] [Reference Citation Analysis (0)] |
| 39. | Vignaux O. Cardiac sarcoidosis: spectrum of MRI features. AJR Am J Roentgenol. 2005;184:249-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 133] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 40. | Greulich S, Deluigi CC, Gloekler S, Wahl A, Zürn C, Kramer U, Nothnagel D, Bültel H, Schumm J, Grün S. CMR imaging predicts death and other adverse events in suspected cardiac sarcoidosis. JACC Cardiovasc Imaging. 2013;6:501-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 341] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 41. | Patel MR, Cawley PJ, Heitner JF, Klem I, Parker MA, Jaroudi WA, Meine TJ, White JB, Elliott MD, Kim HW. Detection of myocardial damage in patients with sarcoidosis. Circulation. 2009;120:1969-1977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 514] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 42. | Ise T, Hasegawa T, Morita Y, Yamada N, Funada A, Takahama H, Amaki M, Kanzaki H, Okamura H, Kamakura S. Extensive late gadolinium enhancement on cardiovascular magnetic resonance predicts adverse outcomes and lack of improvement in LV function after steroid therapy in cardiac sarcoidosis. Heart. 2014;100:1165-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 125] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 43. | Vignaux O, Dhote R, Duboc D, Blanche P, Dusser D, Weber S, Legmann P. Clinical significance of myocardial magnetic resonance abnormalities in patients with sarcoidosis: a 1-year follow-up study. Chest. 2002;122:1895-1901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 158] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 44. | Shimada T, Shimada K, Sakane T, Ochiai K, Tsukihashi H, Fukui M, Inoue S, Katoh H, Murakami Y, Ishibashi Y. Diagnosis of cardiac sarcoidosis and evaluation of the effects of steroid therapy by gadolinium-DTPA-enhanced magnetic resonance imaging. Am J Med. 2001;110:520-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 141] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 45. | Hendel RC, Patel MR, Kramer CM, Poon M, Hendel RC, Carr JC, Gerstad NA, Gillam LD, Hodgson JM, Kim RJ. ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR 2006 appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American College of Radiology, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, American Society of Nuclear Cardiology, North American Society for Cardiac Imaging, Society for Cardiovascular Angiography and Interventions, and Society of Interventional Radiology. J Am Coll Cardiol. 2006;48:1475-1497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1092] [Cited by in RCA: 948] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 46. | Mahrholdt H, Wagner A, Deluigi CC, Kispert E, Hager S, Meinhardt G, Vogelsberg H, Fritz P, Dippon J, Bock CT. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation. 2006;114:1581-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 661] [Cited by in RCA: 589] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 47. | Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, White JA, Abdel-Aty H, Gutberlet M, Prasad S. Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J Am Coll Cardiol. 2009;53:1475-1487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1951] [Cited by in RCA: 1747] [Article Influence: 109.2] [Reference Citation Analysis (0)] |
| 48. | Schumm J, Greulich S, Wagner A, Grün S, Ong P, Bentz K, Klingel K, Kandolf R, Bruder O, Schneider S. Cardiovascular magnetic resonance risk stratification in patients with clinically suspected myocarditis. J Cardiovasc Magn Reson. 2014;16:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 49. | Grün S, Schumm J, Greulich S, Wagner A, Schneider S, Bruder O, Kispert EM, Hill S, Ong P, Klingel K. Long-term follow-up of biopsy-proven viral myocarditis: predictors of mortality and incomplete recovery. J Am Coll Cardiol. 2012;59:1604-1615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 395] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 50. | Kearney MT, Cotton JM, Richardson PJ, Shah AM. Viral myocarditis and dilated cardiomyopathy: mechanisms, manifestations, and management. Postgrad Med J. 2001;77:4-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 109] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 51. | Stensaeth KH, Hoffmann P, Fossum E, Mangschau A, Sandvik L, Klow NE. Cardiac magnetic resonance visualizes acute and chronic myocardial injuries in myocarditis. Int J Cardiovasc Imaging. 2012;28:327-335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 52. | Gutberlet M, Spors B, Thoma T, Bertram H, Denecke T, Felix R, Noutsias M, Schultheiss HP, Kühl U. Suspected chronic myocarditis at cardiac MR: diagnostic accuracy and association with immunohistologically detected inflammation and viral persistence. Radiology. 