Published online Nov 26, 2016. doi: 10.4330/wjc.v8.i11.667
Peer-review started: July 1, 2016
First decision: August 5, 2016
Revised: August 20, 2016
Accepted: September 7, 2016
Article in press: September 8, 2016
Published online: November 26, 2016
Processing time: 148 Days and 21.3 Hours
To evaluate platelet activation markers in psoriasis patients, compared to controls, and investigate their association with the inflammatory burden of psoriasis.
Forty psoriatic patients without cardiovascular disease, and 12 healthy controls were subjected to measurement of baseline platelet CD62P, CD63 and CD42b expression, platelet-leukocyte complexes, i.e., platelet-monocyte complexes (PMC), platelet-neutrophil complexes (PNC) and platelet-lymphocyte complexes, and concentrations of platelet-derived microparticles (PMPs) using flow cytometry. Both larger-size (0.5-0.9 μm) and smaller-size (< 0.5 μm) PMPs were determined. Serum interleukin (IL)-12 and IL-17 levels were also measured by enzyme-linked immunosorbent assay. The severity of psoriasis was evaluated by the Psoriasis Area Severity Index (PASI).
PMP concentrations were significantly higher in psoriasis patients than controls [mean ± standard error of mean (SEM): 22 ± 5/μL vs 11 ± 6/μL; P = 0.018), for both smaller-size (10 ± 2/μL vs 4 ± 2/μL; P = 0.033) and larger-size (12 ± 3/μL vs 6 ± 4/μL; P = 0.014) PMPs. Platelet CD62P, CD63 and CD42b expression and circulating PMC and PNC were similar between the two groups. Lower circulating PLC were observed in psoriasis patients compared to controls (mean ± SEM: 16% ± 3% vs 23% ± 6%; P = 0.047). Larger-size PMPs were related with IL-12 levels (P < 0.001) and smaller-size PMPs with both IL-12 and IL-17 levels (P < 0.001). Total PMPs also correlated with IL-12 (P < 0.001). CD63 expression was positively correlated with both IL-12 and IL-17 (P < 0.05). Increased PASI score was associated with increased levels of larger-size PMPs (r = 0.45; P = 0.011) and increased CD63 expression (r = 0.47; P < 0.01).
PMPs, known to be predictive of cardiovascular outcomes, are increased in psoriasis patients, and associated with high inflammatory disease burden. Enhanced platelet activation may be the missing link leading to cardiovascular events in psoriatic patients.
Core tip: Psoriasis is associated with increased risk of cardiovascular disease. The pathogenic mechanisms shared by the two diseases seem to converge onto “inflammation” phenomenon. Platelets have a potent role in inflammation. Herein we evaluated platelet activation in psoriasis patients compared to healthy controls, and investigated a potential association between platelet activation markers and the inflammatory burden of psoriasis, the latter assessed by serum levels of pivotal pro-inflammatory cytokines implicated in psoriasis. We conclude that the association between psoriasis and atherosclerosis may be related to excessive platelet-derived microparticles (PMPs) formation. The size class of PMPs was taken into consideration in our study.
- Citation: Papadavid E, Diamanti K, Spathis A, Varoudi M, Andreadou I, Gravanis K, Theodoropoulos K, Karakitsos P, Lekakis J, Rigopoulos D, Ikonomidis I. Increased levels of circulating platelet-derived microparticles in psoriasis: Possible implications for the associated cardiovascular risk. World J Cardiol 2016; 8(11): 667-675
- URL: https://www.wjgnet.com/1949-8462/full/v8/i11/667.htm
- DOI: https://dx.doi.org/10.4330/wjc.v8.i11.667
Psoriasis is now considered as an immune-mediated inflammatory disease of the skin affecting about 3% of the adult general population[1]. Although primarily a cutaneous disease, recent research implicates its association with systemic inflammation resulting in increased risk for atherosclerosis and subsequent cardiovascular disease (CVD)[2,3]. The detailed pathophysiological mechanisms which lead psoriasis patients to atherosclerosis remain unclear; however the common inflammatory milieu the two diseases share is of rising significance[3,4].
Hemostasis-maintaining platelets also have relevant functions in inflammation, with recent evidence showing that thrombosis and inflammation are in fact two intrinsically linked processes[5]. Pathomechanisms of psoriasis involve platelet activation, as reported by several investigators so far[6-9]. Increased platelet activation is also implicated in atherosclerotic plaque formation and plaque destabilization[10,11]. Activation of platelets is associated with their degranulation and the subsequent surface expression of antigens, such as CD62P (P-selectin) and CD63, the decreased surface expression of CD42b (GPIb alpha)[12], and the formation of platelet-leukocyte complexes[13]. In addition, the so-called platelet-derived microparticles (PMPs)[14] constitute a marker of platelet activation which, in recent years, has gained emerging importance. PMPs are membrane vesicles of a diameter of 0.1 to 1 μm generated from activated platelets in an exocytotic budding process. They display procoagulant and atherosclerotic properties, being reported to possess 50- to 100-fold higher specific procoagulant activity than activated platelets themselves[15]. PMPs are involved in inflammatory diseases[16], as well as in atherosclerosis and CVD[17,18]. Besides platelet activation, the chronic inflammatory burden of psoriatic patients may also be the trigger for the development of CVD, with interleukin (IL)-12 and IL-17 implicated in the pathogenesis of both diseases[19-21]. Interestingly, IL-17 has recently been shown to facilitate platelet aggregation[22].
