OVERVIEW
This brief overview is essentially medical-centred, in a dual sense: (1) it regards the clinical approach (obviously patient-centred in its scope and ethics) that is, and must be, driven by an individual medical doctor with the most comprehensive as possible skills and expertise; and (2) it regards the clinical innovation and research, which raised and raise from the observation and reasoning of single clinicians; the dissemination of skills, practice, recommendation and guidelines must have the support of the best available clinical evidences, with the feature of appropriateness, sustainability and cost-benefit for the patient and the community.
This is particularly true for a procedure, such as thoracic ultrasound (TUS), basic, if not elementary, quite neglected by the cardiologist. This despite TUS was early concurrently practised with echocardiography[1] and, subsequently, limitedly used by internists, radiologists and in paediatrics[2-9].
The requirement that TUS shares with echocardiography[10] are remarkable. The effort of a good quality imaging, the reproducibility of the procedure by different operators and equipments and the criteria of the indications[9] can be summarized in two sentences: (1) the use of TUS for initial diagnosis is recommended when there is a change in the patient’s clinical status, likely related to pulmonary function; and (2) when new data from a TUS would result in the physician changing the patient’s care.
There is an established agreement for these criteria in cardiological patients for their use in echocardiography[9,10]. Moreover, alike echocardiography, TUS is not recommended as routine testing equally when the patient has no modification in clinical status or when a physician is unlikely to change care for the patient based on the results of testing: These are both two strongly advised points against the use of echocardiography as a routine testing, which are suitable to be transferred also to TUS[9].
Differently from echocardiography, TUS is almost exclusively an imaging tool, without any “functional” application comparable to M- B- and Doppler Echocardiography, no accurate and reproducible dynamic measures, no translational relevance in the description and interpretation of mechanisms of disease[11].
Nonetheless, the knowledge of these limitations and the use of the few direct information provided by TUS are a substantial add-on to the clinical strategy of the cardiologist[12], also in emergency[13-15].
RATIONALE AND KEY POINTS
The customized rules of a journal presentation are: What, who, why, when, where, how.
What
Clinical assessment and workup of patients referred to cardiologists is a task often more comprehensive and not only focused on the heart since it is extended to chest disease. This approach may require more in-depth examination of respiratory co-morbidities due to uncertainty or severity of the clinical presentation.
Who
The cardiologist, facing respiratory associated symptoms and co-morbidities, can usually detect and manage the clinical presentation by physical examination, a thoroughly collected story, and using the current non-invasive procedures at the hands. The cardiologist’s view is of paramount relevance for the clinical reasoning and action of the other specialists. The adjunct of TUS examination is an excellent companion to the other knowledge, procedures and skills.
Why
According to uncertainty or severity of the clinical presentation, the referral to radiologist and to pneumologist is an assignment that is more appropriate if explicitly addressed with objective information, which exclusively can produce evident cost-benefit advantages[16,17]. Some investigation implicitly aims to a clinical risk management analysis with subsequent recommendations. Actually, TUS can be an excellent risk-reducing tool by increasing: (1) diagnostic certainty; (2) shortening time to definitive therapy; and (3) decreasing complications from blind procedures that carry an inherent level of complications. The background and the backbone of it all is an efficient network of timely, coordinated and experienced professionals, and the analysis of obstacles and structural barriers that may take place or that are interposed[17,18].
When
The contribution of the cardiologist in the diagnosis and management of respiratory disease must be well timed. Apart the obvious clinical judgement, the expert task of filtering ecg and echocardiographic information, addressing to the clues of right ventricular impairment, pulmonary embolism and pulmonary hypertension, and other less frequent conditions, including congenital, inherited and systemic disease, is an help for timely diagnosis and therapeutic choice. Even with limited indications, the concurrent use of TUS by the cardiologist is important in this regard.
Where
Despite the evidence of the strict links between cardiac and respiratory medicine, the two ultrasound imaging approaches to heart and chest are still separated. This is due to different skills of physicians, to the different preference of patients in the choice of type of referrals and even due to the suitability of equipments and probes, which are not equally fitting for heart or lung examination and not always already available in the same room or facility.
How
Several respiratory conditions are currently studied and monitored, when not preliminary detected, by echocardiography, which is nowadays an established tool in the workup of pulmonary fibrosis and diffuse interstitial disease. As a further step, the ultrasound (US) approach of the cardiologist should be extended to lung and pleura, achieving simple and straightforward information suitable of articulation within the clinical cardiology frame. Electrocardiography, pulse oximetry and US equipment are the friendly technological extension of the physical examination, if their use is based on adequate knowledge and training of the professionals, on appropriate setting of tools which must be efficient and well working. If these premises lack, overshadowing or misleading artefacts can ensue: Overall, the procedure must be affordable, reliable and comfortable for both the patient and the well-trained doctor[18-20].
CHEST ULTRASOUND: THE THORAX, THE LUNG, THE HEART
Many clinical subsets increasingly use chest ultrasound, i.e., TUS procedures. This is due to the greater availability of portable point-of-care US equipment, suitable also at the patient’s bedside, in the ward, in the emergency and intensive care unit, in outpatient clinics and even at home of the patients themselves.
TUS procedure allows the view of the most superficial parts of the chest: The thorax “wall”, the pleura, which may be a virtual space or a real fluid-filled space in pleural effusion, or may be a mass-occupied space in many cancers, and the lung itself[9]. Some part of the lung, where not overshadowed by ribs or other bones, such as scapula, is therefore clearly visible only if “consolidated”. This happens in atelectasis, pneumonia and cancer, provided that the mass or nodule strictly adheres close to pleura, becoming accessible to micro-invasive procedures[21-23]. There is no TUS established criterion for differentiating the nature of lung consolidation. Physical interaction of the ultrasonic beam at the tissue/air interface strongly influences or frankly impairs transthoracic US imaging, so that TUS cannot detect any mass or nodule, behind more superficial and even small portion of aerated lung, often even clearly defined by radiological procedures.
The heart is one of the organ visible by ultrasound in the thorax - by echocardiography - and, as it is well known, also for this reason the worst enemy of its imaging is the air, the pulmonary air; as a consequence, any cardiologist focuses on the detection of the acoustical windows for achieving and recording useful and better images and videos. Actually, in children and in thin persons, high frequency (6-10 MHz) linear or convex probes[9] enhance the view of the chest wall and of the pleura-lung abnormalities. This is possible because, and only if the structures we are attempting to see are just below our transducers. The yield of a sector or phased array probe is usually more limited; the only notable exception is the detection, also by sector probes, of pleural fluid, which sometimes may be well visible even anteriorly, sometimes not well differentiated by a pericardial effusion requiring a complete lateral and posterior chest assessment by TUS[24]. More easily and with a greater sensibility for small amounts of fluid is the view through a window in the lateral and posterior part of the chest, better with the patient upright or sitting[9].
A LONG STORY: PIONEERS AND CURRENT USERS
TUS is a complementary tool also in cardiology[25-30], along its main use for more specific pleura and lung disease[31-34]. Nonetheless, TUS pioneering and practice began in late 60’s. Then, the most important B-mode studies concurrently, in the same centre in United States, demonstrated the usefulness of TUS, along with the assessment of mitral and tricuspid valve disease[35,36], for the diagnosis of lung consolidation, envisaging an help even in pulmonary embolism[37]. In early 70’s, with the improved quality and greater availability of the US equipments, the use of TUS as an allied support of cardiologists performing echocardiography was recognized and practised. Our practice was done, regretfully, without delivering relevant cardiology publications on this topic, which was considered a parallel but minor informative practice. Thereafter, in late 80’s in France[5], the TUS procedure was optimally developed by pneumologists, as it was in Germany[6-8], and in Italy[3] defining appropriately the criteria of pneumothorax and of lung consolidation. The clinical research and practice of TUS in cardiology in late 90’s in Japan, demonstrated the usefulness of detecting and monitoring pleura effusions[25-28,30], an achievement that others subsequently confirmed[29] enhancing the dissemination of knowledge and interest for TUS in Cardiology. The use in pediatric and newborn intensive care facilities was[2,4] and still is[17,38,39] greatly developed with the contribution of pediatric radiologists. The main barriers to the dissemination of an appropriate TUS practice are the limited availability of clinical application studies within cardiology and pneumology departments, the lack of TUS curricula inside those residency programs[18-20] and the small attention devoted to research and publications in this field by cardiologists.
THE PROCEDURE: NEEDS, FACILITIES, COLLABORATION AND INDICATIONS
There are good reasons for which a cardiologist should seek for the contribution of TUS, and they stems from the referral for a clinical consultation that usually includes echocardiography.
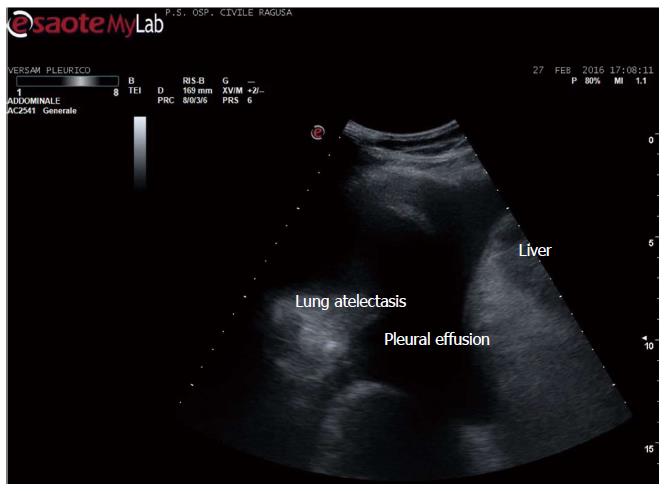
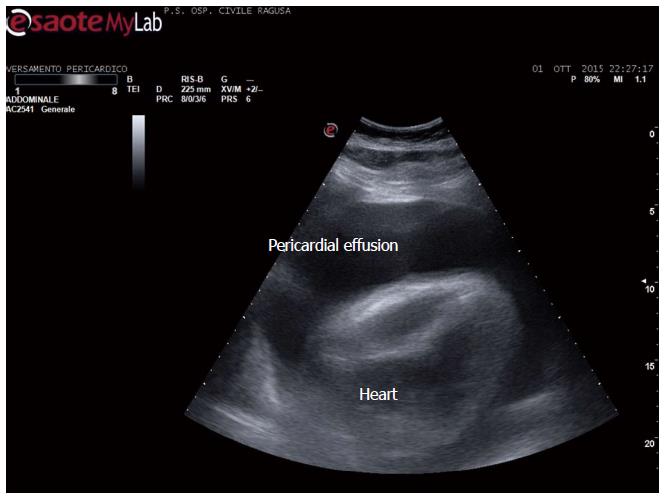
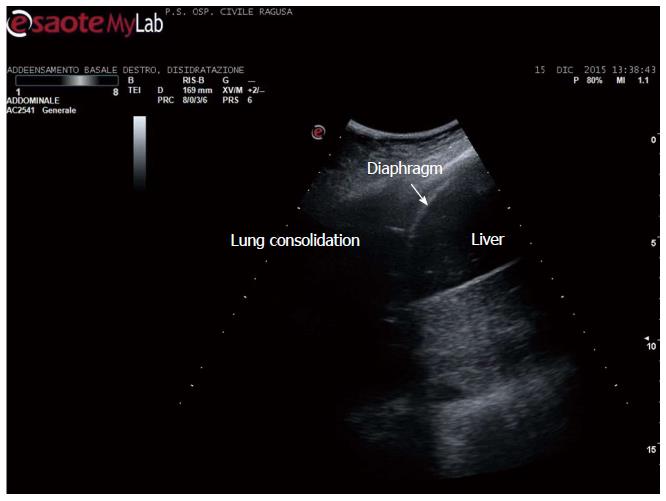
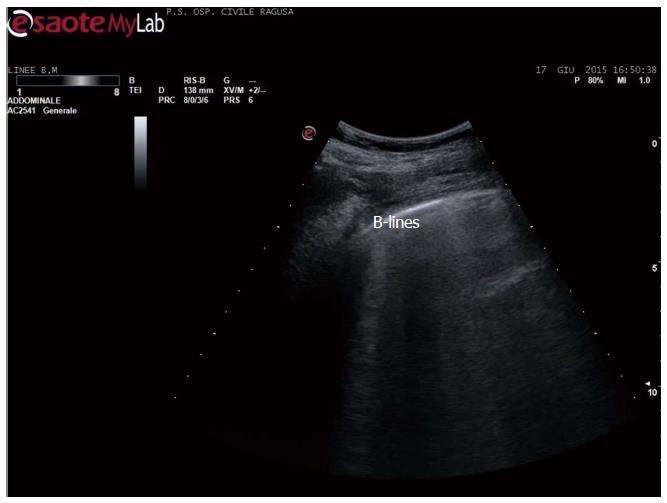
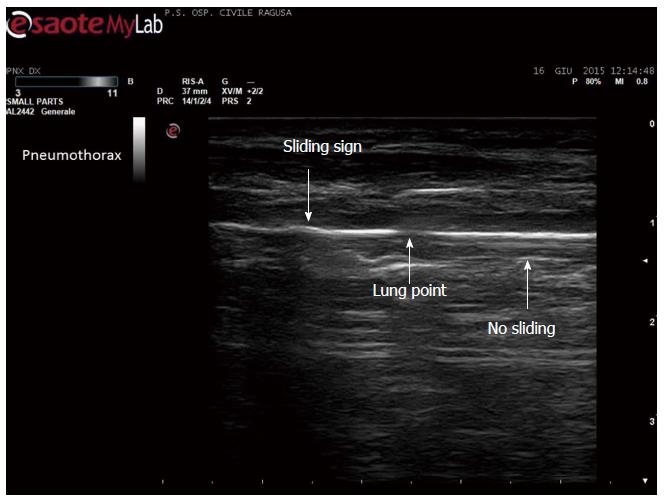
Apart the itemization of the main indications and conditions in which TUS can be of help for the diagnosis and workup of patients, which is below detailed, the focus of the cardiologist performing TUS is clinically-driven by the two more important concurrent conditions: (1) pleural effusion (Figure 1), which is observed even better by video-clip and may be associated with pericardial effusion (Figure 2); (2) lung consolidation (Figure 3) which may be due to pneumonia, as in this case, but which needs a further radiological work-up if there is the suspicion of cancer or lung atelectasis; (3) the appearance of B-lines is a very generic clue (Figure 4), particularly because their count is at best impractical and imprecise, as it is obvious looking at any videoclip; (4) differently, the dynamic view of the disappearance of pleura sliding (Figure 5) is a very specific sign, unless it is observed in the apical part of the lung, where it can be often undetectable for anatomical reasons; however, detection of the absence of pleura sliding is not a very usual need to search for a cardiologists.
Figure 1 Pleural effusion.
Figure 2 Pericardial and pleural effusion.
Figure 3 Lung consolidation.
Community acquired pneumonia in an adult.
Figure 4 B-lines in acute heart failure.
B-lines count is a dynamic observation, essentially qualitative, since the number changes continuously - from 3 to 6 or more - in case of numerous b-lines. Identical artefacts are detectable in other conditions, including pulmonary fibrosis and dyspnoea due to other causes, including BPCO.
Figure 5 Disappearance of pleural sliding, better demonstrated by video.
Which is here showed as a drop in the continuity of the line, not moving side by side (by courtesy of Giuseppe Molino, MD, MCAU Ospedale Civile di Ragusa, Italy).
The cardiologist can perform TUS procedure, after an appropriate training and with the adequate probe implementation of the US equipment, executing an articulated heart-lung US approach.
A list of the main findings attainable by TUS: (1) small or huge pleural effusions. These can be associated with pericardial effusion, and can be isolated pleural fluid effusions, unilateral, bilateral or, as less frequently happens, loculated. If loculated, effusions may be not detected in the lower part of the chest, but only at the level where the fluid is actually restricted. The sensibility of linear vs phased array probes is greater (100% vs 91%) for uncovering pleural effusions; both are more sensible in comparison with chest X-rays[24]; (2) the recognition of pleural effusion is a frequent occurrence in echocardiography out-patient consultations, and follows referrals for dyspnea, due to congestive heart failure and/or respiratory failure; valvular or congenital heart disease; ischemic or primary myocardial heart disease; cancer disease, primitive or metastatic; other conditions.
These last miscellanea should be considered a particularly relevant group, since the detection of not previously suspected pleural-pericardial effusion can be the first evidence of otherwise still non-identified disease, or a clue of greater severity of an already diagnosed disease.
We see in the current practice pleural effusions, often previously undetected, in: Hypothyroidism; rheumatic and auto-immune disease; malnutrition, due to dietary insufficient intake, to intestinal disease (such as coeliac disease) and to liver disease; nephrosis.
The small, quick diagnostic step of the cardiologist can open the road for a more effective diagnosis and treatment of several patients in these cases.
A journey of a thousand miles starts under one’s feet (Lao-Tzu)
The detection and monitoring of pleural effusion was the object of very careful studies, which demonstrated the usefulness of the detection and of the monitoring of pleural effusion in congestive heart failure patients throughout the time-course of management outcome[25-29] and along ecg changes[30].
Pleura-lung consolidation
This is a more detailed imaging diagnosis, non-specific and not suitable for reliably identifying the cause. It can address to subpleural pneumonia areas[31], pleural and/or subpleural nodes[32], atelectasis[33] and loculated-organized pleural effusions[34], without a definite differentiation[9].
This type of report needs a very systematic chest examination, which should not be, usually, a part of the cardiological US procedure, requires more appropriate probes (linear or convex), and a great level of suspicion, apart the skills and a lasting expertise.
Nonetheless, there are several good reason for performing this type of TUS examination, in selected patients, along the echocardiographic examination, when a consolidation is suspected[35-37], if the cardiologist has achieved a reasonable level of skills, expertise and training: (1) newborn and children with fever, even without overt severe respiratory distress[38,39]. In these small patients, the chest X-ray is usually postponed after having achieved the physical examination evidence of pulmonary involvement and, in the recent years, after some evidence at physical examination of TUS subpleural consolidation, particularly if associated with small pleural effusion; (2) adults with respiratory symptoms, with or without fever, particularly in the outbreaks of community pneumonia[40]. Both situations can be associated with previous or active endocarditis, so that this diagnostic step can be useful for completing the elements of the clinical reasoning of the cardiologist; (3) adults with evidence of small pleural effusion without a definite suspicion of pulmonary infection and with the possibility of lung cancer. This is a special case, usually behind the actual skills and expertise of any US professional. Nonetheless, if positive, TUS could hasten the prescription of more efficient imaging (CT or NMR); and (4) pleural line thickening. This is a minor but relevant clue, detected in several diffuse pulmonary interstitial disease[41] and in conditions such as asbestosis[21,42,43]. This can be an early sign of involvement or of worsening of an already known disease, and can help in the decisional tree for the prescription of a radiological examination - CT.
TUS clues of pneumothorax: This is an important application, even without specific criteria, useful in emergency, for the diagnosis of spontaneous or traumatic/post-procedural pneumothorax, particularly in conditions of limited medical resources. TUS diagnosis relies on the sign of the absence of sliding on the pleural view[44,45] and to other less specific signs[9]. With the considerable exception of unavailability of adequate roentgenologic facilities, the TUS diagnosis is a preliminary step to the urgent definition and demonstration - usually by CT - of the chest condition, amenable to the choice of the most appropriate management[46,47].
ABSENCE OF TUS IMAGING
The ring-down artefacts in patients with dyspnea
In patients with severe dyspnea, whatever the cause (pulmonary oedema, congestive heart failure with or without orthopnea, pulmonary fibrosis, and other conditions), the so-called B-lines artifacts[48-53], which prevents the vision of lungs with the remarkable exception of pleural effusion, may overshadow an adequate imaging. Indeed, the clinical reliability of B-lines count is a doubtful stand-alone criterion, particularly because “in patients with a moderate to high pretest probability for acute pulmonary oedema, an US study showing B-lines can be used mainly to strengthen an emergency physician’s working diagnosis of acute pulmonary oedema. In patients with a low pretest probability for acute pulmonary oedema, a negative US study can almost exclude the possibility of acute pulmonary oedema”[54]. These are the conclusion of the most accurate metanalysis on this topic, which substantially asserts that the only information provided by the B-lines artifacts is that one already available on clinical basis. Moreover, over-reliance on such tools could undermine quick clinical decisions in emergency scenarios[55]. Acute severe dyspnea due to causes other than pulmonary oedema, including exacerbation of chronic obstructive pulmonary disease and pulmonary fibrosis, presents with the same B-line profile as acute pulmonary oedema. It is therefore obvious that in a clinical scenario of severe dyspnea, preliminary diagnosis by history and clinical examination takes precedence, and, in fact, it is almost all that the physician and the patient need for effective intervention. Finally yet importantly, the fact that the number and evidence of B-lines is greater according to the age of the patients[56], not to the body size[57], more than to other factors raises further doubts on a realistic use of this criterion for “universal” clinical purposes, as sometimes claimed.
Indeed, the reference tool in acute pulmonary oedema is auscultation, and the level on the chest - basal, middle, and apical - where wet sounds are heard[9]. Most source articles dealing with the B-lines approach do not mention such features and do not mention in the reports the extension of lung involvement. Furthermore, those articles also fail to inform us of the ultrasound time course of the observed pulmonary oedema cases, from the onset to improvement or recovery, or to the worsening of the clinical situation. This is a crucial point. Differently, usefulness of reduction of TUS pleural effusion with clinical improvement is a very well demonstrated and practised approach since several years[9,24-28]. Nonetheless, the reduction of artefacts grossly runs in parallel with the improvement of dyspnea, whatever is the prominent cause, allowing a grossly graded US detection of pleura-lung abnormalities, if any. The prominent role even in emergency of echocardiography over TUS is, also nowadays[58], when still necessary, confirmed again[15]. Unfortunately, TUS does not provide a substantial adjunctive contribution for the diagnosis of pulmonary embolism[59], as hopeful[37].
TUS guidance for intervention procedure
This is probably the most important application of TUS, useful for diagnostic of nodules by fine needle aspirate biopsy, for diagnosis and drainage treatment of pleural-pericardial effusions and, rarely, of cysts, for the guidance toward chest vessels[60] and, in special cases, toward the diaphragm[61]. The role of the cardiologist in these very specific actions is almost null; nonetheless, a trans-thoracic approach of pericardial effusions[62], instead of sub-xiphoid as currently usual, is safe and in some case - abdominal surgery or trauma - the best suited. The use of probes with a central hole - convex or linear - is particularly useful because these probes allow a greater precision in the guidance and in the visual tracing of the needle toward its target[9,16,21,22,63]. The percentage of complications is minimal or absent.
The cardiologist: The culture and the methodology
After its beginning and development in the cardiology and in the radiology units, TUS was quite relegated, if not neglected. It was used mainly in contexts of limited resources, when there was the need of quick diagnosis and, in a very privileged niche, in the laboratories of interventional diagnostic ultrasound, for performing highly focused and precise lung, nodes and pleural biopsies or therapeutical procedures.
The culture of the cardiologists and of the radiologists has the key traits of addressing quality and conformity to the morphology of the US images, of investigating using unambiguous criteria of comparisons between measurements (invasive, non-invasive, anatomic) and of achieving not redundant, not time wasting and not potentially misleading information. With these criteria in mind, the contribution of TUS, apparently limited to a couple of items and information, is, in the hands of the cardiologist performing echocardiography, a valuable add-on for ruling-in or ruling-out pleural effusions. Overall, the cardiologist may detect isolated lung consolidations, possible pneumonia or other masses, and pleural line thickening, as a possible clue of interstitial lung disease. The available information, particularly in point of care ultrasound, suffers from several limitations which were optimally addressed somewhere else: The concept of a focused examination implies that one is addressing binary questions (e.g., does the patient have cholecystitis or not?). In practice, many diagnoses require the assessment of a variety of imaging findings of varying subtlety and often deal in probabilities rather than binary assessments. Lastly, in order to assess the quality and validity of point-of-care ultrasonography and to permit its correlation with other imaging methods, it is essential that images be documented, ideally on the same picture archiving and communication system used for other imaging[64]. It should be strongly considered that “it is not time to mandate training in the performance of lung ultrasound without proving that ultrasound can reliably make an accurate diagnosis”[65]; moreover, “formal training incorporating ultrasound in adequate curricula is crucial for physicians, avoiding simplistic numeric rules, since medicine is not arithmetic”[66]. The trends of contemporary practice and research address to precision, but also to sustainability within a framework of predictive, preventive and personalized medicine and an affordable implementation of clinical risk assessment and management planning[67-70]. TUS is a significant complementary aspect of this strategy, which can be integrated and articulated within the daily work of the cardiologist.