2008;246:401-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 192] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 53. | Wagner A, Schulz-Menger J, Dietz R, Friedrich MG. Long-term follow-up of patients paragraph sign with acute myocarditis by magnetic paragraph sign resonance imaging. MAGMA. 2003;16:17-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 90] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 54. | Austin BA, Tang WH, Rodriguez ER, Tan C, Flamm SD, Taylor DO, Starling RC, Desai MY. Delayed hyper-enhancement magnetic resonance imaging provides incremental diagnostic and prognostic utility in suspected cardiac amyloidosis. JACC Cardiovasc Imaging. 2009;2:1369-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 194] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 55. | Migrino RQ, Christenson R, Szabo A, Bright M, Truran S, Hari P. Prognostic implication of late gadolinium enhancement on cardiac MRI in light chain (AL) amyloidosis on long term follow up. BMC Med Phys. 2009;9:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 56. | Migrino RQ, Harmann L, Christenson R, Hari P. Clinical and imaging predictors of 1-year and long-term mortality in light chain (AL) amyloidosis: a 5-year follow-up study. Heart Vessels. 2014;29:793-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 57. | Maceira AM, Prasad SK, Hawkins PN, Roughton M, Pennell DJ. Cardiovascular magnetic resonance and prognosis in cardiac amyloidosis. J Cardiovasc Magn Reson. 2008;10:54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 214] [Cited by in RCA: 194] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 58. | White JA, Kim HW, Shah D, Fine N, Kim KY, Wendell DC, Al-Jaroudi W, Parker M, Patel M, Gwadry-Sridhar F. CMR imaging with rapid visual T1 assessment predicts mortality in patients suspected of cardiac amyloidosis. JACC Cardiovasc Imaging. 2014;7:143-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 109] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 59. | Banypersad SM, Fontana M, Maestrini V, Sado DM, Captur G, Petrie A, Piechnik SK, Whelan CJ, Herrey AS, Gillmore JD. T1 mapping and survival in systemic light-chain amyloidosis. Eur Heart J. 2015;36:244-251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 260] [Cited by in RCA: 302] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 60. | Karamitsos TD, Piechnik SK, Banypersad SM, Fontana M, Ntusi NB, Ferreira VM, Whelan CJ, Myerson SG, Robson MD, Hawkins PN. Noncontrast T1 mapping for the diagnosis of cardiac amyloidosis. JACC Cardiovasc Imaging. 2013;6:488-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 498] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 61. | Wassmuth R, Abdel-Aty H, Bohl S, Schulz-Menger J. Prognostic impact of T2-weighted CMR imaging for cardiac amyloidosis. Eur Radiol. 2011;21:1643-1650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 62. | Dungu JN, Valencia O, Pinney JH, Gibbs SD, Rowczenio D, Gilbertson JA, Lachmann HJ, Wechalekar A, Gillmore JD, Whelan CJ. CMR-based differentiation of AL and ATTR cardiac amyloidosis. JACC Cardiovasc Imaging. 2014;7:133-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 238] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 63. | Petersen SE, Selvanayagam JB, Wiesmann F, Robson MD, Francis JM, Anderson RH, Watkins H, Neubauer S. Left ventricular non-compaction: insights from cardiovascular magnetic resonance imaging. J Am Coll Cardiol. 2005;46:101-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 804] [Cited by in RCA: 821] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 64. | Ashrith G, Gupta D, Hanmer J, Weiss RM. Cardiovascular magnetic resonance characterization of left ventricular non-compaction provides independent prognostic information in patients with incident heart failure or suspected cardiomyopathy. J Cardiovasc Magn Reson. 2014;16:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 65. | Stacey RB, Andersen MM, St Clair M, Hundley WG, Thohan V. Comparison of systolic and diastolic criteria for isolated LV noncompaction in CMR. JACC Cardiovasc Imaging. 2013;6:931-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 66. | Dodd JD, Holmvang G, Hoffmann U, Ferencik M, Abbara S, Brady TJ, Cury RC. Quantification of left ventricular noncompaction and trabecular delayed hyperenhancement with cardiac MRI: correlation with clinical severity. AJR Am J Roentgenol. 2007;189:974-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 67. | Nucifora G, Aquaro GD, Pingitore A, Masci PG, Lombardi M. Myocardial fibrosis in isolated left ventricular non-compaction and its relation to disease severity. Eur J Heart Fail. 2011;13:170-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 122] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 68. | Wan J, Zhao S, Cheng H, Lu M, Jiang S, Yin G, Gao X, Yang Y. Varied distributions of late gadolinium enhancement found among patients meeting cardiovascular magnetic resonance criteria for isolated left ventricular non-compaction. J Cardiovasc Magn Reson. 