Published online Sep 26, 2015. doi: 10.4330/wjc.v7.i9.571
Peer-review started: April 3, 2015
First decision: April 28, 2015
Revised: July 30, 2015
Accepted: August 20, 2015
Article in press: August 21, 2015
Published online: September 26, 2015
Processing time: 170 Days and 9.9 Hours
AIM: To investigate the contribution of anti-platelet therapy and derangements of pre-operative classical coagulation and thromboelastometry parameters to major bleeding post-coronary artery bypass grafting (CABG).
METHODS: Two groups of CABG patients were studied: Group A, treated with aspirin alone (n = 50), and Group B treated with aspirin and clopidogrel (n = 50). Both had similar preoperative, clinical, biologic characteristics and operative management. Classic coagulation parameters and rotational thromboelastometry (ROTEM) profiles were determined preoperatively for both groups and the same heparin treatment was administered. ROTEM profiles (INTEM and EXTEM assays) were analyzed, both for traditional parameters, and thrombin generation potential, expressed by area-under-curve (AUC).
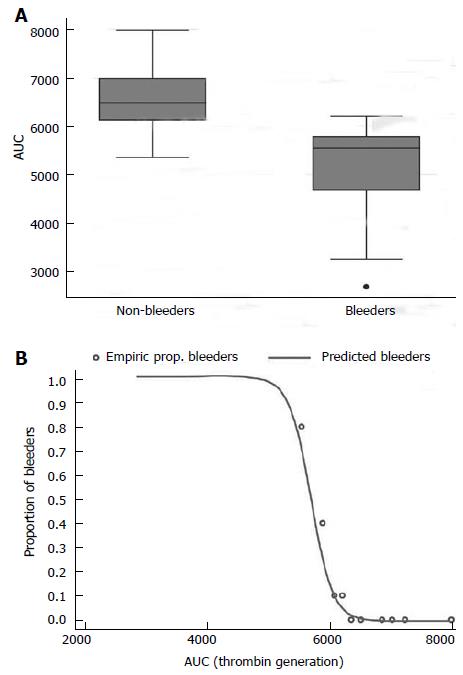
RESULTS: There was no significant difference between rates of major bleeding between patients treated with aspirin alone, compared with those treated with aspirin and clopidogrel (12% vs 16%, P = 0.77). In the 14 cases of major bleeding, pre-operative classic coagulation and traditional ROTEM parameters were comparable. Conversely we observed that the AUC in the EXTEM test was significantly lower in bleeders (5030 ± 1115 Ohm*min) than non-bleeders (6568 ± 548 Ohm*min) (P < 0.0001).
CONCLUSION: We observed that patients with a low AUC value were at a significantly higher risk of bleeding compared to patients with higher AUC, regardless of antiplatelet treatment. This suggests that thrombin generation potential, irrespective of the degree of platelet inhibition, correlates with surgical bleeding.
Core tip: To establish the timing of discontinuation of double antiplatelet therapy before coronary artery bypass grafting (CABG), it is crucial to identify predictors of bleeding. We analysed preoperatively classic parameters and thromboelastometry on 100 patients operated for CABG after presenting with acute coronary syndrome, to investigate the contribution of anti-platelet therapy and derangements of pre-operative coagulation status to major bleeding post-CABG. We observed that patients with a low area-under-curve (AUC) value in EXTEM were at a significantly higher risk of bleeding compared to patients with higher AUC, regardless of anti-platelet treatment. This suggests that thrombin generation potential, irrespective of the degree of platelet inhibition, correlates with surgical bleeding.
-
Citation: Tarzia V, Bortolussi G, Buratto E, Paolini C, Lin CD, Rizzoli G, Bottio T, Gerosa G. Single
vs double antiplatelet therapy in acute coronary syndrome: Predictors of bleeding after coronary artery bypass grafting. World J Cardiol 2015; 7(9): 571-578 - URL: https://www.wjgnet.com/1949-8462/full/v7/i9/571.htm
- DOI: https://dx.doi.org/10.4330/wjc.v7.i9.571
Post-operative bleeding is a major complication following coronary artery bypass grafting (CABG), with excessive bleeding occurring in 10% of patients, causing increased requirements of blood products, re-intervention and mortality[1-3]. Guidelines recommend ceasing clopidogrel therapy 5 d prior to CABG in order to minimise the risk of post-operative haemorrhage[4,5]. However, patients presenting for urgent and emergent CABG are often treated with double antiplatelet therapy, as recommended in major guidelines for the emergency management of acute coronary syndrome (ACS), prior to performing the diagnostic angiogram[6]. Ceasing clopidogrel and waiting the recommended 5 d prior to performing CABG would put these patients at higher risk of adverse coronary events[7]. Furthermore, as newer more potent platelet inhibitors such as ticagrelor and prasugrel become more widely used, patients presenting for CABG will increasingly be treated with these drugs[8-10]. As a result, it would be important to be able to detect patients at risk of excessive bleeding post-CABG, especially among those treated with double anti-platelet therapy. We devised the following study to determine whether bleeding risk could be predicted from the presence of double antiplatelet therapy, classical coagulation parameters or by rotational thrombo-elastometry performed prior to surgery.
Among 905 patients operated for CABG between January 2006 and December 2008, we selected those presenting with ACS, and prospectively enrolled 50 consecutive patients without pre-operative clopidogrel exposure (Group A = 50 pts) and 50 consecutive patients with preoperative clopidogrel exposure within two days prior to intervention (Group B = 50 pts), who fulfilled inclusion criteria. The decision to stop double-antiplatelet therapy was at the discretion of the treating cardiologist. All patients signed informed consent for this observational study on prospectively collected data.
All patients who, within 2 d of surgery, were either on a daily oral regimen of 75 mg of clopidogrel or received a 300 mg oral loading dose prior to percutaneous coronary intervention (PCI), made up the clopidogrel study group. They were compared with a control-group of patients who had no clopidogrel exposure. Both patient groups received aspirin (100 mg) and low-molecular-weight-heparin (nadroparin calcium) prior to surgery. Patients with a history of previous cardiac surgery, concomitant valvular surgery or preoperative exposure to either warfarin or platelet glycoprotein IIb/IIIa inhibitors were excluded.
Recognized risk factors for perioperative bleeding in cardiac surgery were assessed including advanced age, female gender, low weight and renal insufficiency (Table 1).
| Group AASA | Group BASA + Clopidogrel | P | |
| Age | 67 ± 9.4 | 66.3 ± 10.1 | 0.71 (T-t) |
| Gender (M/F) | 42/8 | 45/5 | 0.46 (P-χ2-t) |
| Weight (kg) | 75.2 ± 13 | 76.4 ± 10.3 | 0.60 (T-t) |
| Hypertension | 46/50 (92%) | 50/50 (100%) | 0.11 (F-t) |
| Dyslipidaemia | 45/50 (90%) | 45/50 (90%) | 1 (F-t) |
| Diabetes | 14/50 (28%) | 18/50 (36%) | 0.39 (P-χ2-t) |
| COPD | 12/50 (24%) | 8/50 (16%) | 0.45 (F-t) |
| Renal failure | 7/50 (14%) | 4/50 (8%) | 0.33 (P-χ2-t) |
| History of MI | 28/50 (56%) | 29/50 (58%) | 0.5 (F-t) |
| History of CVA | 4/50 (8%) | 1/50 (2%) | 0.36 (F-t) |
| Urgent surgery | 22/50 (44%) | 25/50 (50%) | 0.34 (F-t) |
| EF% | 56 ± 8.5 | 50 ± 9.5 | 0.04 (KW)/0.03 (LR) |
| LVEDV mL/m2 | 60.8 ± 10.4 | 62.6 ± 12.5 | 0.54 (KW) |
| No. of anastomoses | 2.5 ± 0.8 | 2.5 ± 0.7 | 0.99 (P-χ2-t) |
| No. of grafts | 2.5 ± 0.8 | 2.4 ± 0.7 | 0.99 (P-χ2-t) |
Baseline haematocrit, platelet counts, prothrombin time (PT) and activated partial thromboplastin time (aPTT) were assessed because of their influence on blood product transfusions.
Thromboelastometry was performed on fresh blood within 2 h of being drawn from patients prior to induction of anaesthesia, with a ROTEM® (Tem International GmbH, München, Germany) coagulation analyzer, according to the standard protocols supplied by the manufacturer[11].
Prior to analysis, the samples were stored at room temperature. The sample tubes were gently inverted five times to re-suspend any sedimentation before pipetting the blood. Two standard ROTEM® assays named INTEM® and EXTEM® were performed. INTE® and EXTEM® (ellagic acid and tissue factor activation, respectively) represent assays in which the intrinsic and the extrinsic coagulation pathways are triggered, respectively. The ROTEM® method defines various parameters: Clotting time (CT, s) the time from the beginning of the coagulation analysis until an increase in amplitude of 2 mm, which reflects the initiation phase of the clotting process. Clot formation time (CFT, s) the time between an increase in amplitude of the thrombelastogram from 2 to 20 mm. Alpha-angle (°), the tangent to the clotting curve through the 2 mm point. The CFT and alpha-angle reflect measures of the propagation phase of WB clot formation. Maximum clot firmness (MCF, mm) is the maximum amplitude reached in thromboelastogram and correlates with the platelet count and function as well as the concentration of fibrinogen[12]. Area-under-curve (AUC), defined as the area under 1st derivative (i.e., velocity) curve ending at a time point that corresponds to MCF, reflects thrombin generation potential[13].
All surgical procedures were performed through a median sternotomy and with cardiopulmonary-bypass (CPB), in a standard fashion. A comparable intraoperative heparin anticoagulation regimen was utilized in all patients; initial heparin dose was calculated using a minimum standard of 400 units/kg with additional dosing administered during the procedure, in order to maintain a target activated-clotting-time (ACT) value greater than 480 s.
Post-operative bleeding was carefully managed according to our institution’s diagnostic - therapeutic algorithm[14]. PT, aPTT, platelet count, antithrombin III and ROTEM paramaters (INTEM, EXTEM and HEPTEM) are assessed and any abnormalities are corrected with relevant blood products (platelets, fresh frozen plasma, cryoprecipitate), protamine sulphate, fibrinogen or recombinant factor VIIa according to the underlying cause of bleeding.
Ongoing bleeding at a rate of 500 mL/h in the first hour, 400 mL/h in the first 2 h, 300 mL/h in the first 3 h, 200 mL/h in the first 4 h, despite optimisation of coagulation paramaters or the presence of cardiac tamponade are considered indications for re-intervention for bleeding. The amount of post-operative blood loss and the rate and amount of transfused blood products used both intra- and post-operatively were recorded.
Major bleeding was considered to have occurred when ≥ 3 units of blood, fresh frozen plasma, platelets or surgical revision were required. Chest tube outputs assessed at 12 h were the primary measure of postoperative bleeding. Transfusion quantity was recorded for the three main blood product types (red blood cells, platelets and fresh frozen plasma) during operation and ICU stay. Clinical outcomes specific to CABG recovery included reoperation for bleeding, mortality, acute myocardial infarction, stroke and postoperative atrial fibrillation until discharge. General post-surgical outcomes evaluated were duration of intubation and postoperative length of ICU stay.
The prevalence of bleeding-related haematologic, laboratory and clinical risk factors in the two groups was compared by means of contingency tables of categorical variables and the two-sided Fisher exact test. Two sample t tests with equal or unequal variances were used to compare continuous variables with reasonably normal distribution of the original or transformed units, otherwise Kruskal-Wallis equality-of-populations rank test was used. Distributional differences were further analyzed with Graphic Box plot comparisons between groups. Multivariable stepwise forward and backward logistic regression of the bleeding event vs risk factors was made, with 0.05 significance limits to enter or retain. Confounding factors (age, weight and sex), bleeding related clinical risk factors (hypertension, renal failure, cerebrovascular events and treatment) and the variables enumerated above in the clotting profile paragraph were included. The event probability predicted from the logistic result was compared to the observed empiric probability, calculated on patients’ deciles. A correlation analysis of laboratory ROTEM tests was further performed to identify parameters closely related to the logistic result. All data were manipulated and analyzed using STATA (StataCorp LP, Texas, United States). The statistical review of the study was performed by a biomedical statistician.
The baseline characteristics of those with and without preoperative clopidogrel exposure were comparable in age, gender, weight and renal failure (Table 1). There was a significantly higher incidence of ventricular dysfunction (P = 0.02) in the clopidogrel group. The baseline classic coagulation parameters (hematocrit, platelet count, PT, aPTT) and ROTEM parameters (CT, CFT, MCF, alpha angle and AUC of INTEM and EXTEM) were also comparable between the groups (Table 2).
| Group AASA | Group BASA + Clopidogrel | P (KW) | |
| Hb (g/dL) | 13.44 ± 0.2 | 13.42 ± 0.2 | 0.95 |
| Hematocrit (%) | 39.9 ± 0.6 | 39.3 ± 0.5 | 0.67 |
| Platelet count (103/mm3) | 247 ± 8.6 | 245 ± 0.2 | 0.83 |
| PT (%) | 80.3 ± 1.6 | 80.5 ± 1.7 | 0.95 |
| PTT (%) | 32.2 ± 0.7 | 31.8 ± 0.8 | 0.67 |
| CT-INTEM | 153 ± 16 | 179 ± 15 | 0.87 |
| CFT-INTEM | 57 ± 4 | 63 ± 7 | 0.28 |
| Alpha angle-INTEM | 81 ± 10 | 75 ± 8 | 0.49 |
| MCF-INTEM | 65 ± 4 | 63 ± 5 | 0.51 |
| AUC-INTEM | 6543 ± 520 | 6320 ± 731 | 0.65 |
| CT-EXTEM | 55 ± 6 | 58 ± 7 | 0.52 |
| CFT-EXTEM | 96 ± 8 | 102 ± 9 | 0.51 |
| Alpha angle-INTEM | 71 ± 6 | 66 ± 5 | 0.33 |
| MCF-EXTEM | 65 ± 6 | 61 ± 9 | 0.057 |
| AUC-EXTEM | 6529 ± 643 | 6177 ± 978 | 0.056 |
No statistically significant differences between the two groups in term of postoperative bleeding, blood product transfusions (Table 3) and clinical outcomes (Table 4) were observed.
| Group AASA | Group BASA + Clopidogrel | P | |
| Major bleeding | 6 (12%) | 8 (16%) | 0.77 (F-t) |
| Re-intervention | 1 (2%) | 0 (0%) | 1.0 (F-t) |
| Chest tube output (12 h) | 509 ± 234 | 539 ± 239 | 0.41 (KW) |
| PRBCs (U) | 1 ± 1 | 0.9 ± 0.9 | 0.87 (KW) |
| FFP (U) | 0.5 ± 2 | 0.5 ± 1.9 | 0.99 (KW) |
| PLTs (U) | 0.7 ± 2.7 | 1 ± 2.5 | 0.35 (KW) |
| Group AASA | Group BASA + Clopidogrel | P | |
| AMI | 1 (2%) | 0% | 0.5 (F-t) |
| IABP | 3 (6%) | 2 (4%) | 0.5 (F-t) |
| Stroke | 1 (2%) | 0 (0%) | 0.5 (F-t) |
| Renal failure | 1 (2%) | 1 (2%) | 1.0 (F-t) |
| AF | 12 (24%) | 7 (14%) | 0.15 (F-t) |
| MV (h) | 8 ± 11 | 6 ± 2 | 0.22 (KW) |
| ICU stay (d) | 1.8 ± 3.3 (Med 1) | 1.4 ± 0.9 (Med 1) | 0.77 (KW) |
| Mortality | 1 (2%) | 0 (0%) | 0.5 (F-t) |
| MACE (death, stroke. AMI) | 3 (6%) | 0 (0%) | 0.24 (F-t) |
Patients on double treatment showed a greater frequency of major bleeding (8 vs 6 episodes), greater mean chest tube output at 12 h (539 ± 239 mL vs 509 ± 234 mL), and mean number of blood products transfusions, although these differences were not statistically significant. One patient in the single treatment group required surgical re-intervention due to bleeding.
The length of stay in the ICU (days) and of mechanical ventilation (hours) were comparable between the groups. There was 1 death in group A and 0 in group B, 1 myocardial-infarction in group A and 0 in group B, 1 stroke in group A and 0 in group B. Therefore there was a greater number of major adverse events in the group treated with aspirin alone (3 events, 6%; OR = 0.75; P = 0.24) compared with the double treatment group (0 events, 0%).
From the comparison of the risk-factors for perioperative bleeding (advanced age, female gender, low weight and renal insufficiency), and preoperative haemocoagulatory status between all patients who bled (n = 14) and those who did not, no significant difference was noted. After performing a logistic multivariate analysis (details in Table 5), the backward stepwise variable selection showed a significant independent relationship of bleeding risk only with AUC activity (coefficient –0.0099; OR = 0.99; 95%CI: 0.98-0.99; P < 0.001). In fact, patients who bled had lower AUC-value (Thrombin-generation-potential) in the EXTEM test (5030 ± 1115 Ohm*min) than those who did not bleed (6568 ± 548 Ohm*min) (P < 0.0001) (Figure 1A). We observed a good correlation (r = 0.96) between AUC and MCF, a clot quality indicator, that similarly was increased in patients who did not bleed (65.7 ± 5.6 mm) than those who did (51.9 ± 12.5 mm) (P < 0.0001), although these values were still within the normal range (50-72 mm). At this point we identified a threshold value for AUC and MCF which predicted bleeding in our patients, that is 6000 for AUC and 60 for MCF (Figure 1B). In fact, all patients with an AUC and MCF below these cut off values were at increased risk of major bleeding, and the lower these values the greater the risk. Furthermore patients with higher AUC and MCF values were at a lower risk of significant haemorrhagic events.
| Coefficient | OR | 95%CI | P | |
| Age | -0.14 | 0.87 | 0.73-1.03 | 0.11 |
| Weight | -0.35 | 0.71 | 0.52-0.97 | 0.03 |
| Gender (female) | -10.54 | 0.00003 | 0.06-3.9 | 0.04 |
| EF | -0.02 | 0.98 | 0.83-1.16 | 0.81 |
| AUC EXTEM | -0.0099 | 0.99 | 0.98-0.99 | 0.008 |
| Aspirin only | -1.18 | 0.31 | 0.02-4.58 | 0.39 |
Post-CABG bleeding is a major issue in cardiac surgery and remains difficult to predict. Concerns about bleeding risk have resulted in the recommendation to cease double antiplatelet therapy prior to elective CABG[5]. There are still many patients who present for urgent CABG with double antiplatelet therapy, and increasingly patients will have been exposed to more potent antiplatelet agents such as prasugrel and ticagrelor[8-10]. Hence, we set out to determine if it was possible to predict post-operative bleeding with pre-operative clotting characteristics via analysis of classical coagulation assays (aPTT, PT) as well as a suite of ROTEM analyses. We also compared haemorrhagic and thrombotic complications between single and double treated patients.
Our data showed a higher frequency of major bleeding in the double antiplatelet therapy group (16% vs 12%), but this was not statistically significant (P = 0.77). However, assuming that it represents a true difference, to attain a P value of 0.05 with a test power of 70% the sample size required is 978 patients on each arm. On the contrary the major adverse events (MACE: Death, stroke and myocardial infarct) were limited to group A (6%) (Table 4), but this difference was not statistically significant (P = 0.24).
Comparison of classic and ROTEM parameters between the 14 “bleeder” patients and the “non-bleeders”, showed that differences were limited to AUC and MCF, in the EXTEM test. Patients with lower AUC and MCF values were at a higher risk of suffering major bleeding, regardless of whether they were treated with clopidogrel or not. MCF reveals the quality of the clot and is linked to thrombin generation and the change in AUC observed here is a reflection of the increase in MCF.
Clot generation is a composite process involving both primary and secondary haemostasis, as well as intrinsic and extrinsic pathways. Yet bleeding risk after CABG, the tendency has been to focus on the platelet, probably because of its major role in atherothrombosis[15,16]. And the extensive experience with the use of antiplatelet agents in minimising further ischemic events. Nevertheless, despite receiving the same standard ACS medical treatment some patients bleed while others do not, and although this may be attributed to the recognized response variability (so called “resistance”) observed with clopidogrel or aspirin[15], our results indicate that differences in patients’ thrombin generation potential may be an important determinant of bleeding.
We believe that a broader perspective may be necessary: Platelets and platelet-inhibition play very important roles but their complicate interplay with other pieces of the haemostatic process should be taken into account. ROTEM is appropriate for providing such analyses, and unique in allowing evaluation of every element of thrombosis and clot lysis[17], especially fibrinogen and factor XIII.
Indeed, we found that clot quality is a predictor of post-operative bleeding. We believe that association of MCF and AUC with haemorrhagic risk derives from the role of thrombin, which is fundamental as it affects every step of clot formation: It has a role in activation of fibrinogen to fibrin as the final common pathway from both intrinsic and extrinsic pathways, it activates factor XIII which promotes clot stability by cross linking fibrin polymers within the clot[18] and contributes to platelet activation, acting on at least three diverse platelet receptors[19]. We propose that in post surgical patients a high clot quality prior to surgery, as indicated by higher MCF and AUC values in the EXTEM test, can effectively clot despite the inhibition of platelet activation by aspirin and clopidogrel.
The mechanism by which patients with greater thrombin generation potential are able to form effective clots in the face of double antiplatelet therapy are probably twofold: (1) platelet activation is multifactorial, in addition to adenosine diphosphate (ADP) and thromboxane A2, thrombin also plays a critical role and is the most potent of the platelet activators[15], and patients who are better able to generate thrombin may thus sufficiently activate their platelets despite inhibition of the first two pathways by clopidogrel and aspirin respectively; and (2) patients with a higher MCF and hence higher thrombin generation may be able to affect greater thrombin deposition and stabilisation by factor XIII and hence form a stable clot despite decreased platelet activation and aggregation. In such circumstance the metaphor would be to a brick wall: Despite the presence of fewer effective bricks (platelets) in the presence of clopidogrel therapy, patients with a higher MCF can counterbalance by applying more higher-quality mortar (thrombin), thus producing a stable wall (clot).
Besides characterizing MCF and AUC as factors influencing post-surgical bleeding, we identified a threshold at which the risk becomes significant: an AUC < 6000, corresponding to an MCF of < 60. This is important in two ways: (1) these values are within the normal range and yet the patients are at high risk of post-operative bleeding; and (2) identification of patients at risk may allow improved management in these patients.
While rotational thromboelastometry has been previously studied in relation to post CPB haemorrhage it has demonstrated mixed results. Published studies generally show that ROTEM parameters obtained intra-operatively or-post operative can predict post-operative excessive blood loss, but not those obtained prior to commencing bypass[20-23]. Our results differ from these as we have seen that ROTEM analysis performed prior to the institution of CPB can predict post-operative bleeding. This difference is difficult to explain, but may be related to the use of different ROTEM parameters, the high proportion of patients treated with clopidogrel in our cohort and differing definitions of major post-operative bleeding amongst these studies.
The capacity to recognize patients at high risk of post-operative bleeding may influence the decision to perform CABG in the first instance and further permit adjustment of surgical procedure and post-operative management to reduce the risk of haemorrhage in patients who are treated with CABG. As an example, patients at a high risk of bleeding may be treated with off pump CABG, avoiding CPB and hence lowering the risk of haemorrhagic events[24]. This is extremely important as CPB results in coagulopathy due to activation and consumption of platelets and coagulation factors, such as thrombin, which results in an increased risk of post-operative bleeding[25]. Other management options would include a more targeted use of blood product derived coagulation factor infusions, as well as the potential development of new pharmacological interventions involving replacement of individual clotting factors, such as recombinant activated factor VII, and factor XIII, prothrombin complexes and fibrinogen.
On the other hand, patients with higher thrombin generation potential may represent a subgroup more prone to develop ischemic rather than hemorrhagic complications (myocardial infarction, recurrent angina, stroke), thus deserving double antiplatelet therapy[26].
While these results are promising for the determination of risk of post-operative bleeding in CABG patients, the study does have several limitations. While AUC and MCF were seen to correlate well with bleeding risk in EXTEM analysis, INTEM analysis did not demonstrate such a relationship. This may result from the use of LWMH in all patients, a factor which affects the intrinsic pathway and hence INTEM analyses. Furthermore, ROTEM, while giving a good overall impression of a patient’s haemostatic function, is not the ideal tool for the assessment of platelets, rather a platelet function analyzer would be the gold standard for such analyses[13], which would have allowed better determination of the contribution of antiplatelet response variability to bleeding risk in our cohort[27]. Additionally the sample size in this study was small and the patients were not randomised to the two treatment arms, thus limiting our ability to comment on the differential effect of single and double antiplatelet on postoperative haemorrhage risk. Further studies in groups not treated with LMWH, the use of platelet function analyser and greater sample size and randomization would allow greater understanding of the factors relating to bleeding risk identified in our study. Finally, we cannot generalize our results to newer antiplatelet agents such as prasugrel and ticagrelor.
In conclusion, we have seen that patients with a low AUC value (thrombin-generation-potential) are at a significantly higher risk of bleeding as compared to patients with higher AUC, regardless of whether they were treated with clopidogrel. Hence this study provides a promising insight into the potential role of ROTEM analyses in the prediction of post-CABG bleeding risk; future research on this topic may contribute to a more effective intra- and post- operative management.
Post-operative bleeding is a major complication following coronary artery bypass grafting (CABG). Guidelines recommend ceasing clopidogrel therapy 5 d prior to CABG in order to minimise the risk of post-operative haemorrhage. However, patients presenting for urgent and emergent CABG are often treated with double antiplatelet therapy, as recommended in major guidelines for the emergency management of acute coronary syndrome, prior to performing the diagnostic angiogram. Ceasing clopidogrel and waiting the recommended 5 d prior to performing CABG would put these patients at higher risk of adverse coronary events.
As yet there is no effective way to predict which patients will have significant bleeding after CABG. In fact, it would be important to be able to detect patients at risk of excessive bleeding, especially among those treated with double anti-platelet therapy.
While rotational thromboelastometry has been previously studied in relation to post cardiopulmonary-bypass haemorrhage it has demonstrated mixed results. Published studies generally show that rotational thromboelastometry (ROTEM) parameters obtained intra-operatively or-post operative can predict post-operative excessive blood loss, but not those obtained prior to commencing bypass. The authors devised the following study to determine whether bleeding risk could be predicted from the presence of double antiplatelet therapy, classical coagulation parameters or by rotational thrombo-elastometry performed prior to surgery. The authors found that there was no significant difference between rates of major bleeding between patients treated with aspirin alone, compared with those treated with aspirin and clopidogrel. In the cases of major bleeding, pre-operative classic coagulation and traditional ROTEM parameters were comparable. Conversely the authors observed that the area-under-curve in the EXTEM test was significantly lower in bleeders than non-bleeders, regardless of antiplatelet treatment. This suggests that thrombin generation potential, irrespective of the degree of platelet inhibition, correlates with surgical bleeding. Moreover, the authors were able to define a threshold at which bleeding risk becomes significant.
The ability to identify patients at high risk of post-operative bleeding is important as it may influence the decision to perform CABG in the first instance and further allow tailoring of surgical technique and post-operative management to reduce the risk of haemorrhage, and achieve a more targeted use of blood products. On the other hand, patients with higher thrombin generation potential may represent a subgroup more prone to develop ischemic rather than hemorrhagic complications, thus deserving double antiplatelet therapy.
ROTEM consists in a viscoelastic method for hemostasis testing in whole blood, which can be used to detect clotting disorders and drug effects. Moreover, through appropriate assays, it can provide differential diagnostic information to support decisions in therapy.
This is an interesting manuscript about the predictive factors of bleeding after CABG in patients with previous acute coronary syndrome.
P- Reviewer: De Ponti R, Falconi M, Lazzeri C, Nunez-Gil IJ, Ueda H S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Besser MW, Klein AA. The coagulopathy of cardiopulmonary bypass. Crit Rev Clin Lab Sci. 2010;47:197-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 2. | Despotis G, Eby C, Lublin DM. A review of transfusion risks and optimal management of perioperative bleeding with cardiac surgery. Transfusion. 2008;48:2S-30S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Berger JS, Frye CB, Harshaw Q, Edwards FH, Steinhubl SR, Becker RC. Impact of clopidogrel in patients with acute coronary syndromes requiring coronary artery bypass surgery: a multicenter analysis. J Am Coll Cardiol. 2008;52:1693-1701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 146] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 4. | Dunning J, Versteegh M, Fabbri A, Pavie A, Kolh P, Lockowandt U, Nashef SA. Guideline on antiplatelet and anticoagulation management in cardiac surgery. Eur J Cardiothorac Surg. 2008;34:73-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 224] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 5. | Eagle KA, Guyton RA, Davidoff R, Edwards FH, Ewy GA, Gardner TJ, Hart JC, Herrmann HC, Hillis LD, Hutter AM. ACC/AHA 2004 guideline update for coronary artery bypass graft surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1999 Guidelines for Coronary Artery Bypass Graft Surgery). Circulation. 2004;110:e340-e437. [PubMed] |
| 6. | O’Connor RE, Bossaert L, Arntz HR, Brooks SC, Diercks D, Feitosa-Filho G, Nolan JP, Vanden Hoek TL, Walters DL, Wong A. Part 9: Acute coronary syndromes: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation. 2010;122:S422-S465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 7. | Akowuah E, Shrivastava V, Jamnadas B, Hopkinson D, Sarkar P, Storey R, Braidley P, Cooper G. Comparison of two strategies for the management of antiplatelet therapy during urgent surgery. Ann Thorac Surg. 2005;80:149-152. [PubMed] |
| 8. | Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 9. | Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, De Servi S, Murphy SA. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001-2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 10. | Fitchett D, Mazer CD, Eikelboom J, Verma S. Antiplatelet therapy and cardiac surgery: review of recent evidence and clinical implications. Can J Cardiol. 2013;29:1042-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Calatzis AN, Fritzsche P, Calatzis AL, Kling M, Hipp R, Stemberger A. A comparison of the technical principle of the roTEG coagulation analyzer and conventional thrombelastography system. Ann Hematol. 1996;72:87. |
| 12. | Zuckerman L, Cohen E, Vagher JP, Woodward E, Caprini JA. Comparison of thrombelastography with common coagulation tests. Thromb Haemost. 1981;46:752-756. [PubMed] |
| 13. | Sørensen B, Johansen P, Christiansen K, Woelke M, Ingerslev J. Whole blood coagulation thrombelastographic profiles employing minimal tissue factor activation. J Thromb Haemost. 2003;1:551-558. [PubMed] |
| 14. | Tarzia V, Bottio T, Buratto E, Spiezia L, Simioni P, Gerosa G. The hazard of comparing apples and oranges: the proper indication for the use of recombinant activated clotting factor VII in cardiac surgery. J Thorac Cardiovasc Surg. 2011;142:1588-1589; author reply 1589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Angiolillo DJ, Ueno M, Goto S. Basic principles of platelet biology and clinical implications. Circ J. 2010;74:597-607. [PubMed] |
| 16. | Jennings LK. Mechanisms of platelet activation: need for new strategies to protect against platelet-mediated atherothrombosis. Thromb Haemost. 2009;102:248-257. [PubMed] |
| 17. | Yürekli BP, Ozcebe OI, Kirazli S, Gürlek A. Global assessment of the coagulation status in type 2 diabetes mellitus using rotation thromboelastography. Blood Coagul Fibrinolysis. 2006;17:545-549. [PubMed] |
| 18. | Tanaka KA, Key NS, Levy JH. Blood coagulation: hemostasis and thrombin regulation. Anesth Analg. 2009;108:1433-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 218] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 19. | Lova P, Canobbio I, Guidetti GF, Balduini C, Torti M. Thrombin induces platelet activation in the absence of functional protease activated receptors 1 and 4 and glycoprotein Ib-IX-V. Cell Signal. 2010;22:1681-1687. [PubMed] |
| 20. | Wang JS, Lin CY, Hung WT, O’Connor MF, Thisted RA, Lee BK, Karp RB, Yang MW. Thromboelastogram fails to predict postoperative hemorrhage in cardiac patients. Ann Thorac Surg. 1992;53:435-439. [PubMed] |
| 21. | Wasowicz M, McCluskey SA, Wijeysundera DN, Yau TM, Meinri M, Beattie WS, Karkouti K. The incremental value of thrombelastography for prediction of excessive blood loss after cardiac surgery: an observational study. Anesth Analg. 2010;111:331-338. [PubMed] |
| 22. | Lee GC, Kicza AM, Liu KY, Nyman CB, Kaufman RM, Body SC. Does rotational thromboelastometry (ROTEM) improve prediction of bleeding after cardiac surgery? Anesth Analg. 2012;115:499-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Cammerer U, Dietrich W, Rampf T, Braun SL, Richter JA. The predictive value of modified computerized thromboelastography and platelet function analysis for postoperative blood loss in routine cardiac surgery. Anesth Analg. 2003;96:51-57, table of contents. [PubMed] |
| 24. | Shim JK, Choi YS, Oh YJ, Bang SO, Yoo KJ, Kwak YL. Effects of preoperative aspirin and clopidogrel therapy on perioperative blood loss and blood transfusion requirements in patients undergoing off-pump coronary artery bypass graft surgery. J Thorac Cardiovasc Surg. 2007;134:59-64. [PubMed] |
| 25. | Karkouti K, McCluskey SA, Syed S, Pazaratz C, Poonawala H, Crowther MA. The influence of perioperative coagulation status on postoperative blood loss in complex cardiac surgery: a prospective observational study. Anesth Analg. 2010;110:1533-1540. [PubMed] |
| 26. | Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494-502. [PubMed] |
| 27. | Yu PJ, Cassiere HA, Dellis SL, Manetta F, Stein J, Hartman AR. P2Y12 platelet function assay for assessment of bleeding risk in coronary artery bypass grafting. J Card Surg. 2014;29:312-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |