Published online Nov 26, 2016. doi: 10.4330/wjc.v8.i11.647
Peer-review started: July 1, 2016
First decision: August 5, 2016
Revised: August 26, 2016
Accepted: September 7, 2016
Article in press: September 8, 2016
Published online: November 26, 2016
Processing time: 148 Days and 12.4 Hours
To assess the prevalence, clinical characteristics and independent prognostic impact of atrial fibrillation (AF) in chronic heart failure (CHF) patients, and the potential protective effect of disease-modifying medications, particularly beta-blockers (BB).
We retrospectively reviewed the charts of patients referred to our center since January 2004, and collected all clinical information available at their first visit. We assessed mortality to the end of June 2015. We compared patients with and without AF, and assessed the association between AF and all-cause mortality by multivariate Cox regression and Kaplan-Meyer analysis, particularly accounting for ongoing treatment with BB.
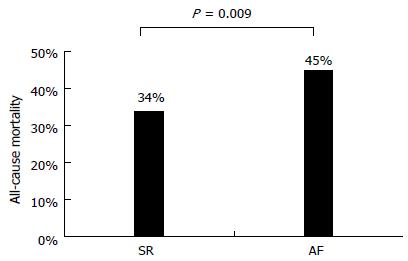
A total of 903 patients were evaluated (mean age 68 ± 12 years, 73% male). Prevalence of AF was 19%, ranging from 10% to 28% in patients ≤ 60 and ≥ 77 years, respectively. Besides the older age, patients with AF had more symptoms (New York Heart Association II-III 60% vs 44%), lower prevalence of dyslipidemia (23% vs 37%), coronary artery disease (28% vs 52%) and left bundle branch block (9% vs 16%). On the contrary, they more frequently presented with an idiopathic etiology (50% vs 24%), a history of valve surgery (13% vs 4%) and received overall more devices implantation (31% vs 21%). The use of disease-modifying medications (i.e., BB and ACE inhibitors/angiotensin receptor blockers) was lower in patients with AF (72% vs 80% and 71% vs 79%, respectively), who on the contrary were more frequently treated with symptomatic and antiarrhythmic drugs including diuretics (87% vs 69%) and digoxin (51% vs 11%). At a mean follow-up of about 5 years, all-cause mortality was significantly higher in patients with AF as compared to those in sinus rhythm (SR) (45% vs 34%, P value < 0.05 for all previous comparisons). However, in a multivariate analysis including the main significant predictors of all-cause mortality, the univariate relationship between AF and death (HR = 1.49, 95%CI: 1.15-1.92) became not statistically significant (HR = 0.98, 95%CI: 0.73-1.32). Nonetheless, patients with AF not receiving BB treatment were found to have the worst prognosis, followed by patients with SR not receiving BB therapy and patients with AF receiving BB therapy, who both had similarly worse survival when compared to patients with SR receiving BB therapy.
AF was highly prevalent and associated with older age, worse clinical presentation and underutilization of disease-modifying medications such as BB in a population of elderly patients with CHF. AF had no independent impact on mortality, but the underutilization of BB in this group of patients was associated to a worse long-term prognosis.
Core tip: In this retrospective analysis atrial fibrillation (AF) was diagnosed in 1 out of 5 patients with chronic heart failure. The arrhythmia was associated with older age, worse clinical presentation and underutilization of disease-modifying medications, particularly beta-blockers (BB) and ACE inhibitors/angiotensin receptor blockers. At a mean follow-up of about 5 years, mortality was significantly higher in patients with AF, and patients with AF not receiving BB treatment were found to have the worst prognosis. However, in a multivariate analysis including main significant predictors of all-cause mortality, such as age, gender, blood pressure, coronary artery disease, comorbidities and medications, the univariate relationship between AF and death became not statistically significant.
- Citation: Gigli L, Ameri P, Secco G, De Blasi G, Miceli R, Lorenzoni A, Torre F, Chiarella F, Brunelli C, Canepa M. Clinical characteristics and prognostic impact of atrial fibrillation in patients with chronic heart failure. World J Cardiol 2016; 8(11): 647-656
- URL: https://www.wjgnet.com/1949-8462/full/v8/i11/647.htm
- DOI: https://dx.doi.org/10.4330/wjc.v8.i11.647
Atrial fibrillation (AF) is the most common arrhythmia and frequently coexists with chronic heart failure (CHF)[1]. It is commonly held that CHF decompensated by a transient AF episode has better prognosis than CHF with permanent AF[2]. However, the real prognostic impact of permanent AF in patients with CHF remains poorly understood[3-6] and a matter of current debate[7,8]. Conflicting data also exist on medical treatment of CHF patients with AF, particularly in the elderly. Indeed, although beta-blockers (BB) are a corner-stone therapy of CHF, their value when AF coexists has recently been questioned[9]. Thus, the aim of this study was to investigate the prevalence, clinical characteristics and prognostic impact of permanent AF in a cohort of unselected CHF patients referred to a single tertiary outpatient clinic. In particular, we assessed whether a diagnosis of permanent AF was independently associated with increased all-cause mortality, and whether this association was influenced by medical therapy with BB.
The study population was drawn from a tertiary CHF outpatient clinic; all patients with a diagnosis of CHF, New York Heart Association (NYHA) functional class between I and III and a readable rest ECG were considered eligible. Data were retrospectively collected by reviewing all available complete records of the first visit at the clinic between January 1st 2004 and May 31st 2015. A total of 941 unique patients were originally included; 23 patients were subsequently excluded because they did not have a readable ECG, and another 10 patients because the heart rhythm was not clearly definable due to pacemaker stimulation. Mortality was ascertained by consulting hospital and administrative databases and death registers. Follow-up was censored at June 30, 2015; survival status was not retrievable in five patients, leaving a final study sample of 903 patients.
All patients signed an informed consent allowing the utilization of their anonymized clinical information for medical research purposes, as approved by the local Institutional Review Board.
Permanent AF (subsequently indicated solely as AF) was defined as a documented history of AF that had persisted for more than 6 mo and was confirmed by a surface ECG at first visit. A diagnosis of coronary artery disease (CAD) was ascertained by coronary angiography, and patients without any luminal stenosis > 50% were considered without CAD. Information regarding previous percutaneous and/or surgical revascularization and previous valve surgery was also routinely collected. The remaining patients with other CHF etiology (including hypertensive cardiac disease, valve disease, tachycardiomyopathy, idiopathic cardiomyopathy) were all incorporated in a single group. Implanted devices were divided as follows: Mono/bicameral pacemakers (PM), biventricular pacemakers (CRT-D/CRT-P) and implantable-cardioverter defibrillators (ICD). Hypertension was defined by a blood pressure ≥ 140/90 and/or the use of antihypertensive medications. Diabetes mellitus was defined by history of diabetes mellitus and/or a random plasma glucose ≥ 200 mg/dL and/or fasting plasma glucose ≥ 126 mg/dL and/or an HbA1c ≥ 7% and/or use of antidiabetic treatments. Dyslipidemia was defined by history of high cholesterol levels and/or a total cholesterol ≥ 200 mg/dL. Present or former smoking was ascertained by medical interview, and patients who had smoked > 100 cigarettes/year were considered as smoker. Cancer history was defined by a previous or current malignancy, regardless of disease status at the time of medical interview. A clinical diagnosis of chronic obstructive pulmonary disease (COPD) was made during the visit based on the presence of a history of COPD, and/or signs and/or symptoms suggestive of COPD including chronic productive cough, chronic wheezing, emphysema or bronchitis.
Lab tests completed within 3 mo from the study visit were considered to identify anemia (hemoglobin levels < 13.5 g/dL in male and < 12.5 g/dL in female patients) and chronic kidney disease (CKD: Estimated glomerular filtration rate < 60 mL/min per 1.73 m2 as calculated from creatinine using the CKD-EPI formula).
The following variables were collected from a basal 12-lead standard ECG: Heart rhythm, heart rate, and presence of a right or left bundle branch block. Left ventricular ejection fraction (LVEF) was derived from a transthoracic echocardiogram obtained within 3 mo from the first visit, and patients with a LVEF > 45% were considered as having a preserved LVEF.
Information regarding ongoing medications was ascertained for each patient, and included CHF-modifying drugs [i.e., BB, ACE inhibitors and/or angiotensin receptor antagonists (ACEi/ARB) and aldosterone antagonists], diuretics (both loop diuretics and thiazides), other blood pressure lowering drugs (such as calcium channel blockers and alpha blockers), digoxin, amiodarone, lipid-lowering drugs (i.e., statins) antiplatelet drugs (including aspirin, clopidogrel and - for very few patients - ticagrelor), and anticoagulants (i.e., warfarin and very few patients with direct factor X or thrombin inhibitors).
Continuous variables were expressed as mean ± standard deviation (SD), and categorical variables as percentages. Characteristics of patients with AF vs sinus rhythm (SR) were compared using student’s t test and χ2 test as appropriate. To define univariate predictors of all-cause mortality, we compared characteristics of dead vs alive patients at the end of follow-up. Univariate and multivariate predictors of mortality were also investigated by Cox regression analysis. Variables with a P value < 0.10 in univariate analysis were selected based on clinical and statistical criteria (i.e., to ease the interpretation of the analysis and to avoid multicollinearity) and introduced into a multivariate model. A backward elimination of variables with a P value > 0.05 was performed to obtain the final multivariate reduced model. Kaplan-Meyer curves were obtained for all-cause mortality in patients with AF vs SR, and also based on the use of BB medications. All analyses were performed using SAS for Windows (version 9.2; SAS Institute Inc, Cary, NC). The statistical review of the manuscript was performed by a biomedical statistician.
From January 2004 to May 2015, a total of 903 patients were evaluated who satisfied our inclusion criteria (mean age 68 ± 12 years, 73% male). Prevalence of AF was 19%, ranging from 10% to 28% in patients ≤ 60 and ≥ 77 years of age, respectively (P < 0.0001). Characteristics of study population by the presence of AF or SR are summarized in Table 1. Patients with AF were significantly more symptomatic in comparison to patients with SR (NYHA class II-III 60% vs 44%). CAD was less common in patients with AF than in those with SR (28% vs 52%), as were previous coronary revascularization (21% vs 37%) and dyslipidemia (23% vs 37%). By contrast, a non-ischemic etiology was more frequent in the AF group (50% vs 24%), as well as a history of previous valve surgery (13% vs 4%). Patients with AF received overall more devices implantation (31% vs 21%). ECG data showed a lower prevalence of left bundle branch block (9% vs 16%) and a higher mean heart rate (80 ± 19 vs 70 ± 13) in patients with AF. Patients with AF were more frequently diagnosed with CHF with preserved LVEF (29% vs 21%).
| Atrial fibrillation (n = 173) | Sinus rhythm (n = 730) | P value | |
| Demographics and physical examination | |||
| Age (yr) | 72 ± 11 | 66 ± 12 | < 0.0001 |
| Age ≥ 65 yr (%) | 81 | 60 | < 0.0001 |
| Male gender (%) | 70 | 73 | 0.42 |
| SBP (mmHg) | 127 ± 18 | 130 ± 19 | 0.10 |
| DBP (mmHg) | 74 ± 10 | 75 ± 10 | 0.47 |
| NYHA II-III (%) | 60 | 44 | 0.0002 |
| Aetiology | |||
| CAD (%) | 28 | 52 | < 0.0001 |
| Previous CABG/PCI (%) | 21 | 37 | < 0.0001 |
| Without CAD (%) | 22 | 24 | 0.58 |
| Others/idiopathic (%) | 50 | 24 | < 0.0001 |
| Valve surgery (%) | 13 | 4 | < 0.0001 |
| Device | |||
| Any PM (%) | 30 | 19 | 0.001 |
| CRT-P/CRT-D (%) | 10 | 7 | 0.14 |
| ICD (%) | 11 | 16 | 0.07 |
| Any device (%) | 31 | 21 | 0.005 |
| History of VT (%) | 2 | 5 | 0.06 |
| Risk factors | |||
| Hypertension (%) | 61 | 60 | 0.81 |
| Diabetes mellitus (%) | 24 | 28 | 0.39 |
| Dyslipidaemia (%) | 23 | 37 | 0.0004 |
| Ever smoke (%) | 27 | 41 | 0.0010 |
| Comorbidities | |||
| Cancer history (%) | 12 | 10 | 0.47 |
| COPD (%) | 14 | 13 | 0.55 |
| Anaemia (%) | 6 | 10 | 0.11 |
| CKD (eGFR < 60) (%) | 7 | 8 | 0.57 |
| ECG | |||
| Heart rate (bpm) | 80 ± 19 | 70 ± 13 | < 0.0001 |
| PM stimulation (%) | 24 | 8 | < 0.0001 |
| Right bundle branch block (%) | 7 | 5 | 0.65 |
| Left bundle branch block (%) | 9 | 16 | 0.01 |
| Echocardiogram | |||
| Preserved LVEF (> 45%) (%) | 29 | 21 | 0.022 |
| LVEF (%) | 38 ± 14 | 35 ± 12 | 0.05 |
| Medications | |||
| Beta-blockers (%) | 72 | 80 | 0.01 |
| ACEi/ARB (%) | 71 | 79 | 0.02 |
| Beta-blockers and ACEi/ARB (%) | 51 | 66 | 0.0003 |
| Aldosterone blockers (%) | 46 | 37 | 0.02 |
| Diuretics (%) | 87 | 69 | < 0.0001 |
| Calcium channel blockers (%) | 6 | 13 | 0.01 |
| Alfa-blockers (%) | 6 | 8 | 0.55 |
| Digoxin (%) | 51 | 11 | < 0.0001 |
| Statin (%) | 28 | 49 | < 0.0001 |
| Amiodarone (%) | 6 | 13 | 0.01 |
| Antithrombotic treatment (%) | 19 | 63 | < 0.0001 |
| OAT (%) | 82 | 16 | < 0.0001 |
| DAPT (%) | 2 | 16 | < 0.0001 |
| OAT and antithrombotic (%) | 8 | 2 | 0.0006 |
| Antithrombotic only (%) | 11 | 61 | < 0.0001 |
When AF was present, there was a significant lower percentage of treatment with disease-modifying medications, including BB (72% vs 80%) and ACEi/ARB (51% vs 66%), as well as a less frequent use of calcium channel blockers (6% vs 13%), statins (28% vs 49%), amiodarone (6% vs 13%) and antithrombotic treatment (19% vs 63%). On the contrary, treatment with diuretics (87% vs 69%), aldosterone blockers (46% vs 37%), digoxin (87% vs 69%) and oral anticoagulants (82% vs 16%) was lower in patients with SR (Table 1).
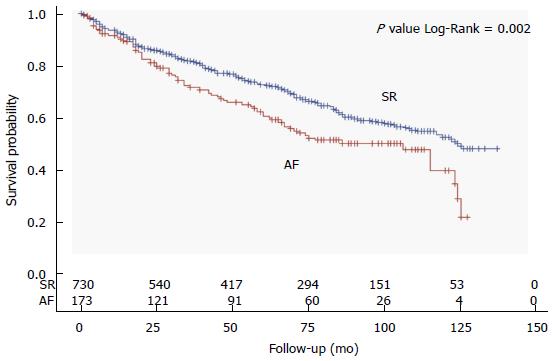
At a mean follow-up of 59 ± 40 mo (range 1 to 137 mo), all-cause mortality was significantly higher in patients with AF as compared to those in SR (45% vs 34%, Figure 1). Patients with AF were more likely to die during the course of our extended follow-up (Figure 2). Table 2 shows univariate associations of variables listed in Table 1 with all-cause mortality. At univariate analysis, patients who died had more frequently a diagnosis of AF than those who survived (23% vs 16%), were significantly older at baseline (71 ± 10 years vs 66 ± 12 years), had lower systolic and diastolic blood pressure (127 ± 19 mmHg vs 130 ± 19 mmHg, 72 ± 10 mmHg vs 76 ± 10 mmHg, respectively) and had more often NYHA class II-III (60% vs 40%), idiopathic etiology of CHF (32% vs 26%), implantable devices (29% vs 19%), PM stimulation (14% vs 9%) and a history of ventricular tachycardia (7% vs 4%). Moreover, diabetes mellitus (32% vs 24%), cancer history (14% vs 8%), COPD (18% vs 10%), chronic anemia (11% vs 8%), CKD (10% vs 6%), and use of diuretics (82% vs 67%), digoxin (26% vs 14%) or aldosterone blockers (45% vs 35%) was more frequent in the group of patients who died at follow-up. On the contrary, variables associated with survival were the presence of dyslipidemia (27% vs 39%), a preserved LVEF (19% vs 24%), and the use of BB (72% vs 82%) and ACEi/ARB (75% vs 79%) (Table 2).
| Death (n = 324) | Alive (n = 579) | P value | HR (95%CI) | P value | |
| Atrial fibrillation (%) | 23 | 16 | 0.0085 | 1.48 (1.14-1.92) | 0.0028 |
| Demographics and physical examination | |||||
| Age (yr) | 71 ± 10 | 66 ± 12 | < 0.0001 | 1.05 (1.04-1.06) | < 0.0001 |
| Male gender (%) | 25 | 29 | < 0.0001 | 2.4 (1.85-3.07) | < 0.0001 |
| SBP (mmHg, 10) | 127 ± 19 | 130 ± 19 | 0.0238 | 0.93 (0.88-0.99) | 0.0228 |
| DBP (mmHg, 10) | 72 ± 10 | 76 ± 10 | < 0.0001 | 0.76 (0.67-0.85) | < 0.0001 |
| NYHA II-III (%) | 60 | 40 | < 0.0001 | 1.7 (1.35-2.10) | < 0.0001 |
| Aetiology | |||||
| CAD (%) | 51 | 46 | 0.18 | ||
| Previous CABG/PCI (%) | 33 | 35 | 0.57 | ||
| Without CAD (%) | 17 | 27 | 0.0002 | 0.53 (0.4-0.71) | < 0.0001 |
| Others/idiopathic | 32 | 26 | 0.049 | 1.29 (1.02-1.63) | 0.0302 |
| Valve surgery (%) | 5 | 6 | 0.57 | ||
| Device | |||||
| Any PM (%) | 28 | 17 | 0.0003 | 1.64 (1.29-2.1) | < 0.0001 |
| CRT-P/CRT-D (%) | 8 | 7 | 0.32 | ||
| ICD (%) | 20 | 13 | 0.0027 | 1.45 (1.1-1.9) | 0.0074 |
| Any device (%) | 29 | 19 | 0.0006 | 1.57 (1.23-2.00) | 0.0002 |
| History of VT (%) | 7 | 4 | 0.0258 | 1.53 (1.01-2.32) | 0.0439 |
| Risk factors | |||||
| Hypertension (%) | 58 | 61 | 0.2619 | ||
| Diabetes mellitus (%) | 32 | 24 | 0.0053 | 1.72 (1.36-2.17) | < 0.0001 |
| Dyslipidaemia (%) | 27 | 39 | 0.0003 | 0.68 (0.53-0.87) | 0.0023 |
| Ever smoke (%) | 32 | 41 | 0.0055 | 0.92 (0.72-1.16) | 0.4668 |
| Comorbidities | |||||
| Cancer history (%) | 14 | 8 | 0.0044 | 1.89 (1.37-2.60) | < 0.0001 |
| COPD (%) | 18 | 10 | 0.0006 | 1.84 (1.4-2.40) | < 0.0001 |
| Anaemia (%) | 11 | 8 | 0.0521 | 2.22 (1.57-3.13) | < 0.0001 |
| CKD (eGFR < 60) (%) | 10 | 6 | 0.0551 | 2.807 (1.85-4.25) | < 0.0001 |
| ECG | |||||
| Heart rate (bpm, 10) | 72 ± 15 | 70 ± 15 | 0.1026 | 1.06 (0.99-1.14) | 0.0805 |
| PM stimulation (%) | 14 | 9 | 0.0438 | 1.56 (1.14-2.14) | 0.0057 |
| Right bundle branch block (%) | 7 | 5 | 0.0866 | 1.38 (0.9-2.1) | 0.1321 |
| Left bundle branch block (%) | 12 | 16 | 0.0761 | 0.75 (0.53-1.05) | 0.0958 |
| Echocardiogram | |||||
| Preserved LVEF (> 45%) (%) | 19 | 24 | 0.0560 | 0.74 (0.56-0.98) | 0.0345 |
| LVEF (%) | 34 ± 12 | 36 ± 11 | 0.0015 | 0.98 (0.97-0.99) | 0.0008 |
| Medications | |||||
| Beta-blockers (%) | 72 | 82 | 0.0003 | 0.67 (0.53-0.85) | 0.0012 |
| ACEi/ARB (%) | 75 | 79 | 0.0994 | 0.69 (0.53-0.88) | 0.0032 |
| Beta-blockers and ACEi/ARB (%) | 32 | 68 | 0.003 | 0.66 (0.53-0.83) | 0.0002 |
| Aldosterone blockers (%) | 45 | 35 | 0.0033 | 1.57 (1.26-1.95) | < 0.0001 |
| Diuretics (%) | 82 | 67 | < 0.0001 | 2.50 (1.87-3.31) | < 0.0001 |
| Calcium channel blockers (%) | 14 | 11 | 0.1588 | ||
| Alfa-blockers (%) | 8 | 7 | 0.8049 | ||
| Digoxin (%) | 26 | 14 | < 0.0001 | 1.60 (1.25-2.05) | 0.0002 |
| Statin (%) | 39 | 48 | 0.0088 | 0.80 (0.64-1.00) | 0.0513 |
| Amiodarone (%) | 12 | 12 | 0.8421 | ||
| Antithrombotic treatment (%) | 56 | 54 | 0.5669 | ||
| OAT (%) | 31 | 27 | 0.2374 | ||
| DAPT (%) | 10 | 15 | 0.0282 | 0.89 (0.62-1.29) | 0.5394 |
| OAT and antithrombotic (%) | 3 | 4 | 0.4946 | ||
| Antithrombotic only (%) | 53 | 50 | 0.4149 | ||
In a multivariate analysis including the main significant predictors of all-cause mortality, the univariate relationship between AF and death (HR = 1.49, 95%CI: 1.15-1.92) became not statistically significant (HR = 0.98, 95%CI: 0.73-1.32, Table 3). In the final reduced multivariate model, independent predictors at baseline of all-cause mortality were the following: Older age, male gender, lower systolic blood pressure, NYHA class II-III, presence of CAD at coronary angiography, presence of an implanted device, diagnosis of diabetes mellitus, COPD or anemia, history of cancer, non-use of ACEi/ARB and statins, and use of diuretics and digoxin (Table 3).
| Univariate | Multivariate full | Multivariate reduced | ||||
| HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | |
| Atrial fibrillation | 1.48 (1.14-1.92) | 0.0028 | 0.98 (0.73-1.32) | 0.8896 | ||
| Age (1 yr) | 1.05 (1.04-1.06) | < 0.0001 | 1.04 (1.03-1.05) | < 0.0001 | 1.04 (1.03-1.05) | < 0.0001 |
| Male gender | 2.4 (1.85-3.07) | < 0.0001 | 1.45 (1.11-1.90) | 0.0068 | 1.48 (1.13-1.93) | 0.0045 |
| SBP (10 mmHg) | 0.93 (0.88-0.99) | 0.0228 | 0.92 (0.86-0.98) | 0.0084 | 0.91 (0.86-0.97) | 0.0057 |
| NYHA II-III | 1.70 (1.35-2.10) | < 0.0001 | 1.3 (1.03-1.65) | 0.0265 | 1.32 (1.05-1.66) | 0.0195 |
| Without CAD | 0.53 (0.4-0.71) | < 0.0001 | 0.61 (0.44-0.84) | 0.0023 | 0.58 (0.43-0.80) | 0.0008 |
| Any device | 1.57 (1.23-2.00) | 0.0002 | 1.65 (1.19-2.29) | 0.0028 | 1.57 (1.23-2.02) | 0.0004 |
| Dyslipidaemia | 0.68 (0.53-0.87) | 0.002 | 0.8 (0.60-1.06) | 0.1151 | ||
| Diabetes mellitus | 1.72 (1.36-2.17) | < 0.0001 | 1.63 (1.27-2.08) | 0.0001 | 1.59 (1.25-2.04) | 0.0002 |
| Cancer history | 1.89 (1.37-2.60) | < 0.0001 | 1.82 (1.31-2.54) | 0.0004 | 1.84 (1.33-2.56) | 0.0003 |
| COPD | 1.84 (1.4-2.4) | < 0.0001 | 1.33 (0.98-1.80) | 0.0707 | 1.38 (1.02-1.86) | 0.0359 |
| Anaemia | 2.22 (1.57-3.13) | < 0.0001 | 1.82 (1.23-2.69) | 0.0027 | 1.95 (1.37-2.79) | 0.0002 |
| CKD (eGFR < 60) | 2.81 (1.85-4.25) | < 0.0001 | 1.42 (0.87-2.29) | 0.1577 | ||
| Preserved LVEF (> 45%) | 0.74 (0.56-0.98) | 0.034 | 0.91 (0.68-1.22) | 0.5369 | ||
| PM stimulation | 1.56 (1.14-2.14) | 0.006 | 0.91 (0.59-1.40) | 0.6561 | ||
| Beta-blockers | 0.67 (0.53-0.85) | 0.001 | 0.83 (0.64-1.09) | 0.1903 | ||
| ACEi/ARB | 0.69 (0.53-0.88) | 0.003 | 0.77 (0.59-1.01) | 0.0634 | 0.73 (0.56-0.94) | 0.0169 |
| Aldosterone blockers | 1.57 (1.26-1.95) | < 0.0001 | 1.11 (0.86-1.43) | 0.429 | ||
| Diuretics | 2.5 (1.87-3.31) | < 0.0001 | 1.51 (1.09-2.10) | 0.0134 | 1.58 (1.17-2.15) | 0.0031 |
| Digoxin | 1.6 (1.25-2.05) | 0.0002 | 1.29 (0.97-1.73) | 0.0807 | 1.31 (1.00-1.72) | 0.0482 |
| Statin | 0.8 (0.64-1.00) | 0.051 | 0.8 (0.60-1.05) | 0.1108 | 0.71 (0.55-0.90) | 0.0057 |
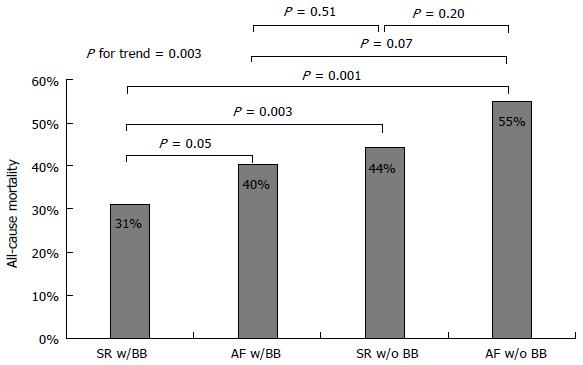
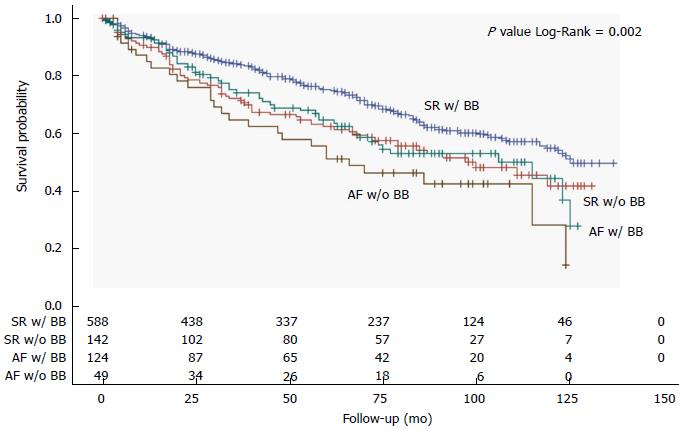
All-cause mortality was studied also through a comparison between patients with SR and patients with AF based on the presence or absence of BB treatment. Patients with AF not receiving BB treatment were found to have the worst prognosis, followed by patients with SR not receiving BB therapy and patients with AF receiving BB therapy, who both had similarly worse survival when compared to patients with SR receiving BB therapy (Figure 3). During the course of follow-up, patients with AF not receiving BB treatment had the worst prognosis, followed by patients with SR not receiving BB therapy together with patients with AF receiving BB therapy, and finally patients with SR receiving BB therapy (Figure 4).
Overall, our data demonstrates that in ambulatory patients with CHF, the presence of permanent AF is associated with worse clinical presentation, underuse of disease-modifying medications including BB, and possibly worse prognosis. After accounting for confounders, we found no independent association between AF and all-cause mortality; nonetheless, we found a significantly worse prognosis in AF patients with CHF not receiving BB treatment.
Patients with AF in our study population were older and had a higher NYHA functional class at presentation, in agreement with other data reported in the literature[10,11]. The presence of AF was also associated with an increased use of symptomatic medications, such as diuretics and digoxin, and a less frequent use of CHF-modifying medications, such as BB and ACEi/ARB. In addition, CAD was less represented among AF patients, whereas the prevalence of valve disease and non-cardiovascular comorbidities was greater in this group of patients, who interestingly also had a higher mean LVEF and more frequently a preserved LVEF (here LVEF > 45%). Recent literature emphasizes the stronger correlation of AF with CHF with preserved LVEF as compared to reduced LVEF[12], though this association was rather weak in our population, possibly because it mainly included CHF patients with reduced LVEF. CHF patients with AF are usually characterized by the presence of multiple comorbidities, and it is still unknown whether the adverse outcomes associated with AF are related to the arrhythmia itself, or to the burden of comorbidities associated with this diagnosis[8].
Contrasting findings have been published regarding a potential independent contribution of AF to increased mortality in patients with CHF. Some studies found AF to be an independent predictor of worse outcomes[13,14] whereas others found no independent association after accounting for confounders[4-6]. Two meta-analyses reported a 30%-40% increased risk of mortality when CHF is associated with a diagnosis of AF[7,8], irrespective of LVEF. In our study population, the coexistence of CHF and permanent AF resulted in a worse outcome, as shown by the Kaplan-Meyer survival curve in Figure 2. However, after adjusting for other significant predictors (including older age, male sex, systolic blood pressure, NYHA class II-III, ischemic etiology, pacemaker implanted, diabetes mellitus, history of cancer, COPD, anemia), AF did not show an independent impact on overall mortality (Table 3). This finding is in accordance with the abovementioned analyses from the COMET[5] and the V-HeFT study[4]. Advanced age and CHF severity have been shown to largely explain the association between AF and mortality in CHF patients, and this was also true in our study population, in which beyond age and NYHA functional class, we demonstrated a significant and independent contribution of non-cardiovascular comorbidities to mortality, including COPD, anemia and a history of cancer.
Although the use of BB in the setting of CHF has recently been disputed[9], we observed the worst prognosis in AF patients not receiving BB medications, while patients with AF receiving BB presented a significant survival benefit similar to those with SR not receiving BB but still lower than those with SR receiving BB treatment (Figures 3 and 4). It is still uncertain whether BB therapy reduces morbidity and mortality in patients with AF, but a class IA indication is given for these medications in patients with CHF and AF to control ventricular rate[15]. Our present results support this recommendation and point against the underuse of BB medications that is generally observed in CHF with AF as compared to those with SR[9].
The contribution of treatment with digoxin to the worse outcome in patients with CHF and AF is a matter of current debate[16]. We observed that digoxin was used in half of our patients with AF, and in only 1 out of 10 patients with SR. These percentages refer to the use of digoxin at first study visit, which happened some years ago starting in 2004, and probably do not reflect the current use of this medication in our clinical practice. Trends in the use of digoxin for AF have been steadily decreasing in the recent years, at least in the American population[17], and this drug has class IIa/B recommendations for rate control treatment of AF in most recent European[15] and American[18] HF guidelines. This is because of an overall neutral effect of this drug on mortality[19], and some observational studies showing an independent association with increased mortality[20]. Accordingly, its utilization was a strong and independent predictor of mortality at multivariate analysis in our retrospective analysis (Table 3).
The presence of implantable devices was associated with increased mortality in our final multivariate model. This finding appears counterintuitive at first, but may have different explanations. In particular, the presence of a device may be representative of a sicker CHF patient, for which the implantation of a device is generally indicated. In addition, when we distinguished patients with only pacing devices from patients with a resynchronizing device (either CRT-P or CRT-D) and patients with an ICD, only patients with a pacing device and an ICD implanted showed a statistically significant worse prognosis (Table 2). Treatment of LV dyssychrony with CRT device is expected to improve EF and symptoms over time, which in turn has a major positive impact on outcomes, including survival[15]. This is also at least partially reflected by the positive prognostic association of the presence of a left bundle branch block that we found in our study population (Table 2), which is likely indicative of the effect of CRT in patients that were implanted with a resynchronizing device after the first study visit at our clinic.
In contrast to what would be expected, dyslipidemia was associated with a reduction of mortality. In the setting of CHF, the presence of low cholesterol levels is known to identify patients with more advanced cardiac disease (i.e., with sarcopenia and possibly cachexia), and low concentrations of low-density lipoproteins have been associated with worse prognosis[15]. Patients with advanced cardiac disease are also less likely to receive lipid-lowering medications such as statins, for which the indication in CHF patients without active CAD is lacking[15]. Thus, the presence of dyslipidemia and the use of statins in our CHF population of advanced age probably indicate a healthier patient, which explain the associations of both these variables with a better prognosis.
Our analysis has several limitations that should be acknowledged. First, this is a retrospective analysis, thus our findings can only be interpreted with the intrinsic limits of this methodology. Second, cardiac rhythm was defined at first study visit, and we cannot exclude subsequent rhythm modifications. Third, we assessed mortality from all causes and could not obtain clear information specifically on cardiovascular and non-cardiovascular mortality. Because a history of cancer was a significant predictor of increased mortality, in an attempt to remove deaths due to malignancy, we performed sensitivity analysis excluding patients with a positive history of cancer. This analysis included 812 patients, of whom 659 with SR (81%) and 153 with AF (19%), and a total of 279 deaths out of the original 324. In this subsample, final results of independent predictors of mortality were substantially unchanged (data not shown). Finally, due to the low number of patients with preserved LVEF, we could not explore the interaction between LVEF and AF on mortality.
Our retrospective cohort study investigating a real-world population of elderly ambulatory CHF patients confirmed the association of AF with older age and worse clinical presentation previously reported in the literature. It further highlighted how a diagnosis of AF also led to an underutilization of disease-modifying medications such as BB and ACEi/ARBs, and to a more frequent use of symptomatic and antiarrhythmic drugs, particularly diuretics and digoxin, which in turn were independently associated with worse prognosis. In multivariate analysis, AF had no independent impact on all-cause mortality, which nonetheless was found to be the highest in AF patients not receiving BB medications. Further prospective randomized studies are needed investigating the independent prognostic impact of BB treatment in CHF with AF.
Atrial fibrillation (AF) frequently coexists with chronic heart failure (CHF). Conflicting data exist on the prevalence, clinical characteristics and medical treatment of HF patients with AF, particularly in the elderly. The independent prognostic impact of AF in these patients also remains unknown, as well as the potential protective effect of disease-modifying medications, particularly beta-blockers (BB).
The independent prognostic impact of AF in patients with CHF is a current matter of debate, and many have argued that this association is solely explained by other conditions associated to this arrhythmia, particularly comorbidities and underuse of disease-modifying medications.
This analysis confirmed the relevant clinical impact of AF in patients with CHF, although like other previous studies in the literature found no independent prognostic impact of this arrhythmia on overall mortality at long-term follow-up after accounting for several important confounders which are frequently found in these elderly CHF patients.
The study findings highlight the underuse of disease-modifying medications in CHF patients with coexisting AF, particularly BB. This is a matter of current debate in the clinical arena, with international guidelines giving a strong recommendation for the use of BB as a first-line treatment to control ventricular rate in euvolemic patients with New York Heart Association class I-III CHF. Efforts need to be done in order to increase the appropriate use of these medications in CHF with AF in the real world.
This interesting study by Gigli et al examined the impact of AF on outcomes in patients with CHF. The authors conclude that AF did not have an independent impact on mortality, but BB use appeared to affect this relationship.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: den Uil CA, Joseph J, Ong HT S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, D’Agostino RB, Murabito JM, Kannel WB, Benjamin EJ. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107:2920-2925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1360] [Cited by in RCA: 1532] [Article Influence: 69.6] [Reference Citation Analysis (0)] |
| 2. | Smit MD, Moes ML, Maass AH, Achekar ID, Van Geel PP, Hillege HL, van Veldhuisen DJ, Van Gelder IC. The importance of whether atrial fibrillation or heart failure develops first. Eur J Heart Fail. 2012;14:1030-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 3. | Nieuwlaat R, Eurlings LW, Cleland JG, Cobbe SM, Vardas PE, Capucci A, López-Sendòn JL, Meeder JG, Pinto YM, Crijns HJ. Atrial fibrillation and heart failure in cardiology practice: reciprocal impact and combined management from the perspective of atrial fibrillation: results of the Euro Heart Survey on atrial fibrillation. J Am Coll Cardiol. 2009;53:1690-1698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Carson PE, Johnson GR, Dunkman WB, Fletcher RD, Farrell L, Cohn JN. The influence of atrial fibrillation on prognosis in mild to moderate heart failure. The V-HeFT Studies. The V-HeFT VA Cooperative Studies Group. Circulation. 1993;87:VI102-VI110. [PubMed] |
| 5. | Swedberg K, Olsson LG, Charlesworth A, Cleland J, Hanrath P, Komajda M, Metra M, Torp-Pedersen C, Poole-Wilson P. Prognostic relevance of atrial fibrillation in patients with chronic heart failure on long-term treatment with beta-blockers: results from COMET. Eur Heart J. 2005;26:1303-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 395] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 6. | Paolillo S, Agostoni P, Masarone D, Corrà U, Passino C, Scrutinio D, Correale M, Cattadori G, Metra M, Girola D. Prognostic role of atrial fibrillation in patients affected by chronic heart failure. Data from the MECKI score research group. Eur J Intern Med. 2015;26:515-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Mamas MA, Caldwell JC, Chacko S, Garratt CJ, Fath-Ordoubadi F, Neyses L. A meta-analysis of the prognostic significance of atrial fibrillation in chronic heart failure. Eur J Heart Fail. 2009;11:676-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 305] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 8. | Wasywich CA, Pope AJ, Somaratne J, Poppe KK, Whalley GA, Doughty RN. Atrial fibrillation and the risk of death in patients with heart failure: a literature-based meta-analysis. Intern Med J. 2010;40:347-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Nielsen PB, Larsen TB, Gorst-Rasmussen A, Skjøth F, Lip GY. β-Blockers in Atrial Fibrillation Patients With or Without Heart Failure: Association With Mortality in a Nationwide Cohort Study. Circ Heart Fail. 2016;9:e002597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Feinberg WM, Blackshear JL, Laupacis A, Kronmal R, Hart RG. Prevalence, age distribution, and gender of patients with atrial fibrillation. Analysis and implications. Arch Intern Med. 1995;155:469-473. [PubMed] |
| 11. | Suman-Horduna I, Roy D, Frasure-Smith N, Talajic M, Lespérance F, Blondeau L, Dorian P, Khairy P. Quality of life and functional capacity in patients with atrial fibrillation and congestive heart failure. J Am Coll Cardiol. 2013;61:455-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 12. | Goyal P, Almarzooq ZI, Horn EM, Karas MG, Sobol I, Swaminathan RV, Feldman DN, Minutello RM, Singh HS, Bergman GW. Characteristics of Hospitalizations for Heart Failure with Preserved Ejection Fraction. Am J Med. 2016;129:635.e15-635.e26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 13. | Dries DL, Exner DV, Gersh BJ, Domanski MJ, Waclawiw MA, Stevenson LW. Atrial fibrillation is associated with an increased risk for mortality and heart failure progression in patients with asymptomatic and symptomatic left ventricular systolic dysfunction: a retrospective analysis of the SOLVD trials. Studies of Left Ventricular Dysfunction. J Am Coll Cardiol. 1998;32:695-703. [PubMed] |
| 14. | Olsson LG, Swedberg K, Ducharme A, Granger CB, Michelson EL, McMurray JJ, Puu M, Yusuf S, Pfeffer MA. Atrial fibrillation and risk of clinical events in chronic heart failure with and without left ventricular systolic dysfunction: results from the Candesartan in Heart failure-Assessment of Reduction in Mortality and morbidity (CHARM) program. J Am Coll Cardiol. 2006;47:1997-2004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 432] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 15. | Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129-2200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10348] [Cited by in RCA: 9356] [Article Influence: 1039.6] [Reference Citation Analysis (3)] |
| 16. | Al-Zakwani I, Panduranga P, Zubaid M, Sulaiman K, Rashed WA, Alsheikh-Ali AA, AlMahmeed W, Shehab A, Al Qudaimi A, Asaad N. Impact of Digoxin on Mortality in Patients With Atrial Fibrillation Stratified by Heart Failure: Findings From Gulf Survey of Atrial Fibrillation Events in the Middle East. J Cardiovasc Pharmacol Ther. 2016;21:273-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Patel N, Ju C, Macon C, Thadani U, Schulte PJ, Hernandez AF, Bhatt DL, Butler J, Yancy CW, Fonarow GC. Temporal Trends of Digoxin Use in Patients Hospitalized With Heart Failure: Analysis From the American Heart Association Get With The Guidelines-Heart Failure Registry. JACC Heart Fail. 2016;4:348-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147-e239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4116] [Cited by in RCA: 4654] [Article Influence: 387.8] [Reference Citation Analysis (1)] |
| 19. | Ziff OJ, Lane DA, Samra M, Griffith M, Kirchhof P, Lip GY, Steeds RP, Townend J, Kotecha D. Safety and efficacy of digoxin: systematic review and meta-analysis of observational and controlled trial data. BMJ. 2015;351:h4451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 232] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 20. | Turakhia MP, Santangeli P, Winkelmayer WC, Xu X, Ullal AJ, Than CT, Schmitt S, Holmes TH, Frayne SM, Phibbs CS. Increased mortality associated with digoxin in contemporary patients with atrial fibrillation: findings from the TREAT-AF study. J Am Coll Cardiol. 2014;64:660-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 165] [Article Influence: 15.0] [Reference Citation Analysis (0)] |