2013;15:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 69. | Basso C, Corrado D, Marcus FI, Nava A, Thiene G. Arrhythmogenic right ventricular cardiomyopathy. Lancet. 2009;373:1289-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 714] [Cited by in RCA: 610] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 70. | Deac M, Alpendurada F, Fanaie F, Vimal R, Carpenter JP, Dawson A, Miller C, Roussin I, di Pietro E, Ismail TF. Prognostic value of cardiovascular magnetic resonance in patients with suspected arrhythmogenic right ventricular cardiomyopathy. Int J Cardiol. 2013;168:3514-3521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 71. | Aquaro GD, Pingitore A, Strata E, Di Bella G, Molinaro S, Lombardi M. Cardiac magnetic resonance predicts outcome in patients with premature ventricular complexes of left bundle branch block morphology. J Am Coll Cardiol. 2010;56:1235-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 72. | Keller DI, Osswald S, Bremerich J, Bongartz G, Cron TA, Hilti P, Pfisterer ME, Buser PT. Arrhythmogenic right ventricular cardiomyopathy: diagnostic and prognostic value of the cardiac MRI in relation to arrhythmia-free survival. Int J Cardiovasc Imaging. 2003;19:537-543; discussion 545-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 73. | te Riele AS, Tandri H, Bluemke DA. Arrhythmogenic right ventricular cardiomyopathy (ARVC): cardiovascular magnetic resonance update. J Cardiovasc Magn Reson. 2014;16:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 101] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 74. | Pfluger HB, Phrommintikul A, Mariani JA, Cherayath JG, Taylor AJ. Utility of myocardial fibrosis and fatty infiltration detected by cardiac magnetic resonance imaging in the diagnosis of arrhythmogenic right ventricular dysplasia--a single centre experience. Heart Lung Circ. 2008;17:478-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 75. | Tandri H, Saranathan M, Rodriguez ER, Martinez C, Bomma C, Nasir K, Rosen B, Lima JA, Calkins H, Bluemke DA. Noninvasive detection of myocardial fibrosis in arrhythmogenic right ventricular cardiomyopathy using delayed-enhancement magnetic resonance imaging. J Am Coll Cardiol. 2005;45:98-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 326] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 76. | Sen-Chowdhry S, Syrris P, Ward D, Asimaki A, Sevdalis E, McKenna WJ. Clinical and genetic characterization of families with arrhythmogenic right ventricular dysplasia/cardiomyopathy provides novel insights into patterns of disease expression. Circulation. 2007;115:1710-1720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 386] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 77. | El Ghannudi S, Nghiem A, Germain P, Jeung MY, Gangi A, Roy C. Left ventricular involvement in arrhythmogenic right ventricular cardiomyopathy - a cardiac magnetic resonance imaging study. Clin Med Insights Cardiol. 2014;8:27-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 78. | Wijnmaalen AP, van der Geest RJ, van Huls van Taxis CF, Siebelink HM, Kroft LJ, Bax JJ, Reiber JH, Schalij MJ, Zeppenfeld K. Head-to-head comparison of contrast-enhanced magnetic resonance imaging and electroanatomical voltage mapping to assess post-infarct scar characteristics in patients with ventricular tachycardias: real-time image integration and reversed registration. Eur Heart J. 2011;32:104-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 166] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 79. | Kohan AA, Levy Yeyati E, De Stefano L, Dragonetti L, Pietrani M, Perez de Arenaza D, Belziti C, García-Mónaco RD. Usefulness of MRI in takotsubo cardiomyopathy: a review of the literature. Cardiovasc Diagn Ther. 2014;4:138-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 80. | Song BG, Chun WJ, Park YH, Kang GH, Oh J, Lee SC, Park SW, Oh JK. The clinical characteristics, laboratory parameters, electrocardiographic, and echocardiographic findings of reverse or inverted takotsubo cardiomyopathy: comparison with mid or apical variant. Clin Cardiol. 2011;34:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 81. | Eitel I, Behrendt F, Schindler K, Kivelitz D, Gutberlet M, Schuler G, Thiele H. Differential diagnosis of suspected apical ballooning syndrome using contrast-enhanced magnetic resonance imaging. Eur Heart J. 2008;29:2651-2659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 170] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 82. | Naruse Y, Sato A, Kasahara K, Makino K, Sano M, Takeuchi Y, Nagasaka S, Wakabayashi Y, Katoh H, Satoh H. The clinical impact of late gadolinium enhancement in Takotsubo cardiomyopathy: serial analysis of cardiovascular magnetic resonance images. J Cardiovasc Magn Reson Off J Soc Cardiovasc Magn Reson. 2011;13:67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 83. | Nakamori S, Matsuoka K, Onishi K, Kurita T, Ichikawa Y, Nakajima H, Ishida M, Kitagawa K, Tanigawa T, Nakamura T. Prevalence and signal characteristics of late gadolinium enhancement on contrast-enhanced magnetic resonance imaging in patients with takotsubo cardiomyopathy. Circ J. 2012;76:914-921. [PubMed] |
| 84. | Rolf A, Nef HM, Möllmann H, Troidl C, Voss S, Conradi G, Rixe J, Steiger H, Beiring K, Hamm CW. Immunohistological basis of the late gadolinium enhancement phenomenon in tako-tsubo cardiomyopathy. Eur Heart J. 2009;30:1635-1642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 99] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 85. | Bellera MN, Ortiz JT, Caralt MT, Pérez-Rodon J, Mercader J, Fernández-Gómez C, Paré C, Heras M. Magnetic resonance reveals long-term sequelae of apical ballooning syndrome. Int J Cardiol. 2010;139:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 86. | De Cobelli F, Esposito A, Belloni E, Pieroni M, Perseghin G, Chimenti C, Frustaci A, Del Maschio A. Delayed-enhanced cardiac MRI for differentiation of Fabry’s disease from symmetric hypertrophic cardiomyopathy. AJR Am J Roentgenol. 2009;192:W97-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 87. | Krämer J, Niemann M, Störk S, Frantz S, Beer M, Ertl G, Wanner C, Weidemann F. Relation of burden of myocardial fibrosis to malignant ventricular arrhythmias and outcomes in Fabry disease. Am J Cardiol. 2014;114:895-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 113] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 88. | Pica S, Sado DM, Maestrini V, Fontana M, White SK, Treibel T, Captur G, Anderson S, Piechnik SK, Robson MD. Reproducibility of native myocardial T1 mapping in the assessment of Fabry disease and its role in early detection of cardiac involvement by cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2014;16:99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 153] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 89. | Imbriaco M, Pisani A, Spinelli L, Cuocolo A, Messalli G, Capuano E, Marmo M, Liuzzi R, Visciano B, Cianciaruso B. Effects of enzyme-replacement therapy in patients with Anderson-Fabry disease: a prospective long-term cardiac magnetic resonance imaging study. Heart. 2009;95:1103-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 90. | Messalli G, Imbriaco M, Avitabile G, Russo R, Iodice D, Spinelli L, Dellegrottaglie S, Cademartiri F, Salvatore M, Pisani A. Role of cardiac MRI in evaluating patients with Anderson-Fabry disease: assessing cardiac effects of long-term enzyme replacement therapy. Radiol Med. 2012;117:19-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 91. | Hughes DA, Elliott PM, Shah J, Zuckerman J, Coghlan G, Brookes J, Mehta AB. Effects of enzyme replacement therapy on the cardiomyopathy of Anderson-Fabry disease: a randomised, double-blind, placebo-controlled clinical trial of agalsidase alfa. Heart. 2008;94:153-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 236] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 92. | Florian A, Ludwig A, Engelen M, Waltenberger J, Rösch S, Sechtem U, Yilmaz A. Left ventricular systolic function and the pattern of late-gadolinium-enhancement independently and additively predict adverse cardiac events in muscular dystrophy patients. J Cardiovasc Magn Reson. 2014;16:81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 93. | Florian A, Ludwig A, Rösch S, Yildiz H, Sechtem U, Yilmaz A. Myocardial fibrosis imaging based on T1-mapping and extracellular volume fraction (ECV) measurement in muscular dystrophy patients: diagnostic value compared with conventional late gadolinium enhancement (LGE) imaging. Eur Heart J Cardiovasc Imaging. 2014;15:1004-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 94. | Tandon A, Villa CR, Hor KN, Jefferies JL, Gao Z, Towbin JA, Wong BL, Mazur W, Fleck RJ, Sticka JJ. Myocardial fibrosis burden predicts left ventricular ejection fraction and is associated with age and steroid treatment duration in duchenne muscular dystrophy. J Am Heart Assoc. 2015;4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 120] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 95. | Hor KN, Wansapura J, Markham LW, Mazur W, Cripe LH, Fleck R, Benson DW, Gottliebson WM. Circumferential strain analysis identifies strata of cardiomyopathy in Duchenne muscular dystrophy: a cardiac magnetic resonance tagging study. J Am Coll Cardiol. 2009;53:1204-1210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 152] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 96. | Hagenbuch SC, Gottliebson WM, Wansapura J, Mazur W, Fleck R, Benson DW, Hor KN. Detection of progressive cardiac dysfunction by serial evaluation of circumferential strain in patients with Duchenne muscular dystrophy. Am J Cardiol. 2010;105:1451-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |