Peer-review started: May 29, 2015
First decision: July 10, 2015
Revised: August 8, 2015
Accepted: November 17, 2015
Article in press: November 25, 2015
Published online: January 26, 2016
Processing time: 239 Days and 11.6 Hours
Atrial fibrillation (AF) is the most common cardiac arrhythmia and a huge public health burden associated with significant morbidity and mortality. For decades an increasing number of patients have undergone surgical treatment of AF, mainly during concomitant cardiac surgery. This has sparked a drive for conducting further studies and researching this field. With the cornerstone Cox-Maze III “cut and sew” procedure being technically challenging, the focus in current literature has turned towards less invasive techniques. The introduction of ablative devices has revolutionised the surgical management of AF, moving away from the traditional surgical lesions. The hybrid procedure, a combination of catheter and surgical ablation is another promising new technique aiming to improve outcomes. Despite the increasing number of studies looking at various aspects of the surgical management of AF, the literature would benefit from more uniformly conducted randomised control trials.
Core tip: The surgical management of atrial fibrillation (AF) is a rapidly developing field. Existing surgical techniques are constantly evolving in order to achieve more minimally invasive procedures. Additionally, the relatively new ablative modalities are being increasingly used, either alone or in conjunction with surgical techniques; attempting overall better and less invasive results. This review looks at the current surgical techniques and ablative modalities available for managing AF, where each section is re-enforced with the current most up to date guidelines on the use of each of these modalities.
- Citation: Kyprianou K, Pericleous A, Stavrou A, Dimitrakaki IA, Challoumas D, Dimitrakakis G. Surgical perspectives in the management of atrial fibrillation. World J Cardiol 2016; 8(1): 41-56
- URL: https://www.wjgnet.com/1949-8462/full/v8/i1/41.htm
- DOI: https://dx.doi.org/10.4330/wjc.v8.i1.41
Atrial fibrillation (AF) is the most common cardiac arrhythmia seen in clinical practice and it has been associated with substantial morbidity and mortality. The lifetime risk of AF between the ages of 40-95 is 1 in 4, as demonstrated by the Framingham study[1]. In the general population AF has been shown to occur in around 1%-2%[2].
Patients suffering from AF are at risk of thromboembolic events, including stroke leading to disability or death. One out of every five strokes is secondary to AF, with those resulting from AF being more disabling and having increased clinical significance[1,3].
The first-line treatment of AF has traditionally been anti-arrhythmic drugs, whether attempting rate and or rhythm control. However, long-term anticoagulation is not without risks as it can interfere with quality of life and increase morbidity. There is still considerable variation in the pharmacological treatments of AF in clinical practice[2].
Catheter based ablation is an alternative to medical therapy, as a minimally invasive intervention which can provide relatively good results[4].
The role of surgery in the management of AF has been introduced following the ground-breaking “Maze” procedure described and developed by Cox et al[5] in a series of publications. The “Maze” procedure has been the cornerstone of surgical management of AF[3-8]. The Cox-Maze III procedure is regarded as the gold-standard surgical treatment of AF having the highest success rates; greater than or equal to 90% long-term freedom from AF[4,9,10]. However, the procedure is highly invasive and technically difficult, requiring a high level of surgical expertise, thus limiting its use to a few specialist centres[9].
These limitations have fuelled further research in the field of surgical management of AF with a view to develop less invasive but equally effective alternative techniques.
This has led to the development and use of multiple ablative devices using various energy sources, as well as less surgically invasive Cox-Maze lesions allowing replacement of incisions with ablation lines[2]. There is still on-going research and development of new techniques, within this rapidly evolving field.
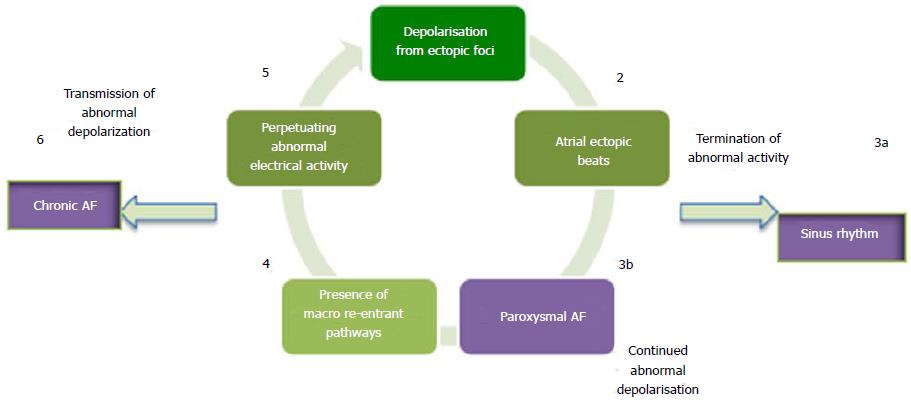
In 1998, Haïssaguerre et al[9] made an important discovery regarding the origin of atrial ectopic beats in paroxysmal AF. At the time it was known that chronic AF was the result of re-entrant circuits, though no information was available about its origin.
The spontaneous initiation of AF was studied using intra-cardiac monitoring, angiography and fluoroscopy looking specifically at the electrical activity preceding the onset of AF. A total of 69 foci were identified as being responsible for the origin of atrial ectopic beats, in a study of 45 patients. The striking majority (94%) of the foci were found to be in the pulmonary veins with others including the right and left atria. Radiofrequency catheter ablation was subsequently utilised to ablate those foci in order to abolish spontaneous depolarisation. Sinus rhythm was achieved and maintained in 28 patients (62%) at a follow up period of 8 ± 6 mo[9]. This discovery led to the pulmonary vein isolation (PVI) approach.
The identification of abnormal depolarisation foci, helped in understanding the origin and development of AF. It became apparent that ectopic atrial depolarisation is responsible for the initiation of AF, whilst macro re-entrant circuits are responsible for its propagation (Figure 1)[10].
AF is defined as a supra-ventricular arrhythmia where asynchronous atrial activation occurs, leading to worsening atrial mechanical function[2,4,11].
According to the definitions given by ACC/ESC/AHA 2006, Society of thoracic surgery (STS), ESC and EACTS clinical guidelines committee, there are five types of AF. These are defined as: First diagnosis, paroxysmal, persistent, long-standing persistent and permanent AF (Table 1).
| Type of AF | Duration | Definition |
| First diagnosis | - | First episode of AF irrespective to duration or severity |
| Paroxysmal | 48 h | Self-terminating (usually within 48 h); may continue for up to 7 d. After 48 h it is unlikely that spontaneous conversion will occur |
| Anticoagulation must be considered | ||
| Persistent | > 7 d | Requires termination by cardio-eversion with drugs or direct current |
| Long standing persistent | ≥ 1 yr | Rhythm control strategy |
| Permanent | - | Presence of arrhythmia is accepted and rhythm control interventions are not pursued1 |
AF can also be classified as primary or secondary according to its origin. Primary AF occurs in patients with no underlying cardiac disease whilst secondary AF occurs as a result of pre-existing cardiac disease. This has implications when looking at the surgical management of AF. Whilst patients with secondary AF benefit from concomitant treatment alongside other cardiac surgical procedures, catheter ablation alone is a more suitable treatment modality for paroxysmal primary AF[12-15].
According to 2012 HRS/EHRA/ESC guidelines the term “Maze” procedure should only be used to refer to the Cox-Maze III lesion set. The above term should not be used for other less extensive lesion sets. Furthermore, the suggested terminology for ablative procedures is as follows: (1) full Cox-Maze lesion set; (2) left atrial appendage (LAA) lesion set; and (3) PVI[4,14].
Cox-Maze I and II: Cox et al[6] first described the Cox-Maze procedure in 1991. The Cox-Maze I (CM-I) procedure was initially developed, consisting of a set of lesions generating an “electrical maze” through the atria. The linked atrial segments were created surgically by cutting and sewing in order to interrupt and eliminate macro re-entrant circuits[7-10]. The Maze I procedure had several limitations including left atrial dysfunction and failure to generate sinus tachycardia on exertion. The Cox-Maze II (CM-II) procedure was subsequently developed aiming to overcome those limitations. In CM-II a revised lesion set was used, in an attempt to improve conduction between the atria. However, this led to a technically more challenging procedure with the same drawbacks as CM-I[7-10].
Cox-Maze III: The Cox-Maze III (CM-III) procedure shortly followed which overcame the limitations of the previous two procedures. Atrial transport function was preserved allowing depolarisation from sinoatrial node to atrioventricular node for efficient atrial contraction. CM-III achieved 93% freedom from AF during an 8.5 year follow up, with successful cardioeversion of all cases on one anti-arrhythmic drug. Additionally, the need for pacemaker was abolished and recurrence of AF reduced[7-10]. Equally encouraging results from the CM-III were generated by the Cleveland Clinic and Mayo Clinic[16,17].