Five studies so far have shown elevated PMPs in psoriasis patients[7,8,23-25], two of them methodologically limited in PMP detection by using ELISA-based assays[7,24]. PMPs were also shown, albeit not always[8,23,25], to correlate with the activity of psoriasis, as assessed by the Psoriasis Area Severity Index (PASI) score[7,24]. However, a possible association of platelet activation with cytokines identified as key players in psoriasis has not yet been examined, to the best of our knowledge. Therefore, the purpose of this investigation was to evaluate platelet activation markers in patients with psoriasis without overt cardiovascular complications, compared to healthy controls, by means of flow cytometry, and to determine the relationship between marker levels and the pro-inflammatory cytokine profile of psoriasis, as this was assessed by IL-12 and IL-17 levels.
This hospital-based cross-sectional study was carried out in 40 patients with psoriasis without coronary artery disease (CAD), and 12 participants selected as healthy controls with age, sex, atherosclerotic risk factors (hypertension, hyperlipidemia, current smoking) and use of anti-hypertensive or lipid-lowering medication, similar to those of the patients with psoriasis (Table 1). Eligible patients were given a diagnosis of plaque psoriasis for at least 6 mo. None of them had received relevant topical medications during the two weeks prior to the study and prior systemic therapy, if any, was interrupted for adequate wash-out period. Exclusion criteria for patients with psoriasis and healthy donors included disorders or drugs affecting platelet activity or likely to influence the outcome of the study, namely obstructive CAD (as defined by the absence of clinical history, angina, and reversible myocardial ischemia during a treadmill test and stress echocardiography), chronic inflammatory disease, psoriatic arthritis, familial hyperlipidemia, diabetes mellitus, moderate or severe valvular heart disease, primary cardiomyopathies, chronic renal failure, malignancies and the use of anti-platelet drugs and systemic steroids. All patients underwent exercise treadmill test and/or stress echocardiography as well as carotid and peripheral artery ultrasonography before blood sampling to exclude the presence of clinical significant CVD. Psoriasis patients were recruited from the inpatients’ section and the outpatients’ clinics of the Department of Dermatology and Venereology of our hospital, while controls were selected from visitors and hospital staff. Written informed consent was obtained from all participants before enrollment in the study. This study was conducted according to the Declaration of Helsinki principles, and was approved by the medical ethical committee of Athens University.
| Variable | Psoriasis (n = 40) | Controls (n = 12) | P value |
| Age, yr | 51 ± 12 | 49 ± 13 | 0.8 |
| Sex (male) (%) | 25 (63) | 7 (58) | 0.8 |
| PASI score | 11 ± 7 | - | - |
| Risk factors (%) | |||
| Hypertension | 14 (35) | 4 (33) | 0.9 |
| Hyperlipidemia | 14 (35) | 4 (33) | 0.9 |
| Current smoking | 19 (48) | 5 (42) | 0.8 |
| Medications (%) | |||
| Anti-hypertensives | 13 (33) | 4 (33) | 0.9 |
| Statins | 16 (40) | 5 (42) | 0.9 |
To avoid artificial platelet activation during collection of samples, blood was taken from the antecubital vein through a 21G needle following light application of a tourniquet; the first 2 mL of blood were discarded to avoid procedurally-induced platelet activation. Subsequently, 4 mL of blood were collected in plastic tubes without anticoagulant for assay of serum IL-12 and IL-17. Finally, 4.5 mL of whole blood were drawn into Vacutainer tubes containing 3.2% sodium citrate stock solution (1:9 volume) and mixed immediately, avoiding frothing during the procedure, for the estimation of platelet activation markers by means of flow cytometry within 45 min after blood collection. All patients and controls had ceased antihypertensive treatment and statins 48 h before blood sampling.
We examined platelet activation state using several markers because it is recognized that platelet activation is a complex process and measuring the classical degranulation markers alone may limit the ability to detect platelet activation under all circumstances.
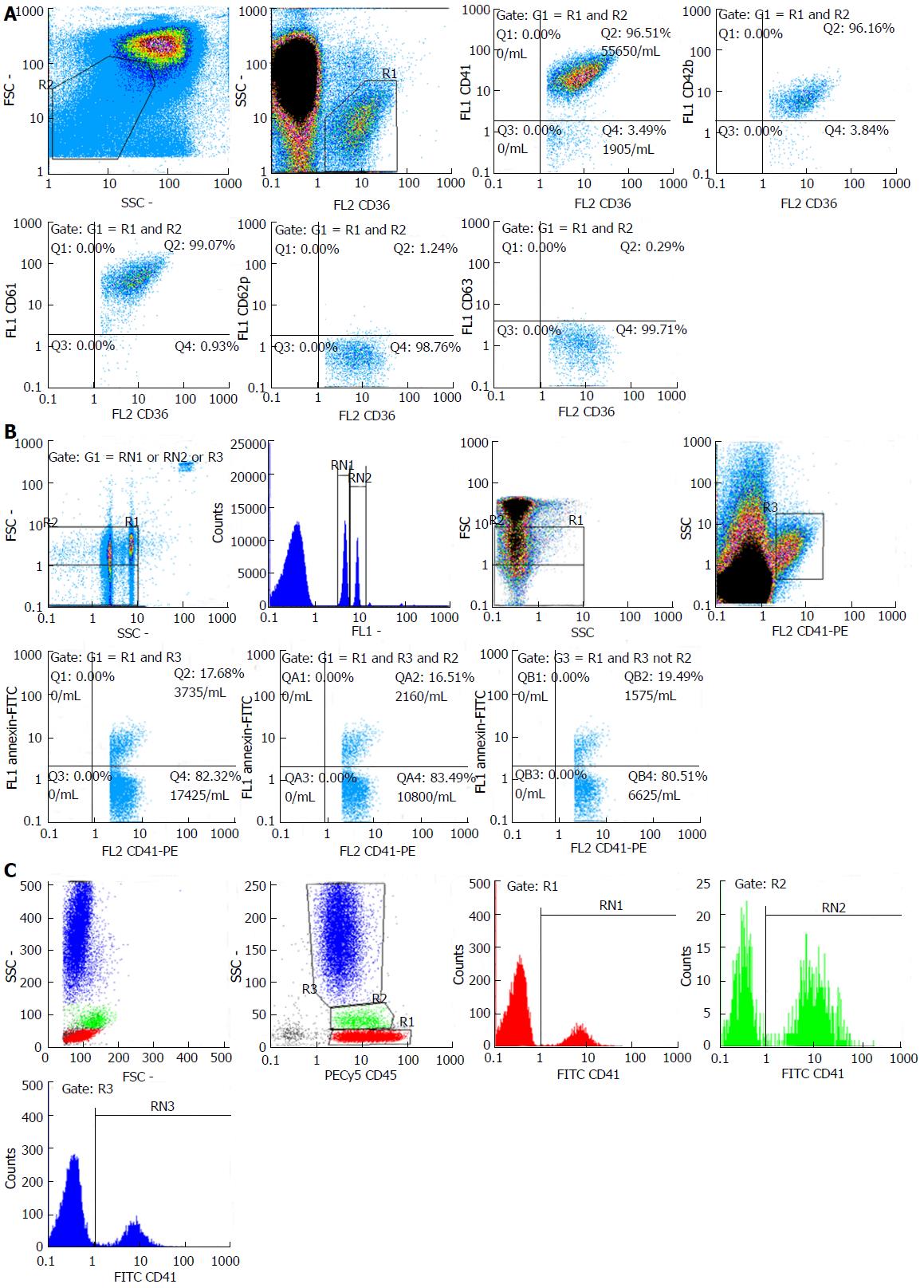
Platelet surface markers: Platelet membrane glycoproteins (GPs) expression was measured from whole blood. Five microliter of blood diluted to 100 μL with PBS per tube were incubated with CD36-PE and FITC labeled monoclonal antibodies against platelet markers that may be expressed in the basal state (CD41, CD42b, CD61) and markers that may be expressed upon activation (CD62P, CD63) (Biolegend, United States) for 10 min at room temperature. One milliliter of PBS was added and samples were analyzed via flow cytometry. Gating was performed using a forward/side scatter (FSC/SSC) dot plot. Expression levels were measured for low FSC/SSC with CD36 positivity using the percentage of platelets with fluorescence over the cutoff set by running 4 samples from control patients (Figure 1A).
PMPs: The technique used for PMP quantification was adapted from a previously described method[26,27]. Plasma was separated from whole blood by centrifugation at 1500 g for 15 min. Recovered plasma was centrifuged for 2 min at 13000 g. Microparticles were labeled using FITC-conjugated Annexin V and PE-conjugated CD41 (Biolegend, United States). Fluorescent-conjugated isotype antibodies were used as controls and a suitable set of beads (Megamix, Biocytex, France) containing three types of beads with a defined size (0.5, 0.9 and 3 μm diameter) was used to identify microparticles via FSC/SSC and determine two PMP-size regions (0.5-0.9 μm and < 0.5 μm PMPs). Samples were diluted to 1 mL using binding buffer and analyzed using absolute counting, when available, on a Partec Cyflow (Partec, Munster, Germany) via volumetric count. PMPs were identified as dual-positive Annexin V-FITC/CD41-PE events in the microparticle region and count/μL was calculated via multiplying the count/mL of the cytometer with the dilution factor of 50 divided by 1000 (Figure 1B).
Platelet-leukocyte complexes: In order to analyze platelet-monocyte complexes (PMC), platelet-neutrophil complexes (PNC) and platelet-lymphocyte complexes (PLC), 100 μL of whole blood, 20 μL of FITC-conjugated anti-CD41 (or negative control antibody) and 20 μL of PECy5-conjugated anti-CD45 (Biolegend, United States) were added into each tube, gently mixed, and incubated in dark, at room temperature for 15 min. Erythrocyte lysis was performed using 2 mL of Quicklysis solution (Cytogons, Spain). Samples were analyzed on a Partec Cyflow Space (Partec, Munster, Germany) within 30 min. Leukocyte populations were gated on a SSC/CD45-PECy5 dot plot and aggregates for each population were calculated as the percentage of monocytes, neutrophils and lymphocytes which were CD41-positive (Figure 1C).
IL-12 was measured in serum using a commercially available kit (Human IL-12 p70 Quantikine HS ELISA Kit; R and D Systems, Minneapolis, United States). This assay detects values as low as 0.5 pg/mL. IL-17 serum levels were also measured by high-sensitivity immunoassay (Human IL-17A High Sensitivity ELISA; eBioscience, Vienna, Austria). The lower limit of detection of the assay was 0.01 pg/mL.
The independent-samples t test was performed to determine the significance level for differences between patient and control groups. Data were expressed as mean ± standard error of mean (SEM). Correlation testing (using Spearman rank correlation coefficient) was performed to assess the strength of relationships between multiple variables. A probability value of < 0.05 was taken to be statistically significant. Statistical Package for Social Sciences version 22.0 (IBM, Chicago, IL) was used for the analysis.
We did not observe any significant difference in baseline characteristics between the two groups (Table 1).
Activation-dependent surface change: No significant difference was observed in CD62P, CD63 or CD42b expression between the two study groups. Mean ± SEM for the fraction of platelets expressing CD62P and CD63 in psoriasis patients and controls were 7 ± 2 vs 6 ± 3; P = 0.748 and 5 ± 2 vs 3 ± 2; P = 0.791, respectively. Mean ± SEM for the fractions of platelets with reduced expression of CD42b was 8 ± 2 vs 10 ± 7; P = 0.397 for psoriasis patients and controls, respectively (Table 2).
| Marker | Psoriasis (n = 40) | Controls (n = 12) | P value |
| CD42b negative platelets (%) | 8 ± 2 | 10 ± 7 | 0.397 |
| CD62P positive platelets (%) | 7 ± 2 | 6 ± 3 | 0.748 |
| CD63 positive platelets (%) | 5 ± 2 | 3 ± 2 | 0.791 |
| Total AV+/CD41+ PMPs1 | 22 ± 5 | 11 ± 6 | 0.0183 |
| < 0.5 μm AV+/CD41+ PMPs1 | 10 ± 2 | 4 ± 2 | 0.0333 |
| 0.5-0.9 μm AV+/CD41+ PMPs1 | 12 ± 3 | 6 ± 4 | 0.0143 |
| Platelet-lymphocyte complexes (%) | 16 ± 3 | 23 ± 6 | 0.0473 |
| Platelet-monocyte complexes (%) | 38 ± 4 | 33 ± 6 | 0.723 |
| Platelet-neutrophil complexes (%) | 27 ± 3 | 29 ± 5 | 0.775 |
| IL-122 | 19 ± 0.5 | 2 ± 0.3 | < 0.0013 |
| IL-172 | 3 ± 0.4 | 0 ± 0.1 | < 0.0013 |
PMPs: PMP concentrations were markedly higher in psoriasis patients compared to controls (mean ± SEM: 22 ± 5/μL vs 11 ± 6/μL; P = 0.018). When considering PMP size, both smaller-size (mean ± SEM: 10 ± 2/μL vs 4 ± 2/μL; P = 0.033) and larger-size (12 ± 3/μL vs 6 ± 4/μL; P = 0.014) PMPs were higher in patients compared to healthy subjects (Table 2).
Platelet-leukocyte complexes: There was no significant difference in the percentage of circulating neutrophils or monocytes in whole blood which formed complexes with platelets between the two groups (mean ± SEM for PMC and PNC in psoriasis patients and controls respectively were 38% ± 4% and 27% ± 3% vs 33% ± 6% and 29% ± 5%; P = 0.723 and P = 0.775, respectively). However, significantly lower circulating PLC were observed in psoriasis patients (mean ± SEM: 16% ± 3% vs 23% ± 6%; P = 0.047) (Table 2).
A significant correlation was established between larger-size PMPs and IL-12 levels (r = 0.55; P < 0.001) and between smaller-size PMPs and levels of both IL-12 and IL-17 (r = 0.58 and r = 0.49 respectively; P < 0.001). Total PMPs also correlated with IL-12 levels (r = 0.56; P < 0.001). CD63 expression correlated well with levels of both IL-12 and IL-17 (r = 0.46; P = 0.011 and r = 0.43; P = 0.015, respectively). Increased PASI score was associated with increased levels of larger-size PMPs (r = 0.45; P = 0.011) and increased CD63 expression (r = 0.47; P < 0.01). Circulating PLC were found to be negatively correlated with PMPs (r = -0.44; P = 0.002), both with smaller-size (r = -0.28; P = 0.048) and larger-size (r = -0.4; P = 0.005) PMPs.
The exact mechanism of predisposition to CVD in psoriasis per se has not been fully elucidated so far. However, several lines of evidence highlight the potent role inflammation plays. Indeed, psoriasis patients, in addition to chronic skin inflammation, display a higher prevalence of CVD risk factors and metabolic syndrome components[28] which lead to systemic inflammation, and therefore atherosclerosis, CVD and myocardial infarction[2,3]. Platelets have an important role in increasing inflammation, and pathogenetic mechanisms of both psoriasis and atherosclerosis may involve platelet activation[6-11,23-25]. The present study demonstrated that circulating platelets are in a state of activation in patients with psoriasis without clinically evident CVD compared to healthy subjects, as shown by a significant increase in circulating PMPs. It reinforces previous findings of elevated circulating PMP levels in psoriasis patients[7,8,23-25] and adds to those findings by demonstrating for the first time, to the best of our knowledge, a positive relationship between PMP concentrations and high inflammatory psoriasis burden, as this was assessed by IL-12 and IL-17 levels, suggesting a close association between PMPs and psoriasis activity. It is also the first study to report a higher level of larger-size PMPs, in addition to small-size ones, in psoriasis patients. It is now accepted that PMPs are separated into four size classes with different active components and different functional effects on platelets and endothelial cells[29], and therefore, elucidation of the size class(es) involved in psoriasis can help clarify PMP involvement in the disease and the mechanisms implicated in exertion of their effects. Pelletier et al[8] had previously showed that only small-size PMPs are increased in psoriasis. The discrepancy with our results may be related to the different working definition of blood-derived PMPs in the two studies, based on the prerequisite or not of Annexin V (a phospholipid-binding protein that binds to exposed phosphatidylserine on the surface of activated platelets) binding. Annexin V positive PMPs are documented to elicit pro-coagulant activity, in contrast to little or no such activity possessed by Annexin V negative PMPs[30].
PMPs are involved in CAD by binding to the endothelium, submatrix of the vascular wall and leukocytes, thereby facilitating thrombus propagation[17,18,31]. They are also known to cause endothelial dysfunction[32]. In the setting of psoriasis per se, PMPs may well contribute to leukocyte recruitment in psoriatic skin lesions, given their known ability to increase leukocyte adhesion to the endothelium and to promote leukocyte activation by modulating leukocyte-leukocyte and leukocyte-endothelial cell interactions[5]. Taken together, elevated levels of PMPs observed in psoriasis patients may be the contributory factor to development of atherosclerosis and the increased cardiovascular risk in those patients by triggering a cascade of events.
In the present study, PMPs proved to be the most “sensitive” index of platelet activation, whereas the classical platelet activation markers CD62P, CD63 and CD42b were not altered. To our knowledge, CD63 or CD42b expression in psoriasis had not been investigated so far. In contrast to our CD62P results, three previous studies have shown enhanced CD62P surface expression in psoriasis[6,9,33]. One other study was in concordance with our findings[34]. Although P-selectin has been considered by many the “gold standard” marker of platelet activation, it was shown that degranulated, P-selectin-positive platelets rapidly lose surface P-selectin to the plasma pool in vivo[35,36]. Therefore, platelets may circulate in an increased state of activation but express normal levels of CD62P. In fact, it has been proposed that CD62P is a more reliable tool for monitoring platelet function at acute but not chronic stimulus of platelets[37]. The majority of our patients did not have a flare of their disease at the time of our study. Regarding platelet-leukocyte complexes as a marker of platelet activation, there is only one previous study measuring PMC and PNC in psoriasis[34], also not managing to highlight a significant increase. There is no report in the literature concerning PLC in psoriasis, to the best of our knowledge. In the setting of CVD, it has been suggested that the formation of PMC is related to the development of atherosclerotic complications being a sensitive marker of platelet activation[38]. Contrary to our expectations for increased PLC in psoriasis pointing to platelet activation, lower PLC were measured in the bloodstream of our psoriasis patients compared to healthy controls. Our finding could be attributed to the adhesion of PLC in the inflamed skin microvasculature, on asymptomatic atherosclerotic lesions or both. Therefore, decreased blood concentration could merely reflect increased sequestration of the generated platelet-lymphocyte aggregates on the vessel wall. With regard to this, it has already been shown in vivo that increased leukocyte rolling in murine skin and subsequent extravasation is due to the aggregate formation of platelets with mononuclear leukocytes[6]. Interestingly, a negative correlation was established in our study between PMP levels and circulating PLC.
Chronic inflammatory skin diseases and atherosclerosis share common pathogenic features in which pro-inflammatory cytokines play an important role[3,4,39]. In the inflammatory microenvironment present in psoriasis, IL-12 and IL-17 are of crucial importance[19]. This is underlined by the fact that the biologic agents ustekinumab and secukinumab are targeted against IL-12 and IL-17, respectively. IL-12 leads to the differentiation of type 1 T helper (Th1) lymphocytes, whereas IL-17A and IL-17F, secreted by type 17 T helper (Th17) cells, activate keratinocytes and induce the production of antimicrobial peptides. Notably, recent interest has focused particularly on IL-17-producing Th17 cells[40]. This cell type is specialized in immunosurveillance of epithelium, and it also secretes IL-22, a key cytokine linking adaptive immune effectors and epithelial dysregulation in psoriasis. Amelioration of epidermal hyperplasia during successful anti-TNF treatment is associated with reduced Th17 responses. Based on the current knowledge, it appears that Th17 cells are responsible for many of the inflammatory and autoimmune responses once attributed to Th1 lymphocytes. Apart from their implication in psoriasis pathogenesis, IL-12 and IL-17 are also involved in the development of atherosclerosis[20,21,41]. In this viewpoint, IL-12 and IL-17 release into the circulation by cell populations in inflamed psoriatic skin could exert harmful atherosclerotic effects. Taken the aforementioned data into consideration, the association of platelet activation markers, namely PMPs and CD63, with the levels of pro-inflammatory cytokines IL-12 and IL-17, demonstrated in our study, comes as no surprise. Interestingly, it has been recently shown that IL-17A can promote platelet function in patients with acute coronary syndrome via activating platelets ERK2 signaling pathway and may provide a novel target for antiplatelet therapies in CAD[22]. On the basis of the ability of IL-17A to promote platelet function, the view that inflammation and platelet activation perpetuate each other and cascade to the development of atherosclerosis is reinforced.
Features of psoriasis pathogenesis, including chronic inflammation and the proven platelet activation, may contribute to atherosclerotic risk in psoriasis. Our study has shown increased levels of PMPs, a marker of platelet activation, in psoriasis patients without overt CVD compared to healthy controls. This difference has been demonstrated for the first time in both smaller-size and larger-size PMPs. As PMPs express procoagulant phospatidylserine activities, facilitate thrombus propagation and provoke endothelial cell damage, elevated PMP levels could provide one of the missing links leading to increased cardiovascular risk in psoriasis. Furthermore, PMPs were higher in those patients with high inflammatory disease burden, as this was assessed by IL-12 and IL-17 levels, as well as in those patients with high PASI score, suggesting a close association between PMPs and psoriasis activity. Given the ability of IL-17A to promote platelet function, this finding is in favor of the view that inflammation and platelet activation may perpetuate each other and cascade to the development of atherosclerosis. Finally, we identified the presence of lower PLC in the bloodstream of psoriasis patients which could be attributed to their adhesion in the inflamed skin microvasculature, on asymptomatic atherosclerotic lesions or both. PLC levels negatively correlated with PMP levels. The clinical relevance of our findings, however, remains still disputed. While there is ample in vitro evidence of the potential downstream biological effects of microparticles (e.g., promotion of coagulation, regulation of inflammation, vascular damage)[42], many of which are known to be important in atherogenesis, in vivo data in patients with psoriasis are lacking. In this setting, PMP generation could merely represent an epiphenomenon related to the inflammation of psoriasis with little in vivo biological activity. Future studies are needed to address whether PMPs are simply biomarkers of inflammatory disease or have a role in psoriasis pathophysiology leading to accelerated atherosclerosis.
The small number of controls and the absence of age/sex matching between patients and controls should be acknowledged as study limitations.
In conclusion, PMPs, known to be predictive of cardiovascular outcomes, are increased in psoriasis patients, and associated with high inflammatory disease burden. Enhanced platelet activation may be the missing link leading to cardiovascular events in psoriatic patients.
Psoriasis is a common immune-mediated inflammatory disease of the skin. Although primarily a cutaneous disease, recent research implicates its association with systemic inflammation resulting in increased risk for atherosclerosis and subsequent cardiovascular disease (CVD). Platelets have an important role in inflammation. Pathogenic mechanisms of both psoriasis and atherosclerosis seem to involve platelet activation.
Enhanced platelet activation in psoriasis patients has already been established, but a potential association between platelet activation markers and the inflammatory burden of psoriasis has not yet been examined.
The present study demonstrated increased platelet activation in patients with psoriasis without clinically evident CVD compared to healthy controls, as shown by a significant increase in circulating platelet-derived microparticles (PMPs), a platelet activation marker which is known to be predictive of cardiovascular outcomes. It reinforces previous findings of elevated circulating PMP levels in psoriasis patients and adds to those findings by demonstrating for the first time, to the best of our knowledge, a positive relationship between PMP concentrations and levels of cytokines identified as key players in psoriasis, namely interleukin (IL)-12 and IL-17, suggesting a close association between PMPs and high inflammatory disease burden. Given the ability of IL-17A to promote platelet function, this finding is in favor of the view that inflammation and platelet activation may perpetuate each other culminating in the development of atherosclerosis. Furthermore, this is the first study, to the best of our knowledge, to report a higher level of larger-size PMPs, additionally to small-size ones, in psoriasis patients. It is now accepted that PMPs are separated into four size classes with different active components and different functional effects on platelets and endothelial cells, and therefore, elucidation of the size class(es) involved in psoriasis can help clarify PMP involvement in the disease and the mechanisms implicated in exertion of their effects. Taken together, the study concludes that the association between psoriasis and atherosclerosis may be related to excessive PMP formation.
Enhanced platelet activation may be the missing link leading to cardiovascular events in psoriatic patients. Future studies are needed to address the in vivo biological activity of PMPs contributing to CVD in patients with psoriasis, as well as the potential role of anti-platelet medications in psoriasis in the context of reducing both psoriasis activity and atherosclerotic risk.
PMPs constitute a marker of platelet activation which, in tecent years, has gained emerging importance. PMPs are membrane vesicles of a diameter of 0.1 to 1 μm generated from activated platelets in an exocytotic budding process. They display procoagulant and atherosclerotic properties, being reported to possess 50- to 100-fold higher specific procoagulant activity than activated platelets themselves.
Interesting and very relevant study regarding the level of markers of platelet activation in psoriasis.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: Greece
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Ayroldi E, Kaliyadan F, Murdaca G S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Gelfand JM, Weinstein R, Porter SB, Neimann AL, Berlin JA, Margolis DJ. Prevalence and treatment of psoriasis in the United Kingdom: a population-based study. Arch Dermatol. 2005;141:1537-1541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 407] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 2. | Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735-1741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1246] [Cited by in RCA: 1447] [Article Influence: 76.2] [Reference Citation Analysis (0)] |
| 3. | Vena GA, Vestita M, Cassano N. Psoriasis and cardiovascular disease. Dermatol Ther. 2010;23:144-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | Ghazizadeh R, Shimizu H, Tosa M, Ghazizadeh M. Pathogenic mechanisms shared between psoriasis and cardiovascular disease. Int J Med Sci. 2010;7:284-289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Wagner DD, Burger PC. Platelets in inflammation and thrombosis. Arterioscler Thromb Vasc Biol. 2003;23:2131-2137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 392] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 6. | Ludwig RJ, Schultz JE, Boehncke WH, Podda M, Tandi C, Krombach F, Baatz H, Kaufmann R, von Andrian UH, Zollner TM. Activated, not resting, platelets increase leukocyte rolling in murine skin utilizing a distinct set of adhesion molecules. J Invest Dermatol. 2004;122:830-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 94] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Tamagawa-Mineoka R, Katoh N, Kishimoto S. Platelet activation in patients with psoriasis: increased plasma levels of platelet-derived microparticles and soluble P-selectin. J Am Acad Dermatol. 2010;62:621-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 8. | Pelletier F, Garnache-Ottou F, Angelot F, Biichlé S, Vidal C, Humbert P, Saas P, Seillès E, Aubin F. Increased levels of circulating endothelial-derived microparticles and small-size platelet-derived microparticles in psoriasis. J Invest Dermatol. 2011;131:1573-1576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Saleh HM, Attia EA, Onsy AM, Saad AA, Abd Ellah MM. Platelet activation: a link between psoriasis per se and subclinical atherosclerosis--a case-control study. Br J Dermatol. 2013;169:68-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Jennings LK. Role of platelets in atherothrombosis. Am J Cardiol. 2009;103:4A-10A. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 139] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 11. | Ikuta T, Naruko T, Ikura Y, Ohsawa M, Fukushima H, Shirai N, Itoh A, Haze K, Ehara S, Sasaki Y. Immunolocalization of platelet glycoprotein IIb/IIIa and P-selectin, and neutrophil-platelet interaction in human coronary unstable plaques. Int J Mol Med. 2005;15:573-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Michelson AD. Flow cytometry: a clinical test of platelet function. Blood. 1996;87:4925-4936. [PubMed] |
| 13. | Rinder HM, Bonan JL, Rinder CS, Ault KA, Smith BR. Activated and unactivated platelet adhesion to monocytes and neutrophils. Blood. 1991;78:1760-1769. [PubMed] |
| 14. | Holme PA, Brosstad F, Solum NO. The difference between platelet and plasma FXIII used to study the mechanism of platelet microvesicle formation. Thromb Haemost. 1993;70:681-686. [PubMed] |
| 15. | Sinauridze EI, Kireev DA, Popenko NY, Pichugin AV, Panteleev MA, Krymskaya OV, Ataullakhanov FI. Platelet microparticle membranes have 50- to 100-fold higher specific procoagulant activity than activated platelets. Thromb Haemost. 2007;97:425-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 397] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 16. | Beyer C, Pisetsky DS. The role of microparticles in the pathogenesis of rheumatic diseases. Nat Rev Rheumatol. 2010;6:21-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 206] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 17. | van der Zee PM, Biró E, Ko Y, de Winter RJ, Hack CE, Sturk A, Nieuwland R. P-selectin- and CD63-exposing platelet microparticles reflect platelet activation in peripheral arterial disease and myocardial infarction. Clin Chem. 2006;52:657-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 149] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 18. | Preston RA, Jy W, Jimenez JJ, Mauro LM, Horstman LL, Valle M, Aime G, Ahn YS. Effects of severe hypertension on endothelial and platelet microparticles. Hypertension. 2003;41:211-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 370] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 19. | Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2361] [Cited by in RCA: 2123] [Article Influence: 132.7] [Reference Citation Analysis (0)] |
| 20. | Fan Z, Yang J, Yang C, Yang J, Guo X. IL-17: A promising therapeutic target for atherosclerosis. Int J Cardiol. 2016;202:930-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Yong K, Dogra G, Boudville N, Chan D, Adams L, Ching H, Lim EM, Lim WH. Interleukin-12 is associated with arterial stiffness in healthy individuals. Am J Hypertens. 2013;26:159-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Zhang S, Yuan J, Yu M, Fan H, Guo ZQ, Yang R, Guo HP, Liao YH, Wang M. IL-17A facilitates platelet function through the ERK2 signaling pathway in patients with acute coronary syndrome. PLoS One. 2012;7:e40641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Takeshita J, Mohler ER, Krishnamoorthy P, Moore J, Rogers WT, Zhang L, Gelfand JM, Mehta NN. Endothelial cell-, platelet-, and monocyte/macrophage-derived microparticles are elevated in psoriasis beyond cardiometabolic risk factors. J Am Heart Assoc. 2014;3:e000507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 24. | Chandrashekar L, Rajappa M, Revathy G, Sundar I, Munisamy M, Ananthanarayanan PH, Thappa DM, Basu D. Is enhanced platelet activation the missing link leading to increased cardiovascular risk in psoriasis? Clin Chim Acta. 2015;446:181-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 25. | Ho JC, Lee CH, Lin SH. No Significant Reduction of Circulating Endothelial-Derived and Platelet-Derived Microparticles in Patients with Psoriasis Successfully Treated with Anti-IL12/23. Biomed Res Int. 2016;2016:3242143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Robert S, Poncelet P, Lacroix R, Arnaud L, Giraudo L, Hauchard A, Sampol J, Dignat-George F. Standardization of platelet-derived microparticle counting using calibrated beads and a Cytomics FC500 routine flow cytometer: a first step towards multicenter studies? J Thromb Haemost. 2009;7:190-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 238] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 27. | Tziatzios G, Polymeros D, Spathis A, Triantafyllou M, Gkolfakis P, Karakitsos P, Dimitriadis G, Triantafyllou K. Increased levels of circulating platelet derived microparticles in Crohn’s disease patients. Scand J Gastroenterol. 2016;51:1184-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Langan SM, Seminara NM, Shin DB, Troxel AB, Kimmel SE, Mehta NN, Margolis DJ, Gelfand JM. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol. 2012;132:556-562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 288] [Cited by in RCA: 343] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 29. | Dean WL, Lee MJ, Cummins TD, Schultz DJ, Powell DW. Proteomic and functional characterisation of platelet microparticle size classes. Thromb Haemost. 2009;102:711-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 154] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 30. | Connor DE, Exner T, Ma DD, Joseph JE. The majority of circulating platelet-derived microparticles fail to bind annexin V, lack phospholipid-dependent procoagulant activity and demonstrate greater expression of glycoprotein Ib. Thromb Haemost. 2010;103:1044-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 236] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 31. | Shantsila E, Kamphuisen PW, Lip GY. Circulating microparticles in cardiovascular disease: implications for atherogenesis and atherothrombosis. J Thromb Haemost. 2010;8:2358-2368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 131] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 32. | Boulanger CM, Scoazec A, Ebrahimian T, Henry P, Mathieu E, Tedgui A, Mallat Z. Circulating microparticles from patients with myocardial infarction cause endothelial dysfunction. Circulation. 2001;104:2649-2652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 356] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 33. | Garbaraviciene J, Diehl S, Varwig D, Bylaite M, Ackermann H, Ludwig RJ, Boehncke WH. Platelet P-selectin reflects a state of cutaneous inflammation: possible application to monitor treatment efficacy in psoriasis. Exp Dermatol. 2010;19:736-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 34. | Pamuk GE, Nuri Pamuk O, Orüm H, Arican O, Turgut B, Demir M. Elevated platelet-monocyte complexes in patients with psoriatic arthritis. Platelets. 2009;20:493-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 35. | Michelson AD, Barnard MR, Krueger LA, Valeri CR, Furman MI. Circulating monocyte-platelet aggregates are a more sensitive marker of in vivo platelet activation than platelet surface P-selectin: studies in baboons, human coronary intervention, and human acute myocardial infarction. Circulation. 2001;104:1533-1537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 513] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 36. | Michelson AD, Barnard MR, Hechtman HB, MacGregor H, Connolly RJ, Loscalzo J, Valeri CR. In vivo tracking of platelets: circulating degranulated platelets rapidly lose surface P-selectin but continue to circulate and function. Proc Natl Acad Sci USA. 1996;93:11877-11882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 424] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 37. | Nagy Jr B, Debreceni IB, Kappelmayer J. Flow cytometric investigation of classical and alternative platelet activation markers. eJIFCC. 2011;23:11 screens Available from: http: //www.ifcc.org/media/215445/05_ Nagy.pdf. |
| 38. | Furman MI, Barnard MR, Krueger LA, Fox ML, Shilale EA, Lessard DM, Marchese P, Frelinger AL, Goldberg RJ, Michelson AD. Circulating monocyte-platelet aggregates are an early marker of acute myocardial infarction. J Am Coll Cardiol. 2001;38:1002-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 305] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 39. | Ikonomidis I, Makavos G, Papadavid E, Varoudi M, Andreadou I, Gravanis K, Theodoropoulos K, Pavlidis G, Triantafyllidi H, Parissis J. Similarities in coronary function and myocardial deformation between psoriasis and coronary artery disease: the role of oxidative stress and inflammation. Can J Cardiol. 2015;31:287-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 40. | Murdaca G, Colombo BM, Puppo F. The role of Th17 lymphocytes in the autoimmune and chronic inflammatory diseases. Intern Emerg Med. 2011;6:487-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 41. | Ranjbaran H, Sokol SI, Gallo A, Eid RE, Iakimov AO, D’Alessio A, Kapoor JR, Akhtar S, Howes CJ, Aslan M. An inflammatory pathway of IFN-gamma production in coronary atherosclerosis. J Immunol. 2007;178:592-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 42. | VanWijk MJ, VanBavel E, Sturk A, Nieuwland R. Microparticles in cardiovascular diseases. Cardiovasc Res. 2003;59:277-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 454] [Article Influence: 20.6] [Reference Citation Analysis (0)] |