Despite its high success rates the procedure remained technically challenging and highly invasive. A median sternotomy and cardiopulmonary bypass (CPB) is required for CM-III, limiting its use to patients undergoing concomitant heart surgery, as it is deemed to be too invasive to be solely performed for AF treatment.
The Cox-Maze IV: A few years later, the Cox-Maze IV (CM-IV) was developed initially described by Gaynor et al[18] in 2004. A different lesion set to CM-III was used and the traditional “cut and sew” technique was replaced with the use of ablative devices. Namely, the combined use of bipolar radiofrequency ablation together with cryoblation was used in that study. Moreover, CM-IV had the added advantage of being technically simpler than CM-III, meaning that it could be used in a greater number of centres. The lesion set used was similar to CM-III and it still had to be performed under CPB[18,19].
Widespread variation has been observed in the lesion sets used to treat AF. Since the publication of CM-III the lesion sets have been altered slightly in CM-IV and are continuously being altered in the current literature. The main reason for the constant alteration of the lesion sets is that surgeons are striving to achieve minimally invasive procedures, with the best clinical outcomes possible[12].
The PVI approach was developed following the discovery of the pulmonary veins being a key area of ectopic depolarisation, resulting in atrial ectopic beats and ultimately leading to AF. The PVI approach has been used in a number of trials and was shown to produce good results in paroxysmal AF.
When first performed by Haïssaguerre et al[9] 62% of patients were free from AF in a median follow up of 7 mo. In studies that followed, empirical isolation of the pulmonary veins became the most common approach as it was observed that the ectopic foci contributing to AF were often variable.
In the study by Haïssaguerre et al[9], 94% of the ectopic foci were found in the pulmonary veins. Thus, one would expect that by ablating those foci freedom from AF would be close to 90%. However, this has not been the case in studies looking at long term (5 year) freedom from AF using PVI, with the success rate being as low as 30%-50% and the recurrence rate up to 70%[20,21].
The reduced effectiveness of PVI can be attributed to incomplete transmural lesions and pulmonary vein reconnection. Another reason for the low success rates is the fact that the pulmonary veins are not the only triggers of AF. Exclusively using PVI may not be sufficient in patients with persistent and long-standing AF, as demonstrated by studies where ablation of additional areas improved the outcomes[22-24]. However, due to large inter-study variation in ablation methodology it is difficult to assess the effectiveness of PVI alone.
The LAA has been associated with occurrence of stroke in patients with AF. Studies have demonstrated that around 90% of the thrombi are found in the LAA, making it the primary source of emboli. This has led to the conclusion that successful closure of the LAA would reduce the risk of such thromboembolic events[25,26]. Several studies have investigated the link between LAA exclusion and reduction in stroke in patients with AF.
Healey et al[27] (2005) performed an RCT of 77 patients undergoing CABG where 52 of them underwent LAA occlusion. However, occlusion was successful in just 66% of the patients. One patient had an intraoperative ischaemic stroke and another had a TIA. Twelve percent of the patients had subsequent self-reported strokes on follow-up done via a questionnaire.
Kanderian et al[28] (2008) demonstrated that 55% of 137 patients who underwent LAA closure had a successful procedure. Eleven percent of the patients that had a successful closure had a subsequent stroke or TIA compared to 15% of those with unsuccessful procedure. These results were nevertheless found to be non-significant.
García-Fernández et al[29] (2003) looked at 205 patients undergoing mitral valve surgery, with 58 of them having LAA ligation. Successful ligation was seen in 89.7% of patients. Absence of LAA ligation was found to be an independent predictor of thromboembolism following mitral valve surgery. Systemic emboli occurred more frequently in the group of patients that did not receive ligation.
Contrasting the above studies, Almahameed et al[30] (2007) found a significantly increased rate of stoke in patients with LAA occlusion, looking at 136 patients that underwent LAA ligation during mitral valve surgery.
The above studies demonstrate the heterogeneity of existing results when looking at LAA exclusion. Success rate is variable between the studies, ranging from 55% to 93%. According to the EACTS guidelines, there is insufficient evidence to prove that LAA exclusion has a benefit in terms of stroke reduction or mortality[2].
Various energy sources have emerged over the last decade striving to replace the traditional “cut and sew” technique by replicating the transmural lesions whilst using a less invasive approach[31]. Nevertheless, the vital pre-requisite for successful AF ablation, as demonstrated by the CM-III lesion set, is that the lesions need to be completely transmural and contiguous bilaterally. In addition, it is fundamental that the lesions are placed in the correct pattern[10,12,18]. Therefore, an important caveat when looking at new ablation techniques is the ability to achieve complete transmurality of lesions. The ablative energy modalities available for surgical treatment of AF are compared in Table 2.
| Ablationmodality | Mode of action | Advantages | Complications | Transmural lesions | Current limitations |
| RFA | Controlled thermal damage and lesions caused by electrical current | Less operating time Reduced technical difficulty | Intercavity thrombus Pulmonary vein stenosis Oesophageal and coronary artery injury | Variable | Confirmation of transmurality Variation between instruments |
| Cryoablation | Targeted scarring by cooling tissue using high-pressure argon and helium Initial cellular destruction followed by fibrosis and full thickness disruption | Visual confirmation of transmurality Less damage to surrounding tissues and vascularity Less endocardial thrombus Electrical isolation of atria | Coronary artery and phrenic nerve injury Atrioesophageal fistula | Yes | Variable success rate |
| Microwave | Production of lesions by thermal injury | Minimal collateral damage Minimal scar formation Lower risk of VTE | Coronary artery damage potential | Variable | Less effective compared to other modalities Limited evidence |
| HIFU | Creation of localised hyperthermic lesions using a focused beam of ultrasound energy | Fast epicardial lesions Future potential advantage visualisation of thickness by ultrasound and tailor made lesions | Atrioesophageal fistula Pericardial effusion Phrenic nerve injury | Yes endocardial only | High rate of complications Limited evidence currently not recommended outside trials |
| Laser | Use of high energy optical beams to create thermal lesions | Well demarcated lesions Non-arrythmogenic Rapid lesions | Crater formation Perforation Tissue loss Poor visibility of scar | Yes | Limited evidence currently not recommended outside trials |
Radiofrequency ablation (RFA) works by conducting an alternating electrical current through the myocardium. The energy from this electrical current gets dissipated through the myocardial tissue as heat, causing coagulative necrosis and resulting in an area of non-conducting myocardium. Complications of RFA include injury to collateral structures such as the pulmonary veins, oesophagus and coronary arteries[14,25].
The effectiveness of unipolar RFA during concomitant cardiac surgery has been investigated by several studies.
Johansson et al[32] (2008) looked at patients undergoing CABG in combination with unipolar RFA. Patients were followed up for 32 ± 11 mo (with intermediate follow up at 3 and 6 mo) looking for sinus rhythm. In the RFA group 62% of patients were in sinus rhythm compared to 33% in the non-RFA group. Patients who were in paroxysmal or persistent AF were more likely to remain in sinus rhythm than patients with permanent AF. The presence of sinus rhythm at 3 mo was found to be a high predictor of the patient remaining in sinus rhythm at further follow up.
Khargi et al[33] (2005) looked at a cohort of patients with permanent AF undergoing open-heart surgery (CABG, aortic and mitral surgery) together with unipolar RFA. Sinus rhythm was observed in 79% of patients undergoing CABG/aortic surgery and 71% of mitral surgery patients. Notably, adding RFA did not increase mortality compared to cardiac surgery alone.
Bukerma et al[34] (2008) looked at patients fulfilling the criteria for permanent AF who underwent concomitant cardiac surgery and unipolar RFA. Contrasting the high success rates seen at the aforementioned studies, just 52% of patients maintained sinus rhythm at 5-year follow up.
All of the above studies used 24 h outpatient holter ECG monitoring to detect the presence of sinus rhythm. An important pitfall is that asymptomatic AF can be easily missed, as the monitoring is not continuous during the follow up period. This needs to be taken into account when designing future studies[2].
Unipolar RFA combined with concomitant cardiac surgery is considered to be effective in restoring sinus rhythm. Higher degrees of success rates are seen in paroxysmal or persistent AF, young age and smaller LAD[2].
The effectiveness of bipolar RFA during concomitant surgery has also been explored.
A meta-analysis conducted by Chiappini et al[35] (2004) looked at 6 non-randomised studies of patients with AF undergoing RFA as an adjunct to cardiac surgery, where 76% freedom from AF was achieved at 13.8 mo follow up with the overall survival rate being 97.1%.
Similarly, von Oppel et al[36] (2009) looked at patients with persistent AF undergoing concomitant cardiac surgery and bipolar RFA compared to cardiac surgery alone. Seventy-five percent of patients in the RFA group were free of AF at a 1-year follow up with more than 60% of patients having restoration of left atrial contraction.
A best evidence topic on the effectiveness of bipolar RFA during concomitant cardiac surgery conducted by Basu et al[37] (2012), revealed that bipolar RFA was more successful in restoring sinus rhythm for at least 1 year when performed together with cardiac surgery. In addition, a high survival rate was observed and the procedure required an average of 15 additional minutes of cross clamp time.
Bipolar RFA used in conjunction with cardiac surgery has a higher success in restoration of sinus rhythm compared to cardiac surgery alone. There is limited evidence to conclude whether bipolar RFA is more effective than unipolar RFA. Further studies comparing the two modalities are needed[2].
Cryoablation works by cryothermal energy, generated by the use of pressurised liquid nitrous oxide resulting in cooling of the surrounding tissue. Tissue injury occurs by the creation of ice crystals within the cells disrupting the cell function and electrical conductivity. Additionally, microvascular disruption ensues resulting in cell death. Complications of cryoablation include phrenic nerve injury, atrioesophageal fistulas and coronary artery injury[14,24]. Several studies have proved the efficacy of cryoablation in the treatment of AF.
The PRAGUE 12 (2012) was a randomised multicentre trial that is considered a milestone trial for AF. It looked at 224 patients with AF undergoing valve replacement or coronary surgery. The patients were randomised in two groups with group A undergoing surgical ablation and group B having no ablation. In the ablation group, 96% had treatment with a cryoprobe. The procedure performed consisted of PVI, mitral annulus lesion, LAA lesion and a connecting lesion. At 1 year follow up 60% of patients that underwent ablation were in sinus rhythm compared to 36% in the untreated group. At 1 year follow up no clinical benefits were seen in patients who underwent AF surgery; however the study is still ongoing and results for the 5-year follow up are yet to be published[38].
Camm et al[39] (2011) conducted a best evidence topic looking at the effectiveness of cryoablation during concomitant cardiac surgery. Nine studies were reviewed, including RCTs and retrospective studies. Cryoablation was found to be an acceptable surgical intervention, achieving sinus rhythm in 60%-82% of patients at 12 mo follow up.
Blomström-Lundqvist et al[40] (2007) conducted a RCT looking at patients undergoing concomitant AF surgery and mitral valve repair. The results demonstrated that undergoing cryoablation for AF treatment significantly increased the return to sinus rhythm. 73.3% of patients in the cryoablation group were found to be in sinus rhythm compared to 42.9% of those undergoing mitral valve surgery alone. However, it is worth noting that patients in the cryoablation group had an increased rate of complications. No significant increase in the mortality or morbidity was seen in either group.
Cryoablation during concomitant cardiac surgery has been proven to achieve good rates of sinus rhythm and it is more successful in patients with paroxysmal AF as opposed to permanent AF. Increased complication rates from cryoablation were seen in just one study. Finally, a lack of 24 h monitoring meant that accurate assessment of AF resolution was difficult[2,4].
Microwave ablation works by producing a well-demarcated lesion through thermal injury. Its main advantage is that it produces good epicardial lesions and can be used in minimally invasive techniques.
MacDonald et al[41] (2011) conducted a best evidence topic regarding the effectiveness of microwave ablation for AF treatment during concomitant heart surgery. Eleven studies were reviewed with a large degree of heterogeneity observed between the studies. The success rate ranged between 65%-87% over a variable follow up period between 6-12 mo. The conclusion was that microwave ablation is not currently recommended due to limited evidence and unclear long-term success rates.
Lin et al[42] (2010) compared microwave ablation to bipolar RFA. A RCT was conducted where patients were randomised to a radiofrequency or a microwave ablation group. Patients were then followed up at 3, 6, 9 and 12 mo and then annually. With a mean follow up of 24 mo, freedom from AF in the radiofrequency group was 88.7% compared to 71.2% in the microwave ablation group (P = 0.0008). Thus bipolar radiofrequency ablation was demonstrated to be superior to microwave ablation.
Kim et al[43] (2010) compared cryoablation to microwave ablation in patients with mitral disease and AF. They demonstrated a 5-year freedom rate from AF of 61.3% in the microwave group compared to 79.9% in the cryoablation group. Additionally, microwave ablation was associated with more frequent AF recurrence rates.
Microwave ablation is currently considered to be less effective than other ablation modalities, based on the limited evidence[2]. Further studies are needed to investigate the effectiveness of microwave ablation as a definite treatment of AF.
High intensity focused ultrasound (HIFU) is a relatively new ablative modality and works by creating a localised thermal lesion using a focused beam of ultrasound energy. HIFU has been proven to create permanent transmural lesions when applied epicardially. It has the advantage that CPB is not needed and can be performed on the beating heart. HIFU can also be delivered via a balloon catheter in order to facilitate circumferencial ablation of pulmonary veins[44].
Neven et al[45] (2011) performed PVI using HIFU and subsequently followed up the patients for 2 years. An oesophageal temperature-guided safety algorithm was used in an attempt to minimise the complications. At 2 year follow up the success rate from the procedure was comparable to that of radiofrequency ablation. However, severe complications were not prevented despite the use of a safety algorithm. Complications included atrioesophageal fistula, pericardial effusion and phrenic nerve palsy. They concluded that HIFU did not meet the safety standards required for AF treatment, mainly because phrenic nerve palsy and atrioesophageal fistula were still common severe complications. The clinical use of HIFU has currently been halted.
Davies et al[46] (2013) followed up 110 patients undergoing HIFU ablation for AF treatment. At a 2-year follow up 49% of patients remained in sinus rhythm. The percentage of patients in sinus rhythm was given for each of the pre-operative AF types: 81% for paroxysmal AF, 56% for persistent AF and 18% for long standing AF. The conclusion was that HIFU is safe and effective for use in paroxysmal AF, however alternative ablation strategies should be considered for persistent and long standing AF.
Klinkenberg et al[47] (2009) and Schmidt et al[48] (2009) have also demonstrated that despite relatively high percentages of freedom from AF there is a high complication rate when using HIFU ablation. They have concluded that further research is needed to assess optimal ablation techniques.
HIFU ablation is currently not recommended outside trials due to the high rates of complications reported and its success rates being inferior to other ablative modalities[2]. Further studies are looking at the success rates of HIFU combined with cardiac surgery are needed, as the evidence available is currently limited.
Laser ablation for AF treatment works by using laser energy to create localised hyperthermic lesions[14,20].
Gal et al[49] (2015) used an endoscopic laser balloon ablation system to perform PVI with 58% of patients remaining free from AF at follow up with no anti-arrhythmic drugs. They concluded that laser ablation has a low risk of complications and its success rate was comparable to other ablation modalities.
Šedivá et al[50] (2014) performed PVI using visually guided laser ablation in 194 patients. In 1 year follow up 82.3% of patients remained free from AF in the paroxysmal AF group and 75% in the persistent AF group; this percentage remained close to 75% in 3 and 4-year follow up. They concluded that visually guided laser ablation is an effective and safe modality to be used in clinical practice with good clinical outcomes and low rates of complications.
Hamman et al[51] (2009) used a diode pumped laser to perform a left modified or complete CM-III lesion set. The results observed were very encouraging with a 95% freedom from AF and 76% freedom from all tachyarrhythmias. However, it needs to be noted that the study was only a small one, consisting of 28 patients.
Currently, laser ablation is not approved for clinical use outside trials due to limited available evidence to support its effectiveness and safety.
As well as using different devices for AF ablation, there are several methods to perform the ablation procedure. Traditionally the two options were catheter and surgical ablation.
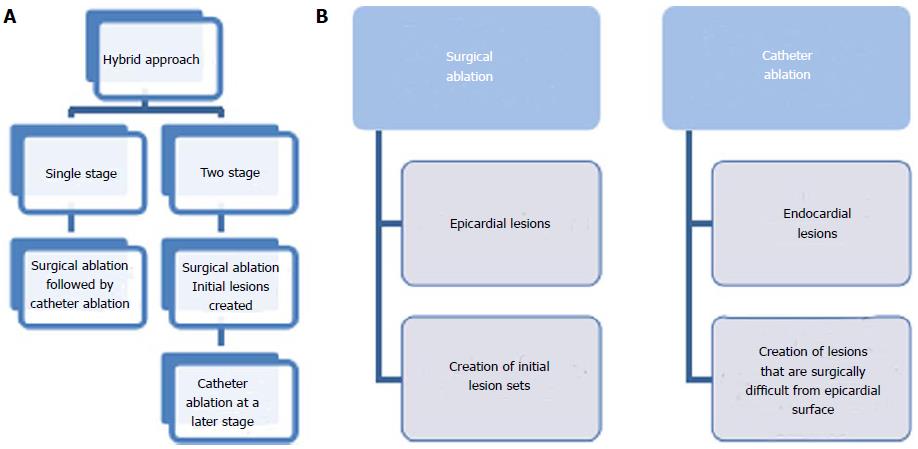
Surgical ablation can be performed with the conventional surgical approach during concomitant cardiac operations or with less invasive approaches such as mini sternotomy, mini thoracotomy or VATS.
Catheter ablation is considered to be the least invasive approach and it is used to create endocardial lesions. In some studies catheter ablation for AF has been shown to be 80% effective, with 70% of patients not requiring any anti-arrhythmic drugs at intermediate follow up[52].
The hybrid approach consists of a combination of surgical and catheter procedures. This has emerged in an attempt to ensure good epicardial and endocardial transmurality of lesions as well as attempting to enhance the long-term success of AF ablation. In essence, this approach aims to overcome the limitations of surgical and catheter procedures alone.
The hybrid procedure can be performed as a single or a two-stage procedure with advantages to each. When performing a single procedure undergoing anaesthesia twice and having further hospital admission are avoided. In contrast, when performing a two-stage approach with an average interval of 1-3 mo, the lesions are more likely to have healed and have stable conductive properties (Figure 2)[53].
Gaita et al[54] (2013) looked at a hybrid approach involving surgical cryoablation and transcatheter RFA in order to perform PVI and left atrial isolation. The procedure was performed in 33 patients, 73% of which were in sinus rhythm, at a mean follow up of 10 ± 3.1 years. At the end of the follow up period 81% of patients with a complete lesion set were in sinus rhythm compared to 43% of those with an incomplete lesion set. Electrophysiological evaluation of lesion transmurality of was used, aiding in significant improvement of outcomes.
Kumar et al[55] (2014) used a hybrid approach consisting of bipolar radiofrequency devices epicardially and cryoballoon endocardially. This approach was found to be feasible and safe, though the results should be interpreted with caution as the study consisted of only 7 patients.
Bulava et al[56] (2015) combined surgical thoracoscopic RFA with catheter RFA performed 6-8 wk later in a staged hybrid method. At 12 mo follow up after a completed hybrid ablation 94% of patients were in sinus rhythm.
A systematic review by Je et al[57] (2015) compared the endocardial Cox-Maze procedure, epicardial surgical ablation and hybrid procedure. The results demonstrated that minimally invasive Cox-Maze procedure with CPB support was the most effective treatment for stand-alone AF with a higher success rate seen at 12 mo following the procedure.
The hybrid approach is a promising new procedure that could significantly improve outcomes for patients undergoing surgical treatment for AF. It has been shown to have a mortality rate close to 0% with long-term success rates approaching 95% similar to the cut and sew Cox maze procedure. Thus, in the mildly symptomatic lone AF population, the hybrid procedure could become the standard of care in the near future[58]. Further studies would be essential in order to identify the combination of modalities that yields the most successful results when performing a hybrid procedure.
Surgical treatment of AF can be performed alone as well as in conjunction to cardiac surgery. Several studies have compared the efficacy of concomitant cardiac and AF surgery to cardiac surgery alone. Mitral valve surgery is the most common procedure that has been combined with AF ablation. It is known that 30%-50% of patients undergoing mitral valve surgery present with AF, which itself leads to increased risk of stroke and reduction in survival rates[59].
Phan et al[59] (2014) conducted a meta-analysis on surgical ablation for AF during mitral valve surgery. The results demonstrated that the addition of AF ablation led to a significantly greater number (64.4% vs 17.9%, P < 0.00001) of patients in sinus rhythm at a follow up period of > 12 mo. There was no increase in mortality, need for pacemaker implantation, stroke or thromboembolism risk.
A RCT conducted by Gillinov et al[60] (2015) consisted of a group of 260 patients with persistent or long-standing persistent AF. It compared patients undergoing mitral valve replacement therapy either with or without surgical ablation. The results showed that addition of AF ablation to mitral valve surgery led to a significant increase (P < 0.001) in the rate of freedom from AF in 1 year. The control group had 29.4% freedom from AF when compared to 63.2% in the ablation group.
The Left atrial radiofrequency ablation during mitral valve surgery: A prospective randomized multicentre study (SAFIR)[61] study (2009) was a multi-centre double-blinded centrally randomised trial involving four university hospitals. It compared patients undergoing left atrial RFA combined with mitral valve replacement to those undergoing mitral valve replacement alone. The results were in favour of the combined ablation and mitral valve replacement. At 12 mo follow up the freedom from AF was 95.2% in the combined group vs 33.3% in the control (P < 0.005).
The Surgical Atrial Fibrillation Suppression Study (2011) was a RCT that looked at patients undergoing RFA performed in conjunction to cardiac surgery. They concluded that surgical RFA for AF during concomitant cardiac surgery significantly reduces AF burden. However, 13% of patients had asymptomatic AF episodes only identified on continuous monitoring. This was deemed to have significant implications for the definition of successful surgical AF ablation as well as the need for post-operative anti-arrhythmic and anticoagulants[62]. Potential disadvantages of this combined approach are the increased rates of permanent pacemaker insertion and increased operative time[27,59-62].
The use of energy sources for AF ablation is recommended in concomitant cardiac surgery because of good evidence supporting the efficacy of the combined procedure[2]. However, further studies are needed to investigate combination of surgical AF ablation with specific cardiac operations. This will help in identifying the combination of ablative modality and cardiac surgery yielding the best results.
Table 1, Table 2, Table 3, Table 4, Table 5, Table 6, Table 7, Table 8 and Table 9 summarise the studies discussed in the sections above, looking at each modality available for surgical AF ablation.
| Indications for concomitant surgical ablation of AF |
| Symptomatic AF refractory or intolerant to at least one Class 1 or 3 antiarrhythmic medication |
| Paroxysmal: Surgical ablation is reasonable for patients undergoing surgery for other indications (IIa, C) |
| Persistent: Surgical ablation is reasonable for patients undergoing surgery for other indications (IIa, C) |
| Longstanding persistent: Surgical ablation is reasonable for patients undergoing surgery for other indications (IIa, C) |
| Symptomatic AF prior to initiation of antiarrhythmic drug therapy with a Class 1 or 3 antiarrhythmic agent |
| Paroxysmal: Surgical ablation is reasonable for patients undergoing surgery for other indications (IIa, C) |
| Persistent: Surgical ablation is reasonable for patients undergoing surgery for other indications (IIa, C) |
| Longstanding persistent: Surgical ablation may be considered for patients undergoing surgery for other indications (IIb, C) |
| Indications for standing alone surgical ablation of AF |
| Symptomatic AF refractory or intolerant to at least one Class 1 or 3 antiarrhythmic medication |
| Paroxysmal: Stand alone surgical ablation may be considered for patients who have not failed catheter ablation but prefer a surgical approach (IIb, C) |
| Paroxysmal: Stand alone surgical ablation may be considered for patients who have failed one or more attempts at catheter ablation (IIb, C) |
| Persistent: Stand alone surgical ablation may be considered for patients who have not failed catheter ablation but prefer a surgical approach (IIb, C) |
| Persistent: Stand alone surgical ablation may be considered for patients who have failed one or more attempts at catheter ablation (IIb, C) |
| Longstanding persistent: Stand alone surgical ablation may be considered for patients who have not failed catheter ablation but prefer a surgical approach (IIb, C) |
| Longstanding persistent: Stand alone surgical ablation may be considered for patients who have failed one or more attempts at catheter ablation (IIb, C) |
| Symptomatic AF prior to initiation of antiarrhythmic drug therapy with a Class 1 or 3 antiarrhythmic agent |
| Paroxysmal: Stand alone surgical ablation is not recommended (III, C) |
| Persistent: Stand alone surgical ablation is not recommended (III, C) |
| Longstanding persistent: Stand alone surgical ablation is not recommended (III, C) |
| Use of ablative modalities |
| Unipolar radiofrequency ablation |
| Concomitant unipolar RFA for AF treatment together with cardiac surgery is effective in restoration of sinus rhythm |
| Success rates vary between 54%-83% at medium term follow up (at least 12 mo) |
| Safe procedure - no additional risks |
| Success rates are higher with: paroxysmal or persistent AF, younger age, smaller LAD |
| Class IIa recommendation based on multiple small retrospective studies (Level C) |
| Bipolar radiofrequency ablation |
| Higher success rates in restoring sinus rhythm compared to no ablation in concomitant cardiac surgery |
| On average the cross clamp time is increased by 15 min |
| There is limited evidence to suggest superiority of bipolar over unipolar RFA |
| 1 prospective trial has provided evidence demonstrating superiority of bipolar RFA over microwave ablation |
| Class I recommendation based on 3 RCTs and multiple small prospective studies (Level A) |
| Cryoablation |
| Acceptable intervention for AF treatment during concomitant surgery with acceptable sinus rhythm conversion rates between 60%-82% at 12 mo |
| Cryoablation is most successful in patients suffering from paroxysmal as opposed to permanent AF (suggested by 6 out of 9 studies reviewed) |
| Class IIa recommendation based on 1 small RCT and multiple prospective and retrospective studies (Level B) |
| Microwave ablation |
| Less effective intervention for AF treatment based on the limited evidence |
| Success rates in the longer term are less clear - the only RCT to date has found outcomes inferior to RFA |
| Class III recommendation based on 1 small RCT and multiple small prospective and retrospective studies (Level B) |
| HIFU |
| Currently not recommended as an intervention for the treatment of AF during concomitant surgery outside clinical trials due to limited evidence |
| Success rates seem to be inferior to those of other devices |
| Significant concerns have been reported |
| Class III recommendation based on cohort studies (Level C) |
| Exclusion of laa and standing alone surgical ablation |
| Exclusion of LAA |
| No proven benefit of surgical LAA exclusion in terms of stroke reduction or mortality |
| Ineffective LAA occlusion and potentially increased stroke risk due to poor technique was seen in many studies |
| Devices designed for LAA exclusion should be preferentially used rather than a cut and sew or stapling technique, if LAA is to be performed |
| Class IIa recommendation based on multiple cohort studies and one pilot RCT (Level B) |
| Stand alone surgical ablation |
| Surgery can be considered for symptomatic patients who are refractory or intolerant to at least 1 anti-arrhythmic medication |
| Considered for patients with paroxysmal, long standing and persistent AF who prefer surgery to catheter ablation or have failed catheter ablation |
| Results of both catheter-based and surgery-based ablation should be discussed with the patient |
| Class IIa recommendations based on 1 RCT and multiple cohort studies (Level B) |
| Procedure | Ref. | Sample size | Mean follow-up period | Outcome | Important findings |
| Cox-Maze | Cox et al[7] | 178 patients | 8.5 yr | 93% freedom from AF | Cox-Maze procedure developed |
| Cox-Maze | McCarthy et al[16] | 100 patients | 3 yr | 90.4% in sinus rhythm or atrial pacing | Associated with low perioperative and late morbidity rates |
| Cox-Maze | Schaff et al[17] | 221 patients | 6 yr | 90% in sinus rhythm | CM procedure was useful in patients requiring valvuloplasty for mitral regurgitation |
| Modified Cox-Maze with bipolar RFA | Gaynor et al[18] | 40 patients | 6 mo | 91% in sinus rhythm | Modification of CM-III shortened and simplified the procedure with no change in short-term efficacy |
| Procedure | Ref. | Sample size | Mean follow-up period | Outcome | Important findiNGS |
| PVI | Haïssaguerre et al[9] | 45 patients | 8 ± 6 mo | Sinus rhythm achieved in 28 patients (62%) | 69 foci identified as the source of ectopic atrial beats in 45 patients |
| PVI | Chao et al[21] | 88 non-paroxysmal AF patients | 36.8 mo | The long-term freedom period of AF was 28.4% after a single procedure | CHADS2 score of >/3 and left atrial diameter found to be significant predictors of recurrences |
| LAA obliteration | Healy et al[27] | RCT - 77 patients with risk factors for stroke | 8 wk follow-up with trans-oesophageal echocardiography | Complete occlusion achieved in 45% (5/11) of patients through the use of sutures and in 72% (24/33) using a stapler | Surgical LAA can be safely done during a routine CABG; expertise is key to its success rates |
| LAA excision or exclusion | Kenderian et al[28] | 137 patients | Post-operative trans-oesophageal echocardiography | Successful LAA closure 73% with surgical excision and 23% with suture exclusion. Evidence of stroke in 11% of successful LAA closure and 15% of unsuccessful LAA closure (P = 0.61) | High proportion of surgical LAA closure. LAA excision more successful than exclusion |
| LAA obliteration + Mitral valve replacement | García-Fernández et al[29] | 58 patients | 69.4 mo trans-oesophageal echocardiography | 46% of patients had an embolism. Risk of embolism increased by 11.6 in incomplete/absence of LAA ligation | Absence of LAA ligation and presence of left atrial thrombus identified as independent predictors for stroke |
| LAA exclusion during mitral valve surgery | Almahameed et al[30] | 136 patients | 3.6 ± 1.3 yr | 12.3% of patients had thromboembolic events, 71% of which occurred in patients undergoing mitral valve repair | There were more thromboembolic events in patients not prescribed warfarin on discharge |
| Procedure | Ref. | Sample size | Mean follow-up period | Outcome | Important findings |
| Concomitant RFA | Johansson et al[32] | 39 patients undergoing CABG | 32 ± 11 mo | 62% freedom from AF in ablation group compared to 33% in non-ablation group | Sinus rhythm at 3 mo was highly predictive of long-term sinus rhythm |
| Concomitant RFA | Khargi et al[33] | 128 patients in permanent AF (Group 1: mitral valve surgery, group 2: aortic valve surgery or CABG) | 3, 6 and 12 mo ECG and sinus rhythm confirmed with 24hrs ECG | 71% post-operative sinus rhythm in group 1 vs 79% in group 2 | Concomitant RFA in mitral valve surgery and aortic valve surgery or CABG is equally effective |
| Concomitant RFA | Beukema et al[34] | 258 patients with permanent AF | 43.7 ± 25.9 mo | Sustained sinus rhythm in 69% of patients at 1 yr, 56% at 3 yr, 52% at 5 yr and 57% at the latest follow up | RF modified maze procedure abolished AF in the majority of patients |
| Concomitant RFA | Chiappini et al[35] | Review of 6 studies - 451 patients in total | 13.8 ± 1.9 mo | 97.1% overall survival rate, 76.3% ± 5.1% overall freedom from AF | RFA is a safe and efficient procedure to cure AF in patients undergoing concomitant heart surgery |
| Concomitant RFA | Von Opell et al[36] | 49 patients with AF of more than 6 mo duration | At discharge, 3 and 12 mo post procedure | Return to sinus rhythm 29% 57% and 75% (at discharge, 3 mo and 12 mo post-procedure) in the cardioblate group vs 20%, 43% and 29% respectively in the control group | Concomitant RFA resulted in 75% conversion rate to sinus rhythm compared to the control group (39%) |
| Concomitant RFA | Budera et al[38] | Multicentre RCT involving 224 patients with AF undergoing cardiac surgery with ( n = 117) or without ablation (n = 107) | 30 d | At 1 yr follow up, 60.2% of patients were in sinus rhythm in the ablation group compared to 35.5% in the control group. 1 yr mortality was 16.2% and 17.4% respectively | Concomitant ablation increases postoperative sinus rhythm with no effect on peri-operative complications |
| Concomitant RFA | Blomström-Lundqvist et al[40] | Double-blind randomized study of 69 patients undergoing mitral valve surgery with or without epicardial left atrial cryoablation | 6 and 12 mo | At 6 mo follow-up, 73.3% of patients in the cryoablation group regained sinus rhythm vs 45.7% of patients with mitral valve surgery alone (P = 0.024). At 12 mo follow-up, the results were 73.3% vs 42.9% respectively (P = 0.013) | Concomitant left atrial epicardial cardioablation is significantly better in regaining sinus rhythm in patients with permanent AF compared to mitral valve surgery alone |
| Concomitant RFA | Chevalier et al[61] | Prospective, multicentre, double-blinded RCT involving 43 patients with mitral valve disease and permanent AF | 12 mo | At 12 mo, sinus rhythm was maintained without any arrhythmia recurrences in 57% of patients in the RFA group vs 4% in the control group (undergoing mitral valve surgery only) | Left atrial RFA is an effective procedure in patients suffering with long-term AF and co-existing valvular disease |
| Concomitant RFA | Veasey et al[62] | 100 patients in paroxysmal or persistent AF undergoing cardiac surgery were enrolled | 6 mo | 75% freedom of AF at 6 mo follow-up post concomitant RFA. The AF burden decreased from 56.2% post-operatively to 27.5% at 6 mo post-operatively. 13% of patients had asymptomatic AF episodes identified via continuous monitoring | Concomitant RFA successfully reduces AF burden but based on these results, the importance of post-operative antiarrhythmic medication and anticoagulation should be evaluated |
| Procedure | Ref. | Sample size | Mean follow-up period | Outcome | Important findings |
| HIFU | Neven et al[45] | Two-year follow-up of 28 people with paroxysmal AF 9 (n = 19) and persistent AF (n = 9) undergoing | Median follow-up 738 d | Following a median follow-up of 738 d, 79% of patients were free of AF. Following a repeat procedure with radiofrequency ablation, 18% of patients maintained freedom of AF | Success rates of HIFU are comparable to radiofrequency ablation but complication rates remain higher for HIFU |
| HIFU | Klinkenberg et al[47] | 15 patients with AF refractory to antiarrhythmic medication underwent HIFU for PVI | 24 mo | At 6 mo 40% of patients with 1 epicardial PVI gained sinus rhythm. After 1.3 ± 0.6 yr, 27% of patients had sinus rhythm after 1 epicardial pulmonary vein isolation | Success rate was low in epicardial pulmonary vein isolation done through right-sided VATS using HIFU and was associated with substantial complications |
| HIFU | Schmidt et al[48] | 22 patients with paroxysmal AF who underwent PVI using HIFU | median follow-up of 342 d | 71% of patients remained free of any AF/AT recurrence without antiarrhythmic drugs after a procedure | The 12F-HIFU induces a very rapid pulmonary venous isolation in patients |
| Procedure | Ref. | Sample size | Mean follow-up period | Outcome | Important findings |
| Hybrid approach | Kuman et al[55] | A cohort of 7 patients with AF undergoing a hybrid procedure | Follow-up at 3, 6, 9 and 12 mo post-procedure | After a follow-up of 40 ± 3 mo, 6 out of 7 patients were in sinus rhythm | The hybrid approach is a safe and feasible technique to AF ablation |
| Hybrid approach | Bulava et al[56] | 50 consecutive patients with long-standing AF who underwent the procedure | Follow-up at 3, 6, 9 and 12 mo post-procedure and thereafter after every 6 mo | 94% of patients were in sinus rhythm, 12 mo after the procedure No arrhythmias were present in any patient after 12 mo | The hybrid approach is extremely effective in maintaining sinus rhythm compared to radiofrequency catheter ablation or surgical ablation alone |
| Hybrid approach vs Cox-Maze vs epicardial ablation | Je et al[57] | Systematic review of 37 studies with a total of 1877 patients | 12 mo | Operative mortality for the Cox-Maze, epicardial ablation and hybrid approach were 0%, 0.5% and 0.9% At 12 mo, rates of sinus rhythm restoration for the above were 93%, 80% and 70% respectively | The Cox-Maze procedure with cardiopulmonary bypass revealed the highest success rate 12 mo post-procedure compared to the hybrid approach and epicardial approach |
A best evidence topic was conducted by Michael Gray et al[63] looking at the safety of stopping anticoagulants following successful surgery for AF.
Looking at compiled data of 10 000 patient-years follow up, they concluded that discontinuation of warfarin following AF surgery is safe. An annual thromboembolic stroke rate of 0.3%-8% in patients who were discontinued off warfarin was seen in these studies, where warfarin was stopped after AF surgery at a mean of 3.6 mo (0-8 mo) after AF surgery. It is worth noting here that care needs to be taken when interpreting those results, since the scarcity of good quality RCTs means that their conclusion was mainly based on low quality evidence such as observational studies.
Stroke risk also varied according to the procedure performed. PVI performed as an isolated procedure, as well as being the procedure most extensively evaluated, was shown to have the lowest stroke risk off warfarin (0%-0.4% per annum). In contrast, concomitant cardiac surgery such as mitral valve repair was found to considerably increase the thromboembolic stroke rate. Thus, mitral valve surgery was found to be risk factor for late thromboembolic stroke in patients undergoing concomitant AF surgery.
In summary, the best evidence topic concluded that discontinuation of warfarin at 3 mo post-operatively would be feasible in selected patients, following consideration of the patient’s individual risk factor profile[63].
The above is in agreement with the 2012 HRS/EHRA/ESC Guidelines, which concluded that surgical intervention for AF is not recommended solely to discontinue warfarin or other anticoagulants. Furthermore, cessation of anticoagulants in patients post-ablation is not recommended if the stroke risk is high as measured on CHAD52. However, if a patient is not high risk on CHAD52 and has been in sinus rhythm for a significant continuous period they could change their warfarin to aspirin alone[14].
The EACTS clinical guidelines committee also recommend cessation of anticoagulants at 3 mo following established AF ablation procedures; provided that the patient is in sustained sinus rhythm and that their stroke-risk profile has been considered and deemed to be low[2].
The STS workforce on evidence-based surgery published a document on reporting results from AF surgery. This included reporting regular interval ECG assessments and encouraging the widespread use of implantable recording devices to assess AF[64].
The STS workforce in accordance with 2012 HRS/EHRA/ESC Guidelines, recommend that the entrance and exit block should be reported and demonstrated intra-operatively[14,63]. Additionally, the 2012 HRS/EHRA/ESC Guidelines recommend follow-up of at least one year with a minimum of 24-72 h holter monitoring, trans-telephonic monitoring, 30 d auto event triggered monitoring or outpatient telemetry[2,14].
The EACTS Guidelines recommend routine testing of entrance and exit block after AF surgery to establish creation of effective lesion sets. They also recommend following the 2012 HRS/EHRA/ESC Guidelines for reporting results of surgery and in publications[2].
The 2012 HRS/EHRA/ESC Guidelines have summarised the indications for surgical interventions of AF. Their statements were in accordance with their 2006 guidelines and their subsequent updates. The guidelines include the indications for concomitant surgical ablation of AF (Table 3) and the use of ablative modalities in each AF category (Table 4)[14].
The surgical management of AF is a rapidly evolving field with multiple studies exploring the various surgical options available. The current literature includes a few RCTs as well as several prospective, retrospective and cohort studies. When looking at these studies, there are certain limitations that need to be considered.
First, there is a variable definition of “success” when reporting results. This is often vaguely described as “freedom from AF” though not being further defined. Conversely, a few studies report “success” as patients being in sinus rhythm at follow up. Standardisation on reporting the outcomes of studies would be invaluable when looking at results from different studies. This would enable accurate comparisons in order to draw reliable conclusions. Guidelines aiding standardisation of results reporting have been published by STS[64] and following them has been recommended by EACTS and HRS/EHRA/ECAS[2,14].
Secondly, there is vast heterogeneity regarding the follow up periods used in each study. Follow up periods ranging from 1 mo to 5 years have been seen across different studies. The lack of a standardised follow up interval makes it very difficult to reliably compare results. The STS workforce has published a document regarding the reporting of results and recommended follow up periods in surgery for AF[64].
When looking at the current literature it is evident that an array of lesion sets is being used when performing AF surgery. An explanation for this can be the move towards minimally invasive surgery attempting to yield good results, whilst using less invasive techniques. Additionally, it is widely accepted that the CM-III procedure has a limited use due to its technically challenging nature. This has led to the development of other lesion sets that are less invasive and easier to perform. It would be worthwhile establishing some common lesion sets that could be used in studies. Currently, the EACTS recommended using the terminology “Maze” procedure, “PVI” and “LAA” when describing the different lesion sets[2]. This would eliminate the bias of using slightly different lesion sets in each study and enable the results of the studies to be compared accurately.
Furthermore, there is limited data available on the comparison of different energy sources in AF ablation. Several studies have looked at the use of individual ablative modalities for AF treatment during concomitant cardiac surgery[59-62]. However, very few studies compared the ablative modalities to each other[42,43]. Future studies performing this comparison would be vital, as evidence is needed in order to identify the ablation modalities with the highest success rates and least complications.
Looking at the patient population recruited in the studies, there is a large variation between AF types in the patients included. A few studies have included a population with a single AF type such as permanent AF and looked at ablation modalities in that population[33,34,36,50]. More studies looking at patient populations with specific AF types would be useful in finding out which ablation modality works better for each AF type.
Additionally, the hybrid approach is a promising new procedure that could potentially improve success rates in the surgical management of AF. Over the recent years an increasing number of studies have been looking at this new approach[53-57]. More studies exploring the hybrid approach are needed in order to obtain reliable results as to whether the hybrid procedure should be clinically recommended for the surgical management of AF, as well as identify the best combination of modalities to be used in this procedure.
Finally, an important consideration for future studies is the big gap in literature when looking for RCTs, in order to obtain reliable results and be able to make good clinical recommendations. There is definitely a need for more, well-conducted prospective RCTs looking the various ablative modalities[65].
In conclusion, AF is a public health burden associated with substantial morbidity and mortality. Surgical management of AF is currently recommended in paroxysmal or persistent AF during concomitant heart surgery. Stand alone surgical ablation for AF can be considered with caution in patients who are intolerant or refractory to antiarrhythmic medication. Several studies have produced promising results using the new ablative modalities, which emerged over the last few years. Nevertheless, there is still a requirement for additional high quality RCTs in order to be able to make reliable evidence-based recommendations regarding the surgical management of AF.
P- Reviewer: Peteiro J, Raja SG S- Editor: Qiu S L- Editor: A E- Editor: Wu HL
| 1. | Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 2. | Dunning J, Nagendran M, Alfieri OR, Elia S, Kappetein AP, Lockowandt U, Sarris GE, Kolh PH. Guideline for the surgical treatment of atrial fibrillation. Eur J Cardiothorac Surg. 2013;44:777-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Boriani G, Diemberger I, Biffi M, Martignani C, Branzi A. Pharmacological cardioversion of atrial fibrillation: current management and treatment options. Drugs. 2004;64:2741-2762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, Kim YH, Klein G, Packer D, Skanes A. Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation. 2005;111:1100-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1080] [Cited by in RCA: 1056] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 5. | Cox JL, Schuessler RB, Boineau JP. The surgical treatment of atrial fibrillation. I. Summary of the current concepts of the mechanisms of atrial flutter and atrial fibrillation. J Thorac Cardiovasc Surg. 1991;101:402-405. [PubMed] |
| 6. | Cox JL, Canavan TE, Schuessler RB, Cain ME, Lindsay BD, Stone C, Smith PK, Corr PB, Boineau JP. The surgical treatment of atrial fibrillation. II. Intraoperative electrophysiologic mapping and description of the electrophysiologic basis of atrial flutter and atrial fibrillation. J Thorac Cardiovasc Surg. 1991;101:406-426. [PubMed] |
| 7. | Cox JL, Schuessler RB, D’Agostino HJ, Stone CM, Chang BC, Cain ME, Corr PB, Boineau JP. The surgical treatment of atrial fibrillation. III. Development of a definitive surgical procedure. J Thorac Cardiovasc Surg. 1991;101:569-583. [PubMed] |
| 8. | Cox JL. The surgical treatment of atrial fibrillation. IV. Surgical technique. J Thorac Cardiovasc Surg. 1991;101:584-592. [PubMed] |
| 9. | Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P, Clémenty J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5432] [Cited by in RCA: 5387] [Article Influence: 199.5] [Reference Citation Analysis (0)] |
| 10. | Cox JL. The longstanding, persistent confusion surrounding surgery for atrial fibrillation. J Thorac Cardiovasc Surg. 2010;139:1374-1386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, Hindricks G, Kirchhof P. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation--developed with the special contribution of the European Heart Rhythm Association. Europace. 2012;14:1385-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 881] [Cited by in RCA: 967] [Article Influence: 74.4] [Reference Citation Analysis (0)] |
| 12. | Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Le Heuzey JY, Kay GN, Lowe JE. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e257-e354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1263] [Cited by in RCA: 1385] [Article Influence: 72.9] [Reference Citation Analysis (0)] |
| 13. | Calkins H, Brugada J, Packer DL, Cappato R, Chen SA, Crijns HJ, Damiano RJ, Davies DW, Haines DE, Haissaguerre M. HRS/EHRA/ECAS expert Consensus Statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2007;4:816-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1013] [Cited by in RCA: 974] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 14. | Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, Crijns HJ, Damiano RJ Jr, Davies DW, DiMarco J. Heart Rhythm Society Task Force on Catheter and Surgical Ablation of Atrial Fibrillation.2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm. 2012;9:632-696.e21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1237] [Cited by in RCA: 1316] [Article Influence: 101.2] [Reference Citation Analysis (0)] |
| 15. | Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, Van Gelder IC, Al-Attar N, Hindricks G, Prendergast B. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J. 2010;31:2369-2429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3227] [Cited by in RCA: 3332] [Article Influence: 222.1] [Reference Citation Analysis (0)] |
| 16. | McCarthy PM, Gillinov AM, Castle L, Chung M, Cosgrove D. The Cox-Maze procedure: the Cleveland Clinic experience. Semin Thorac Cardiovasc Surg. 2000;12:25-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 165] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 17. | Schaff HV, Dearani JA, Daly RC, Orszulak TA, Danielson GK. Cox-Maze procedure for atrial fibrillation: Mayo Clinic experience. Semin Thorac Cardiovasc Surg. 2000;12:30-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 155] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 18. | Gaynor SL, Diodato MD, Prasad SM, Ishii Y, Schuessler RB, Bailey MS, Damiano NR, Bloch JB, Moon MR, Damiano RJ. A prospective, single-center clinical trial of a modified Cox maze procedure with bipolar radiofrequency ablation. J Thorac Cardiovasc Surg. 2004;128:535-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 223] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 19. | Damiano RJ, Bailey M. The Cox-Maze IV procedure for lone atrial fibrillation. Multimed Man Cardiothorac Surg. 2007;2007:mmcts.2007.002758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Ouyang F, Tilz R, Chun J, Schmidt B, Wissner E, Zerm T, Neven K, Köktürk B, Konstantinidou M, Metzner A. Long-term results of catheter ablation in paroxysmal atrial fibrillation: lessons from a 5-year follow-up. Circulation. 2010;122:2368-2377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 530] [Cited by in RCA: 570] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 21. | Chao TF, Tsao HM, Lin YJ, Tsai CF, Lin WS, Chang SL, Lo LW, Hu YF, Tuan TC, Suenari K. Clinical outcome of catheter ablation in patients with nonparoxysmal atrial fibrillation: results of 3-year follow-up. Circ Arrhythm Electrophysiol. 2012;5:514-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 22. | Lin YJ, Chang SL, Lo LW, Hu YF, Chong E, Chao TF, Chung FP, Liao J, Li CH, Tsao HM. A prospective and randomized comparison of limited versus extensive atrial substrate modification after circumferential pulmonary vein isolation in nonparoxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 2014;25:803-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Narayan SM, Krummen DE, Shivkumar K, Clopton P, Rappel WJ, Miller JM. Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation) trial. J Am Coll Cardiol. 2012;60:628-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 877] [Cited by in RCA: 880] [Article Influence: 67.7] [Reference Citation Analysis (0)] |
| 24. | Bunch TJ, Cutler MJ. Is pulmonary vein isolation still the cornerstone in atrial fibrillation ablation? J Thorac Dis. 2015;7:132-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 25. | Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg. 1996;61:755-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1072] [Cited by in RCA: 1201] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 26. | Petersen P, Godtfredsen J. Risk factors for stroke in chronic atrial fibrillation. Eur Heart J. 1988;9:291-294. [PubMed] |
| 27. | Healey JS, Crystal E, Lamy A, Teoh K, Semelhago L, Hohnloser SH, Cybulsky I, Abouzahr L, Sawchuck C, Carroll S. Left Atrial Appendage Occlusion Study (LAAOS): results of a randomized controlled pilot study of left atrial appendage occlusion during coronary bypass surgery in patients at risk for stroke. Am Heart J. 2005;150:288-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 367] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 28. | Kanderian AS, Gillinov AM, Pettersson GB, Blackstone E, Klein AL. Success of surgical left atrial appendage closure: assessment by transesophageal echocardiography. J Am Coll Cardiol. 2008;52:924-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 411] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 29. | García-Fernández MA, Pérez-David E, Quiles J, Peralta J, García-Rojas I, Bermejo J, Moreno M, Silva J. Role of left atrial appendage obliteration in stroke reduction in patients with mitral valve prosthesis: a transesophageal echocardiographic study. J Am Coll Cardiol. 2003;42:1253-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 255] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 30. | Almahameed ST, Khan M, Zuzek RW, Juratli N, Belden WA, Asher CR, Novaro GM, Martin DO, Natale A. Left atrial appendage exclusion and the risk of thromboembolic events following mitral valve surgery. J Cardiovasc Electrophysiol. 2007;18:364-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Comas GM, Imren Y, Williams MR. An overview of energy sources in clinical use for the ablation of atrial fibrillation. Semin Thorac Cardiovasc Surg. 2007;19:16-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 32. | Johansson B, Houltz B, Berglin E, Brandrup-Wognsen G, Karlsson T, Edvardsson N. Short-term sinus rhythm predicts long-term sinus rhythm and clinical improvement after intraoperative ablation of atrial fibrillation. Europace. 2008;10:610-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 33. | Khargi K, Lemke B, Deneke T. Concomitant anti-arrhythmic procedures to treat permanent atrial fibrillation in CABG and AVR patients are as effective as in mitral valve patients. Eur J Cardiothorac Surg. 2005;27:841-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 34. | Beukema WP, Sie HT, Misier AR, Delnoy PP, Wellens HJ, Elvan A. Intermediate to long-term results of radiofrequency modified Maze procedure as an adjunct to open-heart surgery. Ann Thorac Surg. 2008;86:1409-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Chiappini B, Di Bartolomeo R, Marinelli G. Radiofrequency ablation for atrial fibrillation: different approaches. Asian Cardiovasc Thorac Ann. 2004;12:272-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | von Oppell UO, Masani N, O’Callaghan P, Wheeler R, Dimitrakakis G, Schiffelers S. Mitral valve surgery plus concomitant atrial fibrillation ablation is superior to mitral valve surgery alone with an intensive rhythm control strategy. Eur J Cardiothorac Surg. 2009;35:641-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 37. | Basu S, Nagendran M, Maruthappu M. How effective is bipolar radiofrequency ablation for atrial fibrillation during concomitant cardiac surgery? Interact Cardiovasc Thorac Surg. 2012;15:741-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 38. | Budera P, Straka Z, Osmančík P, Vaněk T, Jelínek Š, Hlavička J, Fojt R, Červinka P, Hulman M, Šmíd M. Comparison of cardiac surgery with left atrial surgical ablation vs. cardiac surgery without atrial ablation in patients with coronary and/or valvular heart disease plus atrial fibrillation: final results of the PRAGUE-12 randomized multicentre study. Eur Heart J. 2012;33:2644-2652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 39. | Camm CF, Nagendran M, Xiu PY, Maruthappu M. How effective is cryoablation for atrial fibrillation during concomitant cardiac surgery? Interact Cardiovasc Thorac Surg. 2011;13:410-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 40. | Blomström-Lundqvist C, Johansson B, Berglin E, Nilsson L, Jensen SM, Thelin S, Holmgren A, Edvardsson N, Källner G, Blomström P. A randomized double-blind study of epicardial left atrial cryoablation for permanent atrial fibrillation in patients undergoing mitral valve surgery: the SWEDish Multicentre Atrial Fibrillation study (SWEDMAF). Eur Heart J. 2007;28:2902-2908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 106] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 41. | MacDonald DR, Maruthappu M, Nagendran M. How effective is microwave ablation for atrial fibrillation during concomitant cardiac surgery? Interact Cardiovasc Thorac Surg. 2012;15:122-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 42. | Lin Z, Shan ZG, Liao CX, Chen LW. The effect of microwave and bipolar radio-frequency ablation in the surgical treatment of permanent atrial fibrillation during valve surgery. Thorac Cardiovasc Surg. 2011;59:460-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 43. | Kim JB, Cho WC, Jung SH, Chung CH, Choo SJ, Lee JW. Alternative energy sources for surgical treatment of atrial fibrillation in patients undergoing mitral valve surgery: microwave ablation vs cryoablation. J Korean Med Sci. 2010;25:1467-1472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 44. | Vanelli P, Rossi R, Gelpi G, Cagnoni G, Contino M, Bosisio E, Vago G, Antona C. Chronic histological transmurality of high-intensity focused ultrasound ablation. Ann Thorac Surg. 2012;93:2053-2056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 45. | Neven K, Metzner A, Schmidt B, Ouyang F, Kuck KH. Two-year clinical follow-up after pulmonary vein isolation using high-intensity focused ultrasound (HIFU) and an esophageal temperature-guided safety algorithm. Heart Rhythm. 2012;9:407-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 46. | Davies EJ, Bazerbashi S, Asopa S, Haywood G, Dalrymple-Hay M. Long-term outcomes following high intensity focused ultrasound ablation for atrial fibrillation. J Card Surg. 2014;29:101-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 47. | Klinkenberg TJ, Ahmed S, Ten Hagen A, Wiesfeld AC, Tan ES, Zijlstra F, Van Gelder IC. Feasibility and outcome of epicardial pulmonary vein isolation for lone atrial fibrillation using minimal invasive surgery and high intensity focused ultrasound. Europace. 2009;11:1624-1631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 48. | Schmidt B, Chun KR, Metzner A, Fuernkranz A, Ouyang F, Kuck KH. Pulmonary vein isolation with high-intensity focused ultrasound: results from the HIFU 12F study. Europace. 2009;11:1281-1288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 49. | Gal P, Smit JJ, Adiyaman A, Ramdat Misier AR, Delnoy PP, Elvan A. First Dutch experience with the endoscopic laser balloon ablation system for the treatment of atrial fibrillation. Neth Heart J. 2015;23:96-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 50. | Šedivá L, Petrů J, Škoda J, Janotka M, Chovanec M, Reddy V, Neužil P. Visually guided laser ablation: a single-centre long-term experience. Europace. 2014;16:1746-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 51. | Hamman BL, Theologes TT. Surgical treatment of atrial fibrillation with diode-pumped laser. Proc (Bayl Univ Med Cent). 2009;22:230-233. [PubMed] |
| 52. | Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, Kim YH, Klein G, Natale A, Packer D. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:32-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1367] [Cited by in RCA: 1445] [Article Influence: 90.3] [Reference Citation Analysis (0)] |
| 53. | Wang PJ. Hybrid epicardial and endocardial ablation of atrial fibrillation: is ablation on two sides of the atrial wall better than one? J Am Heart Assoc. 2015;4:e001893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 54. | Gaita F, Ebrille E, Scaglione M, Caponi D, Garberoglio L, Vivalda L, Barbone A, Gallotti R. Very long-term results of surgical and transcatheter ablation of long-standing persistent atrial fibrillation. Ann Thorac Surg. 2013;96:1273-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 55. | Kumar N, Pison L, La Meir M, Maessen J, Crijns HJ. Hybrid approach to atrial fibrillation ablation using bipolar radiofrequency devices epicardially and cryoballoon endocardially. Interact Cardiovasc Thorac Surg. 2014;19:590-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 56. | Bulava A, Mokracek A, Hanis J, Kurfirst V, Eisenberger M, Pesl L. Sequential hybrid procedure for persistent atrial fibrillation. J Am Heart Assoc. 2015;4:e001754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 57. | Je HG, Shuman DJ, Ad N. A systematic review of minimally invasive surgical treatment for atrial fibrillation: a comparison of the Cox-Maze procedure, beating-heart epicardial ablation, and the hybrid procedure on safety and efficacy. Eur J Cardiothorac Surg. 2015;48:531-540; discussion 540-541. [PubMed] |
| 58. | Von Oppell UO, Dimitrakakis G. eComment. atrial fibrillation ablation - are we approaching an equivalent standard of cure? Interact Cardiovasc Thorac Surg. 2012;14:450-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 59. | Phan K, Xie A, Tian DH, Shaikhrezai K, Yan TD. Systematic review and meta-analysis of surgical ablation for atrial fibrillation during mitral valve surgery. Ann Cardiothorac Surg. 2014;3:3-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 60. | Gillinov AM, Gelijns AC, Parides MK, DeRose JJ, Moskowitz AJ, Voisine P, Ailawadi G, Bouchard D, Smith PK, Mack MJ. Surgical ablation of atrial fibrillation during mitral-valve surgery. N Engl J Med. 2015;372:1399-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 354] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 61. | Chevalier P, Leizorovicz A, Maureira P, Carteaux JP, Corbineau H, Caus T, DeBreyne B, Mabot P, Dechillou C, Deharo JC. Left atrial radiofrequency ablation during mitral valve surgery: a prospective randomized multicentre study (SAFIR). Arch Cardiovasc Dis. 2009;102:769-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 62. | Veasey RA, Segal OR, Large JK, Lewis ME, Trivedi UH, Cohen AS, Hyde JA, Sulke AN. The efficacy of intraoperative atrial radiofrequency ablation for atrial fibrillation during concomitant cardiac surgery-the Surgical Atrial Fibrillation Suppression (SAFS) Study. J Interv Card Electrophysiol. 2011;32:29-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 63. | Michael Gray R, Nagendran M, Maruthappu M. Is it safe to stop anticoagulants after successful surgery for atrial fibrillation? Interact Cardiovasc Thorac Surg. 2011;13:642-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 64. | Shemin RJ, Cox JL, Gillinov AM, Blackstone EH, Bridges CR. Guidelines for reporting data and outcomes for the surgical treatment of atrial fibrillation. Ann Thorac Surg. 2007;83:1225-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 93] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 65. | Harling L, Athanasiou T, Ashrafian H, Nowell J, Kourliouros A. Strategies in the surgical management of atrial fibrillation. Cardiol Res Pract. 2011;2011:439312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |