Published online Sep 26, 2015. doi: 10.4330/wjc.v7.i9.525
Peer-review started: May 26, 2015
First decision: June 24, 2015
Revised: July 10, 2015
Accepted: July 24, 2015
Article in press: July 27, 2015
Published online: September 26, 2015
Processing time: 119 Days and 23.6 Hours
Physicians cannot rely solely on the angiographic appearance of epicardial coronary artery stenosis when evaluating patients with myocardial ischemia. Instead, sound knowledge of coronary vascular physiology and of the methods currently available for its characterization can improve the diagnostic and prognostic accuracy of invasive assessment of the coronary circulation, and help improve clinical decision-making. In this article we summarize the current methods available for a thorough assessment of coronary physiology.
Core tip: Assessment of the coronary circulation in the cathlab cannot be limited to angiography nowadays. The interventional cardiologist needs to be aware of current knowledge on coronary physiology and of the methods and measurements available for its characterization in clinical practice and research. In this article we review the main methods to assess the functional severity of coronary stenosis, myocardial blood flow, microvascular circulation, and endothelial function.
- Citation: Díez-delhoyo F, Gutiérrez-Ibañes E, Loughlin G, Sanz-Ruiz R, Vázquez-Álvarez ME, Sarnago-Cebada F, Angulo-Llanos R, Casado-Plasencia A, Elízaga J, Diáz FFA. Coronary physiology assessment in the catheterization laboratory. World J Cardiol 2015; 7(9): 525-538
- URL: https://www.wjgnet.com/1949-8462/full/v7/i9/525.htm
- DOI: https://dx.doi.org/10.4330/wjc.v7.i9.525
Physicians rely on angiography to assess the coronary vasculature of patients with symptoms of myocardial ischemia. However, angiography has a low interobserver agreement[1], and acknowledged limitations for the assessment of myocardial ischemia in a variety of settings, such as intermediate, eccentric or diffuse coronary stenosis[2]. Notably, intermediate lesions are the most frequently found in coronary angiography[3]. The importance of determining which lesions truly produce ischemia and thus require intervention is underscored by clinical trials showing that revascularization of non-ischemia inducing stable lesions does not improve patient outcomes, and may in fact be deleterious[4-6]. Also, a considerable percentage of patients referred to the catheterization laboratory for angina, or even myocardial infarction, have angiographically normal, or only mildly diseased, coronary arteries[7], which highlights the importance of factors beyond epicardial fixed stenosis in the development of myocardial ischemia. Among these factors, coronary microvascular disease and coronary tone dysregulation due to endothelial dysfunction are frequent causes of myocardial ischemia[8-10]. Furthermore, a normal coronary angiogram does not accurately predict prognosis in all patients, since patients with microvascular or endothelial dysfunction have an increased risk of major adverse cardiac events[11].
This article reviews the most important methods currently available to the invasive cardiologist for a comprehensive physiological assessment of the coronary circulation. After a brief reminder of the coronary structure and the physiology of flow regulation, we will discuss the contemporary methods used to assess coronary flow, flow reserve, epicardial stenosis, microvascular function, and, finally, endothelial function.
From a physiological perspective, the coronary circulation is structured in three main compartments. The first compartment (R1) is formed by the large epicardial coronary arteries, with a size over 500 μm. These are the conduction vessels, which offer minimal resistance to flow under normal conditions, accounting for less than 10% of the overall resistance of the coronary circulation. Accordingly, blood pressure remains unaltered along these vessels. The second compartment (R2) is formed by extramyocardial prearterioles, 100-500 μm in diameter. The third coronary compartment is formed by arterioles (< 100 μm) and capillary vessels. The second and third compartments, generically known as “microcirculation”, are accountable for over 90% of the total coronary resistance, and therefore are the main regulators of flow[3,12].
The microvascular compartments are responsible for coronary autoregulation of flow. Because myocardial extraction of oxygen is very high at rest, an increase in oxygen demands must be met with an increase in coronary blood flow. Intramyocardial arterioles respond to metabolic signals, directly diffused from the myocardium, with vasodilation or vasoconstriction. When arterioles relax to reduce resistance and increase myocardial flow, proximal prearterioles and large epicardial arteries respond with flow-mediated dilation, which happens mainly through the release of nitric oxide by the endothelium[12].
In pathological conditions, when a severe epicardial stenosis develops, epicardial resistance increases causing a pressure drop in the distal circulation. This is registered by the prearterioles, which respond with vasodilation to maintain a normal flow and pressure in the arteriolar compartment. It is through this autoregulation that the coronary circulation manages to maintain myocardial blood flow within a normal range in the face of moderate or even severe coronary atherosclerosis. It is also because of this mechanism that the functional significance of a coronary stenosis may be obscured to the interventional cardiologist at rest, and become evident only under conditions of maximal hyperemia.
There are currently two methods available for measuring coronary blood flow in clinical practice: Doppler velocity and thermodilution. Both methods require engaging the coronary artery with a guide catheter, and introducing an intracoronary diagnostic 0.014 wire in the vessel. Heparin must be administered before the procedure, at the same doses as used during percutaneous coronary intervention.
Coronary blood flow can be assessed by blood velocity measurement using an intracoronary Doppler wire (Flowire, Volcano Corp, San Diego, CA, United States)[13]. After engaging the coronary artery with a guiding catheter and administering heparin, the Doppler wire is positioned into the artery, usually at a proximal segment. Doppler-derived blood flow velocity is recorded in a dedicated console, and the average peak velocity (APV) is calculated with the use of integrated automatic software. Coronary flow is then estimated from the APV and the crossectional vessel area, 5 mm distal to the tip of the wire. The vessel area can be calculated from the angiographic vessel diameter, or directly measured by intravascular ultrasound or optical coherence tomography. Thus, the formula to calculate the coronary blood flow by Doppler is:
CBF = 0.5 x APV x (D2π)/4
where CBF is coronary blood flow (cm3/s); APV is average peak velocity (cm/s); and D is coronary diameter (cm).
Coronary blood flow can be estimated by the indicator dilution method, using an intracoronary thermodilution wire (PressureWire, St Jude Medical Inc.; St. Paul, MN, United States)[14]. This wire has two temperature sensors, located at its proximal and distal parts. The wire is introduced into the coronary artery, until the more distal sensor is at least 50 mm away from the catheter tip. A bolus of 3 mL of saline injected through the guiding catheter produces a change in temperature that is recorded by both sensors, and a thermodilution curve is recorded. This is repeated 3 times and the results are averaged. Flow is derived from the thermodilution formula:
CBF = V/Tmn
were CBF is coronary blood flow (cm3/s); V is vessel volume (cm3) between the injection site and measuring site; and Tmn is mean transit time (s), which is calculated by the system console from the thermodilution curve.
As can be appreciated, both methods for measuring coronary blood flow require estimation of the vessel crossectional area or the vessel internal volume, which introduces a source of inaccuracy, and limits their actual use in clinical practice. Fortunately, however, both methods are well suited for a simple and reliable estimation of the most important flow-derived measurement: coronary flow reserve.
Coronary flow reserve (CFR) is defined as the ratio between coronary blood flow at maximal hyperemia and at baseline condition[15]. It expresses the capacity of the coronary circulation to respond to a physiological increase in oxygen demands with a corresponding increase in blood flow. In animals and healthy subjects CFR is usually over 3, meaning their coronary circulation can triple the baseline flow when needed. In humans with chest pain and angiographically normal coronary arteries, however, the average CFR is lower, at 2.7 ± 0.6[16]. Therefore, a cutoff value of 2.0 has been widely accepted for CFR in the clinical practice[3]. Because it is a ratio of two flows, CFR is dimensionless.
Two different methods of assessing CFR invasively are available, based on the previously described systems for coronary flow measurement: Doppler and thermodilution. In both cases, flow determination is made at baseline, and then repeated under maximal hyperemia. Hyperemia can be achieved with several drugs, such as papaverine, nitroprusside or adenosine, but most often the latter is used. A complete description of the main substances used in the catheterization laboratory is shown in Table 1.
| Substance | Doses | Site of action | Endothelium response | Effect |
| Adenosine | Iv: 140 μg/kg per minute Ic: 20-150 μg bolus | Microvascular | Independent | Direct vasodilation |
| Acetylcholine | Ic: 10-6 M/10-5 M/ 10-4 M | Micro and macrovascular | Dependent | Vasodilation if normal endothelial function; vasoconstriction if endothelial dysfunction |
| Nitroglycerin | Ic: 200 μg bolus | Macrovascular | Independent | Vasodilation |
| Nitroprusside | Ic 0.3-0.9 μg/kg bolus | Micro and macrovascular | Independent | Vasodilation |
| Papaverine | Ic: 8-20 mg bolus | Micro and macrovascular | Independent | Enzyme Phosphodiesterase inhibition Vasodilation |
| Regadenoson | Iv: 400 μg bolus | Microvascular | Independent | Adenosine receptor agonist vasodilation |
Adenosine produces a direct, endothelium-independent vasodilation of the coronary microcirculation, while having no appreciable effect on the epicardial vessel. It can be administered intravenously, at a dose of 140 µg/kg per minute[17], which usually achieves maximal stable hyperemia in 2-3 min. A central vein or a large brachial one must be used for the drug to reach sufficient concentration in the coronary circulation. Alternatively, hyperemia can be achieved by a single intracoronary bolus of adenosine administered through the guiding catheter. Doses for intracoronary injection range from 30-60 μg in the left coronary artery, and 20-30 μg in the right[3]. Higher doses, however, have been shown to be safe[18,19].
CFR can be assessed by coronary Doppler, using the method described above for flow measurement[13]. After engaging the artery with a guiding catheter, the wire is advanced and positioned at a segment where a stable Doppler signal is obtained, away from a coronary stenosis and branch ostia. The wire must not move between baseline and hyperemia measurements. Thus, since adenosine does not induce epicardial vasodilation, and the wire position does not change throughout the procedure, the crossectional area of the vessel can be assumed constant and removed from the flow equation, which leaves a simplified formula for CFR estimation:
CFR = APVh/APVb
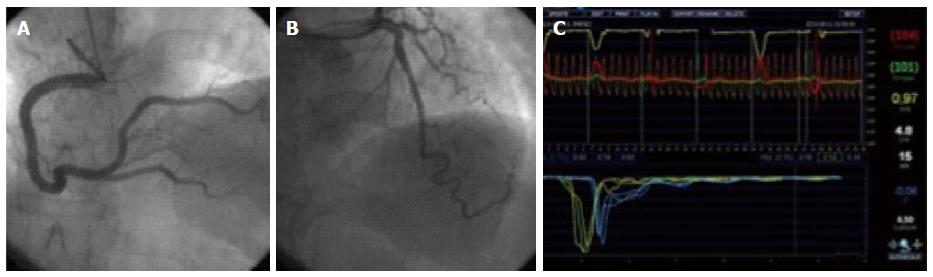
where APVh is average peak velocity (cm/s) during maximal hyperemia, and APVb is average peak velocity (cm/s) at baseline condition. Figure 1 shows an example of CFR measurement with intracoronary Doppler.
Similarly, CFR can be calculated with the use of the thermodilution technique, by comparing mean transit time between hyperemia and baseline[17]. The pressure-temperature wire must be advanced distally into the artery, since mean transit time is more reproducible when the distal thermistor is at least 50 mm away from the catheter tip[14]. Care should be taken not to move the wire from the original position where baseline measurements are made. After three baseline thermodilution curves are obtained, hyperemia is achieved by intravenous infusion of adenosine.It is important to use intravenous administration, because the effects of an intracoronary bolus only last a few seconds, and will thus not suffice to obtain the thermodilution curves under stable maximal hyperemia. Finally, CFR is calculated with the equation:
CFR = Tmn.b/Tmn.h
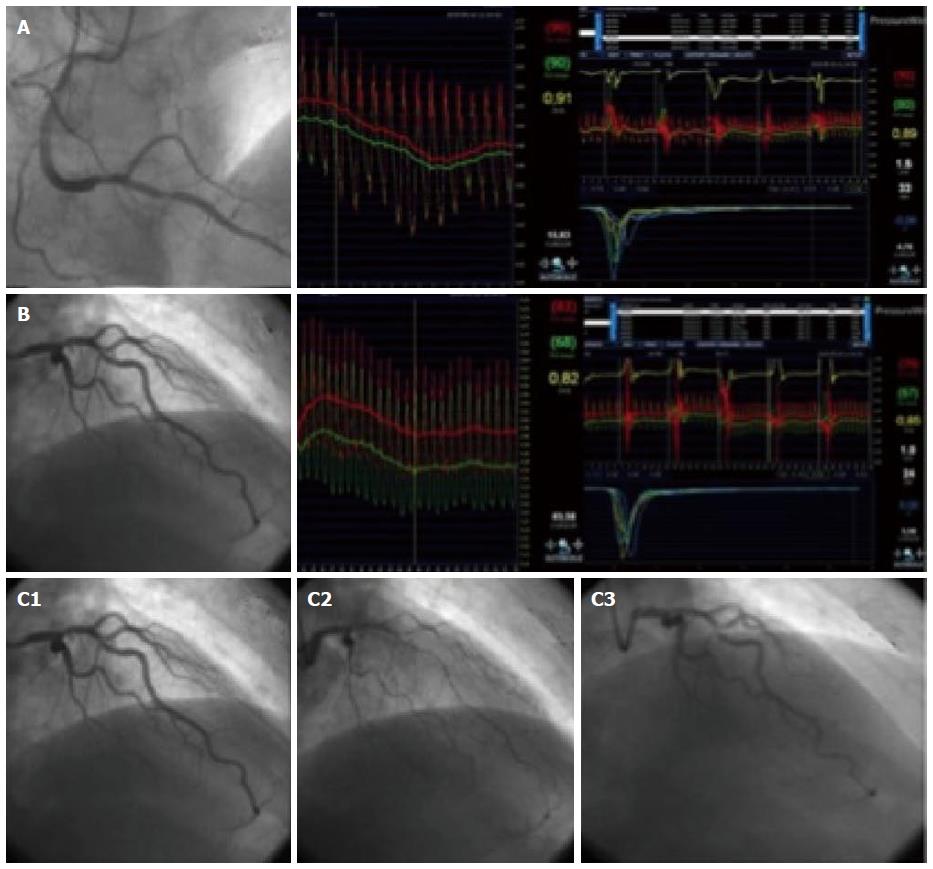
where Tmn.b is mean transit time at baseline (s), and Tmn.h is mean transit time during hyperemia (s). Note that, because transit time is inversely related to flow, in this equation the hyperemia factor is in the denominator, and the baseline in the numerator, conversely to the Doppler method. An example of this technique can be found in Figure 2.
Since both thermodilution and Doppler techniques require the use of adenosine, the most common side effects associated are those described to this substance: bradycardia, hypotension, flushing, dyspnea and chest discomfort; however, the effects of adenosine disappear in seconds after the infusion is stopped or the bolus is administered, so concerning side effects are exceptional. Probably the only truly serious complication of adenosine administration is persistent bronchospasm, which is why it should be avoided in asthmatic patients[20]. If bronchospasm occurs, adenosine may be antagonized with theophylline[21].
The procedure is safe in experienced hands. However, physicians must be aware of the generic potential complications related to the insertion of guiding catheters and wires in the coronary arteries, such as coronary thrombosis, dissection, and spasm.
CFR has two main limitations. First, when an abnormal CFR value is obtained in a stenotic artery, it does not pinpoint the exact level at which flow is limited - that is, it does not help differentiate between epicardial and microvascular flow limitation. Second, given that CFR arises from the ratio of hyperemic to baseline flow, any disturbances in the baseline condition of the patient (tachycardia, stress, vasoactive drugs, abnormal loading conditions, etc.) will alter the ratio, thus possibly rendering a falsely abnormal CFR value.
Since CFR assesses the whole coronary vascular tree (i.e., macrovascular and microvascular compartments), a normal CFR value reflects a basically healthy coronary circulation. The best CFR cutoff value for the detection of inducible ischemia is around 2.0, according to most studies[22-27]. The same cutoff value is useful to decide safe deferral of percutaneous coronary intervention when assessing angiographically intermediate lesions[28,29]. A reduced coronary flow reserve, on the other hand, is an independent predictor of poor clinical outcomes in diverse settings, such as angina without severe coronary stenosis[30] and percutaneous coronary intervention[31].
In conclusion, CFR is a simple measurement of flow, which evaluates both the epicardial and microvascular compartments, and usually reflects normal physiology and good prognosis when it is found to be within normal limits. But other measures are needed in order to specifically investigate epicardial and microvascular disease. These measures are described and discussed below.
As we described earlier in this article, the small vessels of the heart, below 400 μm, are responsible for most of the coronary resistance, and therefore for the regulation of myocardial flow. These vessels autoregulate their resistance with the purpose of maintaining a constant myocardial blood flow independently of blood pressure, across a wide range of pressures. Similarly, when a coronary stenosis appears, the microvascular circulation regulates the resistance to flow to compensate for the stenosis.
In a state of steady maximal hyperemia, as can be achieved with adenosine, coronary autoregulation is abolished, and coronary blood flow is directly proportional to blood pressure. This principle has been used to substitute the measurements of flow by the more simple and reproducible measurements of pressure[32]. Because epicardial stenoses produce a pressure drop due to friction and separation of flow across the obstruction, different pressures can be obtained proximally and distally to a coronary stenosis, and, in a state of hyperemia, these pressures can be considered proportional to flow. Thus fractional flow reserve (FFR) arises from the ratio of the maximal flow achievable by the coronary artery with the epicardial stenosis, compared with the theoretical maximal flow of the same artery without the stenosis. Because in normal conditions the intracoronary pressure does not vary along the epicardial artery, the blood pressure at the tip of the guiding catheter is chosen to represent the theoretical pressure of the non-stenotic artery; this is compared to the pressure detected by a pressure wire distal to the stenosis. Although theoretically venous pressure should be taken into account, in clinical practice it is disregarded, and the simplified equation for FFR is used:
FFR = Pd/Pa
where Pd is the mean pressure distal to the stenosis (mmHg), recorded by the pressure wire, and Pa is the mean aortic pressure (mmHg), recorded by the tip of the guiding catheter.
To measure FFR, the coronary artery should be engaged with a guiding catheter, as with previous methods. Intracoronary nitroglycerin is administered to abolish epicardial reactivity, and a pressure wire (PressureWire, St Jude Medical Inc., St. Paul, MN, United States; or PrimeWire Prestige, Volcano Corp., San Diego, CA, United States) is advanced into the artery. Pressure is balanced against the fluid filled guiding catheter placing the wire transducer at the tip of the catheter, and then the lesion under investigation is crossed with the wire. Maximal hyperemia is induced, and the simultaneous pressure tracings of the wire and the catheter are recorded. FFR is automatically calculated with integrated software, using the mentioned equation.
Hyperemia is usually achieved with adenosine, either intravenous (140 μg/kg per minute)[33] or by intracoronary bolus. Adenosine bolus doses vary from 40 to 150 μg[33,34], although higher doses have been proposed and deemed safe[18]. Alternatively, other drugs may be used, such as papaverine, at doses of 8-20 mg[33]; regadenoson, in a single intravenous bolus of 400 μg[35]; or nitroprusside, in intracoronary bolus of 0.3-0.9 μg/kg[36,37]. These three have the advantage of providing a longer hyperaemic plateau than intracoronary adenosine, but have been less extensively tested in the clinical setting.
The theoretical normal value of FFR in a coronary artery without stenosis is 1, since there should be no appreciable pressure drop along the vessel. A cutoff value of 0.75 (expressing a maximal-flow reduction of 25% attributable to the epicardial stenosis) accurately predicts inducible ischemia[38-40]. The most important clinical studies, however, set a cutoff of 0.8 for safe deferral of coronary intervention[41,42], and accordingly, the current European[43] and American[44] guidelines for revascularization recommend intervention in cases of coronary stenosis with FFR ≤ 0.8. As pointed out by Pijls et al[33], less than 10% of lesions fall into this grey area between 0.75 (almost certain ischemia) and 0.8 [safe deferral of percutaneous coronary intervention (PCI)]. In these cases, sound clinical judgement should be applied. It should also be noted that, while great emphasis has been made on cutoff points, the FFR values express a continuous rather than dichotomic function of risk[45], which highlights the importance of keeping a clinical perspective and evaluating the global risk/benefit profile all treatment options[46].
FFR is a feasible and reproducible technique, minimally modified by the baseline characteristics and hemodynamic status of the patient. Despite its reproducibility, some pitfalls and limitations have been reported.
Pharmacological side effects of adenosine are the same as those described in CFR. Accordingly, the patient must be in a stable hemodynamic condition, and adenosine should be avoided in patients with bronchospasm, severe hypotension, bradycardia or conduction disturbances.
In patients with sequential lesions or diffuse coronary disease, FFR does not provide precise information on which specific lesion is responsible for the ischemia. In such patients, the pressure pullback recording during stable hyperemia may allow identification of the culprit lesion. In order to perform this pullback recording, intravenous adenosine should be used, since an intracoronary bolus will not provide stable hyperemia.
Attention should always be paid to careful technique. The pressure wire signal must be carefully balanced at the tip of the catheter, and the balancing checked at the end of the procedure to rule out pressure drift. The tip of the catheter must be correctly positioned, to avoid damping of the pressure signal and obstruction of the coronary ostium. Submaximal hyperemia may occur, especially if a small peripheral vein is used. In this case, a central vein or an intracoronary bolus should be used to obtain maximal hyperemia.
Finally, FFR is not recommended to assess unstable thrombotic coronary lesions, i.e., in acute myocardial infarction, or when thrombus or instability are evident, since pressure drop across the stenosis would provide an incomplete assessment of the risk associated with this kind of lesions.
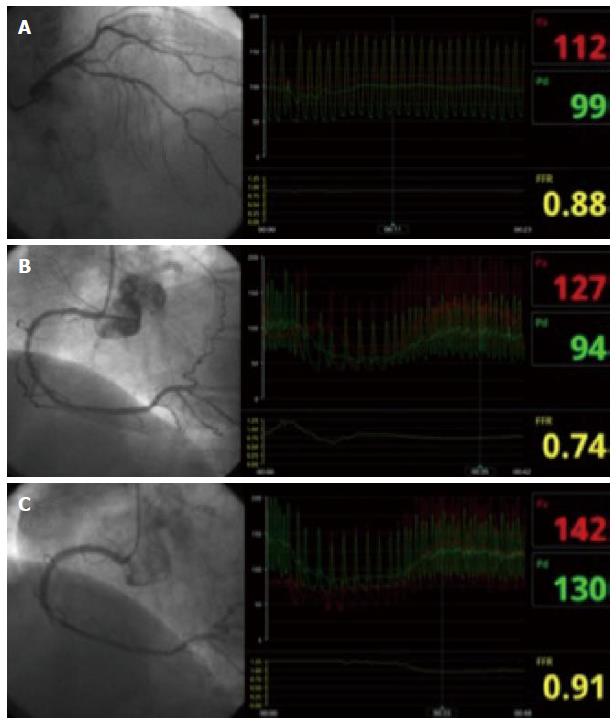
In conclusion, FFR has become an indispensable tool in the catheterization laboratory to make decisions on revascularization of intermediate lesions. It can be performed in almost all elective clinical situations, providing functional information with relevant clinical implications and limited pitfalls. Figure 3 shows an example of an FFR procedure to decide revascularization in multivessel disease.
One of the few pitfalls of FFR is the necessity for maximal hyperemia. Without maximal microvascular vasodilation, the linear relation between pressure and flow that supports the use of FFR disappears. Coronary resistance, however, is not constant throughout the cardiac cycle; and we have already mentioned how, in some cases, stable maximal hyperemia is suboptimal or dubious, and in other rare cases adenosine may be contraindicated. To overcome these difficulties, a new physiologic measure of obstruction has been developed: instantaneous wave free ratio (iFR).
Using wave intensity analysis of simultaneous pressure-velocity recordings, Sen et al[47] identified a period of the cardiac cycle when there are no compression or expansion waves, and the coronary microvascular resistance at rest is minimal and stable, and very similar to the averaged resistance achieved with adenosine. The wave-free period is calculated beginning 25% of the way into diastole, and ending 5 ms before the end (covering around 75% of diastole).
A pressure wire (Verrata, Volcano Corp, San Diego, United States) is inserted in the coronary artery, following the same steps and precautions as with FFR, except that no hyperemia is induced. The wire is attached to a console with built-in proprietary software, which identifies the diastolic period of interest and automatically calculates iFR. The formula of iFR is identical to FFR, but instead of the averaged pressure of the whole cardiac cycle, it only uses the averaged pressure of the mentioned interval:
iFR = Pd/Pa
iFR is highly reproducible and has excellent correlation with FFR (r = 0.90; P < 0.001)[47]. A cutoff value of ≤ 0.90 has been set, which has an overall diagnostic accuracy of 80% to predict an FFR ≤ 0.80[48]. Because dichotomic agreement is logically lower around the cutoff values, a hybrid strategy has been proposed and tested[49,50], which would involve using an upper cutoff of 0.93, above which the coronary stenosis is considered non-significant, and a lower cutoff of 0.86, below which the stenosis is considered significant and PCI is indicated. When iFR falls between these two values (0.86-0.93, the “adenosine zone”), FFR is indicated, and the clinical decision is made according to the FFR value. This strategy may allow for two thirds of patients to be studied without hyperemia, maintaining a 95% agreement with an FFR-for-all strategy[50]. Benefits of this strategy would include reductions in cost and time, and assessment of patients who are unsuitable candidates for adenosine administration such as those with asthma, bradycardia and hypotension. Upcoming important clinical trials, such as the SYNTAX-II[51] should establish the clinical usefulness of this strategy.
Both CFR and FFR show good correlation with non-invasive testing for inducible ischemia. However, when both measurements are made, many patients exhibit a degree of discordance between CFR and FFR determination of ischemia[52,53]. In this context, an index of epicardial resistance that includes information from flow and pressure may have an additional value. The Hyperemic Stenosis Resistance index (HSR) is calculated from simultaneous pressure and Doppler intracoronary tracings, using a pressure-flow guidewire (Combowire, Volcano Corp, San Diego, United States). HSR is defined as the ratio of hyperemic stenosis pressure gradient (Pa-Pd) and hyperemic average peak velocity:
HSR = (Pa - Pd)/APV
where HSR represents hyperemic stenosis resistance (mmHg × s/cm); Pa represents mean aorta pressure (mmHg); Pd distal pressure (mmHg); and APV average peak velocity (cm/s). In normal epicardial arteries, no pressure gradient is expected, so the HSR should be 0. The best cutoff value has been identified at 0.8, and it shows better diagnostic accuracy for ischemia than FFR and CFR[52]. The main limitation of HSR is that it requires the use of a Doppler-pressure wire, which increases the cost of the procedure. Also, it is less validated in clinical practice than FFR and CFR. An index of stenosis resistance derived from thermodilution flow calculations has not been validated to date.
As mentioned above, the main determinant of myocardial blood flow in physiological conditions is the microcirculation. FFR, iFR and HSR give specific information on the resistance to flow of epicardial stenoses; on the other hand, CFR provides an estimation of the overall resistance to flow in the coronary circulation. Thus, if a patient has a low CFR with normal FFR, this is usually attributed to microvascular dysfunction. This may not always be correct, however, as diffuse epicardial disease or altered resting conditions may have an impact of CFR independent of the microvascular circulation[53]. For this reason, resistance indices that provide specific information about the microcirculation have been developed.
Using a Combowire for simultaneous determination of intracoronary pressure and Doppler velocity during adenosine-induced hyperemia, a resistance index called hyperemic microvascular resistance (HMR)[54], can be determined from this equation:
HMR = Pd/APV
where HMR is hyperemic microvascular resistance (mmHg×s/cm); Pd is the pressure in the distal part of the artery (mmHg); and APV is the average peak velocity at the same point (cm/s). Note that venous pressure is here disregarded to simplify the calculations.
There are no clearly set cutoff values for HMR, but Meuwissen et al[54,55] have shown that the median HMR of patients with abnormal CFR is 2.4, compared with 1.9 of patients with normal CFR.
Fearon et al[56] first described the index of microcirculatory resistance (IMR) in 2003, representing minimal microcirculatory resistance measured during conditions of hyperemia. IMR is calculated from the average pressure in the distal part of the coronary artery and the coronary blood flow measured by thermodilution, with the use of a specific pressure wire (PressureWire, Radi Medical Systems, St Jude Medical Inc.; St. Paul, Minn). Mean transit time correlates inversely with flow, so its inverse is used to substitute absolute flow. Theoretically, wedge coronary pressure and central venous pressure should be included in the equation to account for collateral flow and loading conditions. However, as this is usually impractical in the clinical setting, it is most common to disregard both measurements and use the simplified equation:
IMR = Pd × Tmn
where IMR is index of microcirculatory resistance (mmHg×s); Pd is distal pressure (mmHg); and Tmn is mean transit time (s) - all measured during stable hyperemia. In practice, this simplified equation is usually correct, unless there is severe epicardial disease in the artery, in which case collateral flow may be a confounding factor. In such circumstances, the wedge coronary pressure can be measured during PCI, or alternatively an empirical corrected formula[57] may be used:
IMR = Pa×Tmn× [1.35× (Pa/Pd)- 0.32]
An IMR higher than 25 is considered abnormal[58], expressing a damaged coronary microcirculation.
Elevated IMR is related to adverse clinical outcomes in acute myocardial infarction[59], percutaneous intervention[60], and angina with apparently normal epicardial arteries[61].
In conclusion, when simultaneous measurement of pressure and flow indexes is performed, the calculation of a microvascular resistance index (either Doppler or thermodilution derived) adds specific information on the status of the microcirculation, and allows for a better diagnostic and prognostic assessment.
The vascular endothelium is a monolayer of cells that covers the internal lumen of all the blood vessels, separating the blood from the vascular wall and organ tissues. The endothelium is a major determinant of coronary resistance and flow. In response to physiological triggers, the vascular endothelium regulates arterial smooth muscle tone through the release of vasodilators - mainly nitric oxide and prostacyclin - and vasoconstrictors, such as endothelin-1. When the vascular endothelium is damaged or dysfunctional, this function of coronary flow regulation is altered, which results in an insufficient vasodilation, or even paradoxical vasoconstriction, in response to a physiological increase in oxygen demands, such as exercise or stress. This flow dysregulation can be the cause of chronic angina or acute coronary syndromes, even in the absence of coronary epicardial stenosis.
The coronary vasomotion can be assessed directly and invasively by coronary angiography, using mainly acetylcholine as a trigger of endothelium-dependent vascular reactions[62-64]. In the presence of a healthy endothelium, acetylcholine at the doses used induces NO release, which results in coronary vasodilation, both epicardial and microvascular; conversely, if the endothelium is dysfunctional, NO release will be blunted and the predominant net effect will be vasoconstriction due to muscarinic stimulation of smooth muscle. Macrovascular vasodilation or vasoconstriction is evaluated by successive angiographies; the microvascular compartment, being the major determinant of flow velocity, is evaluated using intracoronary Doppler.
Before the endothelial function test, no nitroglycerin should be administered, to allow for epicardial reactivity. Ideally, the patient should be off vasoactive medication for 48 h, although in clinical practice this is not always feasible. The coronary artery - most often the left - is engaged with a guiding catheter, and a baseline angiography is performed to serve as a reference. A coronary microcatheter is advanced into the proximal part of a main vessel, usually the left anterior descending artery, and a Doppler wire (FloWire or ComboWire, Volcano Corp., San Diego, United States) is advanced through the microcatheter into the artery. After checking for signal quality, a baseline tracing of Doppler is recorded. Next, three consecutive infusions of acetylcholine are administered through the microcatheter, at concentrations of 10-6 mol/L, 10-5 mol/L and 10-4 mol/L, at a rate of 1 mL/min, 2-3 min per infusion. After each infusion, the Doppler APV and a coronary angiogram are recorded. Finally, 200 μg of nitroglycerine are administered intracoronary, to evaluate macrovascular endothelium-independent response.
The epicardial endothelial response is considered normal if vasodilation - or at least no vasoconstriction - is observed. The response is considered pathological if a reduction ≥ 20% in coronary artery diameter occurs. A normal microvascular response to acetylcholine would be a 50% increase in APV. If a lower vasodilation, or even vasoconstriction occurs, the microvascular endothelial response is considered abnormal[64].
Most studies o endothelial function have followed this protocol approximately. It has the advantage of evaluating both the macrovascular and microvascular compartments, and the added safety of injecting the drug directly into the LAD. However, other protocols have been described and found safe. The study of the microvascular response by thermodilution, although seldom used, is feasible and has been validated[65]. Some groups[9,66] inject acetylcholine directly into the left main artery (at increasing doses ranging from 2 to 100 μg, for example 2-20-100 μg; each infusion over 3 min), and perform exclusively macrovascular angiographic assessment. This approach, although admittedly less complete than the first, still frequently offers valuable information.
Acetylcholine should be avoided in patients with severe intestinal and/or urologic obstructive disease, as it may enhance muscular contractions. Special attention should be paid to patients with bradycardia or hypotension. Generally, the coronary spasm related to macrovascular endothelial dysfunction is easily reverted with intracoronary nitroglycerin. In any case, vasospasm may cause serious complications, so the patient must be monitored and the procedure must be conducted with utmost care.
Endothelial dysfunction limits maximal coronary flow, and can be the cause of angina without epicardial stenosis[67,68]. It is an important risk factor for poor outcomes in this setting[11], as well as in stable coronary artery disease[69], acute myocardial infarction[70], heart failure[71], and heart transplant[72]. A more detailed account of the importance of endothelial function in coronary heart disease can be found in our recent review[73]. The finding of severe acetylcholine-induced spasm can also assist the physician in the optimization of the medical therapy.
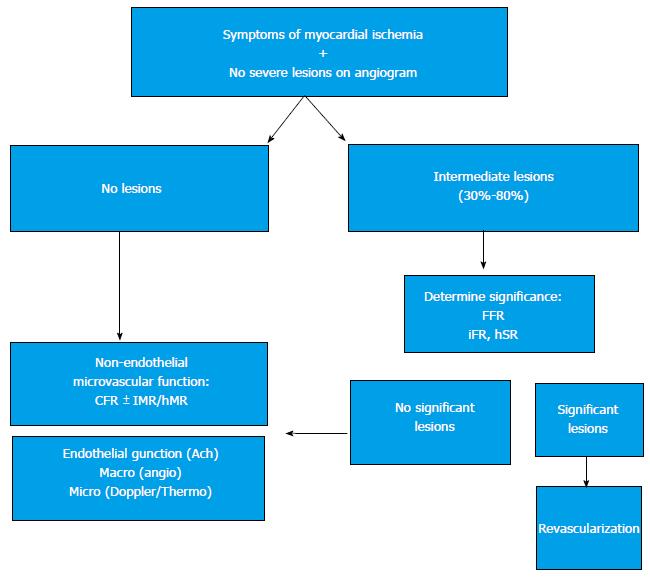
Myocardial ischemia should not be considered to happen exclusively in the presence of critical coronary epicardial stenoses. The physiological significance of intermediate lesions cannot be properly assessed by angiography, and in this case a pressure wire should always be used to decide intervention or deferral. In the absence of significant coronary stenoses, a complete evaluation of the microcirculation and the endothelial function can help identify the fundamental problem, or at the very least reassure the patient and the physician.
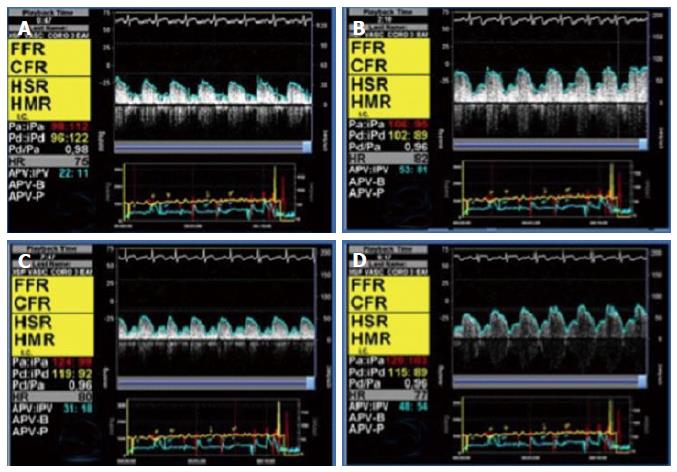
When studying a patient with stable angina or acute coronary syndrome, the interventional cardiologist should not be content with an angiography showing non-significant epicardial disease. If there are intermediate lesions (30%-70%), FFR should be performed to rule out ischemic lesions; if the arteries are clearly non-stenotic, or if FFR is normal, we propose that microvascular endothelium-independent (CFR and microvascular resistance), and macro and microvascular endothelium-dependent function should be assessed. This thorough protocol can be performed in a matter of minutes, and with a very low risk[10]. Recent studies[9,10] show that, in most patients with angina who are extensively evaluated, an alteration can be found that explains the symptoms. Figure 4 shows an example from our centre following this protocol in a complex patient. Figure 5 summarizes this diagnostic algorithm.
In other clinical settings, such as stenting, myocardial infarction and heart transplant, vascular function affects clinical outcomes, and can serve as a prognostic marker. Also, coronary physiological parameters can be of interest as surrogate markers of safety and efficacy in clinical trials for new devices, such as drug eluting stents[66,74] and bioabsorbable scaffolds[75]. The interventional cardiologist should be acquainted with the methods used to perform these measurements and their interpretation. Table 2 summarizes the main parameters available to date.
| Technique | Cutoff value | Implications | Commentary |
| CFR | < 2 | Unspecific macrovascular and microvascular inability to increase flow | Patients with CFR > 2 have favorable outcomes |
| FFR | ≤ 0.8 | Functionally significant epicardial stenosis | Extensive clinical validation |
| Requires vasodilation | |||
| iFR | ≤ 0.9 | Functionally significant epicardial stenosis | Functionally significant epicardial stenosis |
| Vasodilation-Independent | |||
| HSR | 0.8 mmHg × s/cm | Functionally significant epicardial stenosis | Requires doppler-pressure wire |
| Convenient in the presence FFR/CFR discordances | |||
| HMR | > 2 mmHg × s/cm | Microvascular dysfunction | Requires doppler-pressure wire |
| IMR | > 25 mmHg × s | Microvascular dysfunction | Thermodilution method |
Coronary physiology assessment in the catheterization laboratory is essential to help decision making in patients with coronary artery disease, providing functional and prognostic information. Physicians, especially interventional cardiologists should implement its use in daily clinical practise.
P- Reviewer: Haidara M, Nishio K S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Zir LM, Miller SW, Dinsmore RE, Gilbert JP, Harthorne JW. Interobserver variability in coronary angiography. Circulation. 1976;53:627-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 618] [Cited by in RCA: 541] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 2. | Topol EJ, Nissen SE. Our preoccupation with coronary luminology. The dissociation between clinical and angiographic findings in ischemic heart disease. Circulation. 1995;92:2333-2342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 767] [Cited by in RCA: 687] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 3. | Kern MJ, Lerman A, Bech JW, De Bruyne B, Eeckhout E, Fearon WF, Higano ST, Lim MJ, Meuwissen M, Piek JJ. Physiological assessment of coronary artery disease in the cardiac catheterization laboratory: a scientific statement from the American Heart Association Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology. Circulation. 2006;114:1321-1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 358] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 4. | Boden WE, O’Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris CL. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503-1516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3259] [Cited by in RCA: 3208] [Article Influence: 178.2] [Reference Citation Analysis (0)] |
| 5. | Shaw LJ, Berman DS, Maron DJ, Mancini GB, Hayes SW, Hartigan PM, Weintraub WS, O’Rourke RA, Dada M, Spertus JA. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation. 2008;117:1283-1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Pijls NH, van Schaardenburgh P, Manoharan G, Boersma E, Bech JW, van’t Veer M, Bär F, Hoorntje J, Koolen J, Wijns W. Percutaneous coronary intervention of functionally nonsignificant stenosis: 5-year follow-up of the DEFER Study. J Am Coll Cardiol. 2007;49:2105-2111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1099] [Cited by in RCA: 1166] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 7. | Patel MR, Peterson ED, Dai D, Brennan JM, Redberg RF, Anderson HV, Brindis RG, Douglas PS. Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010;362:886-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1067] [Cited by in RCA: 1230] [Article Influence: 82.0] [Reference Citation Analysis (0)] |
| 8. | Summers MR, Lerman A, Lennon RJ, Rihal CS, Prasad A. Myocardial ischaemia in patients with coronary endothelial dysfunction: insights from body surface ECG mapping and implications for invasive evaluation of chronic chest pain. Eur Heart J. 2011;32:2758-2765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Ong P, Athanasiadis A, Borgulya G, Vokshi I, Bastiaenen R, Kubik S, Hill S, Schäufele T, Mahrholdt H, Kaski JC. Clinical usefulness, angiographic characteristics, and safety evaluation of intracoronary acetylcholine provocation testing among 921 consecutive white patients with unobstructed coronary arteries. Circulation. 2014;129:1723-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 269] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 10. | Lee BK, Lim HS, Fearon WF, Yong AS, Yamada R, Tanaka S, Lee DP, Yeung AC, Tremmel JA. Invasive evaluation of patients with angina in the absence of obstructive coronary artery disease. Circulation. 2015;131:1054-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 276] [Article Influence: 27.6] [Reference Citation Analysis (1)] |
| 11. | Schächinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101:1899-1906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1753] [Cited by in RCA: 1709] [Article Influence: 68.4] [Reference Citation Analysis (0)] |
| 12. | Crea F, Lanza G, Camici P. Physiology of coronary microcirculation. 1ST ed. Coronary microvascular dysfunction. Springer. 2014;3-26. |
| 13. | Doucette JW, Corl PD, Payne HM, Flynn AE, Goto M, Nassi M, Segal J. Validation of a Doppler guide wire for intravascular measurement of coronary artery flow velocity. Circulation. 1992;85:1899-1911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 695] [Cited by in RCA: 694] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 14. | De Bruyne B, Pijls NH, Smith L, Wievegg M, Heyndrickx GR. Coronary thermodilution to assess flow reserve: experimental validation. Circulation. 2001;104:2003-2006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 212] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 15. | Gould KL, Lipscomb K. Effects of coronary stenoses on coronary flow reserve and resistance. Am J Cardiol. 1974;34:48-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 646] [Cited by in RCA: 582] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 16. | Kern MJ, Bach RG, Mechem CJ, Caracciolo EA, Aguirre FV, Miller LW, Donohue TJ. Variations in normal coronary vasodilatory reserve stratified by artery, gender, heart transplantation and coronary artery disease. J Am Coll Cardiol. 1996;28:1154-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Pijls NH, De Bruyne B, Smith L, Aarnoudse W, Barbato E, Bartunek J, Bech GJ, Van De Vosse F. Coronary thermodilution to assess flow reserve: validation in humans. Circulation. 2002;105:2482-2486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 247] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 18. | De Luca G, Venegoni L, Iorio S, Giuliani L, Marino P. Effects of increasing doses of intracoronary adenosine on the assessment of fractional flow reserve. JACC Cardiovasc Interv. 2011;4:1079-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 19. | López-Palop R, Carrillo P, Frutos A, Cordero A, Agudo P, Mashlab S, Bertomeu-Martínez V. Comparison of effectiveness of high-dose intracoronary adenosine versus intravenous administration on the assessment of fractional flow reserve in patients with coronary heart disease. Am J Cardiol. 2013;111:1277-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Golzar Y, Doukky R. Regadenoson use in patients with chronic obstructive pulmonary disease: the state of current knowledge. Int J Chron Obstruct Pulmon Dis. 2014;9:129-137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Doré AS, Robertson N, Errey JC, Ng I, Hollenstein K, Tehan B, Hurrell E, Bennett K, Congreve M, Magnani F. Structure of the adenosine A(2A) receptor in complex with ZM241385 and the xanthines XAC and caffeine. Structure. 2011;19:1283-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 460] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 22. | Joye JD, Schulman DS, Lasorda D, Farah T, Donohue BC, Reichek N. Intracoronary Doppler guide wire versus stress single-photon emission computed tomographic thallium-201 imaging in assessment of intermediate coronary stenoses. J Am Coll Cardiol. 1994;24:940-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 23. | Miller DD, Donohue TJ, Younis LT, Bach RG, Aguirre FV, Wittry MD, Goodgold HM, Chaitman BR, Kern MJ. Correlation of pharmacological 99mTc-sestamibi myocardial perfusion imaging with poststenotic coronary flow reserve in patients with angiographically intermediate coronary artery stenoses. Circulation. 1994;89:2150-2160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 175] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 24. | Deychak YA, Segal J, Reiner JS, Rohrbeck SC, Thompson MA, Lundergan CF, Ross AM, Wasserman AG. Doppler guide wire flow-velocity indexes measured distal to coronary stenoses associated with reversible thallium perfusion defects. Am Heart J. 1995;129:219-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 49] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Heller LI, Cates C, Popma J, Deckelbaum LI, Joye JD, Dahlberg ST, Villegas BJ, Arnold A, Kipperman R, Grinstead WC. Intracoronary Doppler assessment of moderate coronary artery disease: comparison with 201Tl imaging and coronary angiography. FACTS Study Group. Circulation. 1997;96:484-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 90] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 26. | Schulman DS, Lasorda D, Farah T, Soukas P, Reichek N, Joye JD. Correlations between coronary flow reserve measured with a Doppler guide wire and treadmill exercise testing. Am Heart J. 1997;134:99-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Danzi GB, Pirelli S, Mauri L, Testa R, Ciliberto GR, Massa D, Lotto AA, Campolo L, Parodi O. Which variable of stenosis severity best describes the significance of an isolated left anterior descending coronary artery lesion? Correlation between quantitative coronary angiography, intracoronary Doppler measurements and high dose dipyridamole echocardiography. J Am Coll Cardiol. 1998;31:526-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Kern MJ, Donohue TJ, Aguirre FV, Bach RG, Caracciolo EA, Wolford T, Mechem CJ, Flynn MS, Chaitman B. Clinical outcome of deferring angioplasty in patients with normal translesional pressure-flow velocity measurements. J Am Coll Cardiol. 1995;25:178-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 102] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | Ferrari M, Schnell B, Werner GS, Figulla HR. Safety of deferring angioplasty in patients with normal coronary flow velocity reserve. J Am Coll Cardiol. 1999;33:82-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Britten MB, Zeiher AM, Schächinger V. Microvascular dysfunction in angiographically normal or mildly diseased coronary arteries predicts adverse cardiovascular long-term outcome. Coron Artery Dis. 2004;15:259-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 31. | Albertal M, Voskuil M, Piek JJ, de Bruyne B, Van Langenhove G, Kay PI, Costa MA, Boersma E, Beijsterveldt T, Sousa JE. Coronary flow velocity reserve after percutaneous interventions is predictive of periprocedural outcome. Circulation. 2002;105:1573-1578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | Pijls NH, van Son JA, Kirkeeide RL, De Bruyne B, Gould KL. Experimental basis of determining maximum coronary, myocardial, and collateral blood flow by pressure measurements for assessing functional stenosis severity before and after percutaneous transluminal coronary angioplasty. Circulation. 1993;87:1354-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 807] [Cited by in RCA: 802] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 33. | Pijls NH, Sels JW. Functional measurement of coronary stenosis. J Am Coll Cardiol. 2012;59:1045-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 194] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 34. | Casella G, Leibig M, Schiele TM, Schrepf R, Seelig V, Stempfle HU, Erdin P, Rieber J, König A, Siebert U. Are high doses of intracoronary adenosine an alternative to standard intravenous adenosine for the assessment of fractional flow reserve? Am Heart J. 2004;148:590-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 35. | Nair PK, Marroquin OC, Mulukutla SR, Khandhar S, Gulati V, Schindler JT, Lee JS. Clinical utility of regadenoson for assessing fractional flow reserve. JACC Cardiovasc Interv. 2011;4:1085-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 36. | Li S, Deng J, Wang X, Zhao X, Han Y. Efficiencies of intracoronary sodium nitroprusside on fractional flow reserve measurement. Int J Clin Exp Med. 2015;8:2679-2683. [PubMed] |
| 37. | Wang X, Li S, Zhao X, Deng J, Han Y. Effects of intracoronary sodium nitroprusside compared with adenosine on fractional flow reserve measurement. J Invasive Cardiol. 2014;26:119-122. [PubMed] |
| 38. | Pijls NH, Van Gelder B, Van der Voort P, Peels K, Bracke FA, Bonnier HJ, el Gamal MI. Fractional flow reserve. A useful index to evaluate the influence of an epicardial coronary stenosis on myocardial blood flow. Circulation. 1995;92:3183-3193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 589] [Cited by in RCA: 554] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 39. | Caymaz O, Fak AS, Tezcan H, Inanir S S, Toprak A, Tokay S, Turoglu T, Oktay A. Correlation of myocardial fractional flow reserve with thallium-201 SPECT imaging in intermediate-severity coronary artery lesions. J Invasive Cardiol. 2000;12:345-350. [PubMed] |
| 40. | Fearon WF, Takagi A, Jeremias A, Yeung AC, Joye JD, Cohen DJ, Chou TM, Kern MJ, Yock PG. Use of fractional myocardial flow reserve to assess the functional significance of intermediate coronary stenoses. Am J Cardiol. 2000;86:1013-1014, A10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 41. | Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van’ t Veer M, Klauss V, Manoharan G, Engstrøm T, Oldroyd KG. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360:213-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2974] [Cited by in RCA: 3058] [Article Influence: 191.1] [Reference Citation Analysis (0)] |
| 42. | De Bruyne B, Fearon WF, Pijls NH, Barbato E, Tonino P, Piroth Z, Jagic N, Mobius-Winckler S, Rioufol G, Witt N. Fractional flow reserve-guided PCI for stable coronary artery disease. N Engl J Med. 2014;371:1208-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 772] [Cited by in RCA: 826] [Article Influence: 75.1] [Reference Citation Analysis (0)] |
| 43. | Kolh P, Windecker S, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Jüni P. 2014 ESC/EACTS Guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur J Cardiothorac Surg. 2014;46:517-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 591] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 44. | Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011;124:2574-2609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 390] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 45. | Johnson NP, Tóth GG, Lai D, Zhu H, Açar G, Agostoni P, Appelman Y, Arslan F, Barbato E, Chen SL. Prognostic value of fractional flow reserve: linking physiologic severity to clinical outcomes. J Am Coll Cardiol. 2014;64:1641-1654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 488] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 46. | Petraco R, Sen S, Nijjer S, Echavarria-Pinto M, Escaned J, Francis DP, Davies JE. Fractional flow reserve-guided revascularization: practical implications of a diagnostic gray zone and measurement variability on clinical decisions. JACC Cardiovasc Interv. 2013;6:222-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 134] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 47. | Sen S, Escaned J, Malik IS, Mikhail GW, Foale RA, Mila R, Tarkin J, Petraco R, Broyd C, Jabbour R. Development and validation of a new adenosine-independent index of stenosis severity from coronary wave-intensity analysis: results of the ADVISE (ADenosine Vasodilator Independent Stenosis Evaluation) study. J Am Coll Cardiol. 2012;59:1392-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 534] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 48. | Jeremias A, Maehara A, Généreux P, Asrress KN, Berry C, De Bruyne B, Davies JE, Escaned J, Fearon WF, Gould KL. Multicenter core laboratory comparison of the instantaneous wave-free ratio and resting Pd/Pa with fractional flow reserve: the RESOLVE study. J Am Coll Cardiol. 2014;63:1253-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 280] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 49. | Petraco R, Park JJ, Sen S, Nijjer SS, Malik IS, Echavarría-Pinto M, Asrress KN, Nam CW, Macías E, Foale RA. Hybrid iFR-FFR decision-making strategy: implications for enhancing universal adoption of physiology-guided coronary revascularisation. EuroIntervention. 2013;8:1157-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 50. | Escaned J, Echavarría-Pinto M, Garcia-Garcia HM, van de Hoef TP, de Vries T, Kaul P, Raveendran G, Altman JD, Kurz HI, Brechtken J. Prospective Assessment of the Diagnostic Accuracy of Instantaneous Wave-Free Ratio to Assess Coronary Stenosis Relevance: Results of ADVISE II International, Multicenter Study (ADenosine Vasodilator Independent Stenosis Evaluation II). JACC Cardiovasc Interv. 2015;8:824-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 161] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 51. | Farooq V. Syntax ii: Pci of 3-vessel disease applying clinical, anatomical and functional parameters. EuroPCR. 2014;2014. |
| 52. | Meuwissen M, Siebes M, Chamuleau SA, van Eck-Smit BL, Koch KT, de Winter RJ, Tijssen JG, Spaan JA, Piek JJ. Hyperemic stenosis resistance index for evaluation of functional coronary lesion severity. Circulation. 2002;106:441-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 142] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 53. | van de Hoef TP, van Lavieren MA, Damman P, Delewi R, Piek MA, Chamuleau SA, Voskuil M, Henriques JP, Koch KT, de Winter RJ. Physiological basis and long-term clinical outcome of discordance between fractional flow reserve and coronary flow velocity reserve in coronary stenoses of intermediate severity. Circ Cardiovasc Interv. 2014;7:301-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 293] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 54. | Meuwissen M, Chamuleau SA, Siebes M, Schotborgh CE, Koch KT, de Winter RJ, Bax M, de Jong A, Spaan JA, Piek JJ. Role of variability in microvascular resistance on fractional flow reserve and coronary blood flow velocity reserve in intermediate coronary lesions. Circulation. 2001;103:184-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 188] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 55. | Meuwissen M, Chamuleau SA, Siebes M, de Winter RJ, Koch KT, Dijksman LM, van den Berg AJ, Tijssen JG, Spaan JA, Piek JJ. The prognostic value of combined intracoronary pressure and blood flow velocity measurements after deferral of percutaneous coronary intervention. Catheter Cardiovasc Interv. 2008;71:291-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 56. | Fearon WF, Balsam LB, Farouque HM, Caffarelli AD, Robbins RC, Fitzgerald PJ, Yock PG, Yeung AC. Novel index for invasively assessing the coronary microcirculation. Circulation. 2003;107:3129-3132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 515] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 57. | Yong AS, Layland J, Fearon WF, Ho M, Shah MG, Daniels D, Whitbourn R, Macisaac A, Kritharides L, Wilson A. Calculation of the index of microcirculatory resistance without coronary wedge pressure measurement in the presence of epicardial stenosis. JACC Cardiovasc Interv. 2013;6:53-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 127] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 58. | Kobayashi Y, Fearon WF. Invasive coronary microcirculation assessment--current status of index of microcirculatory resistance. Circ J. 2014;78:1021-1028. [PubMed] [DOI] [Full Text] |
| 59. | Fearon WF, Low AF, Yong AS, McGeoch R, Berry C, Shah MG, Ho MY, Kim HS, Loh JP, Oldroyd KG. Prognostic value of the Index of Microcirculatory Resistance measured after primary percutaneous coronary intervention. Circulation. 2013;127:2436-2441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 341] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 60. | Ng MK, Yong AS, Ho M, Shah MG, Chawantanpipat C, O’Connell R, Keech A, Kritharides L, Fearon WF. The index of microcirculatory resistance predicts myocardial infarction related to percutaneous coronary intervention. Circ Cardiovasc Interv. 2012;5:515-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 61. | Luo C, Long M, Hu X, Huang Z, Hu C, Gao X, Du Z. Thermodilution-derived coronary microvascular resistance and flow reserve in patients with cardiac syndrome X. Circ Cardiovasc Interv. 2014;7:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 62. | Ludmer PL, Selwyn AP, Shook TL, Wayne RR, Mudge GH, Alexander RW, Ganz P. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med. 1986;315:1046-1051. [PubMed] |
| 63. | Cox DA, Vita JA, Treasure CB, Fish RD, Alexander RW, Ganz P, Selwyn AP. Atherosclerosis impairs flow-mediated dilation of coronary arteries in humans. Circulation. 1989;80:458-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 265] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 64. | Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1336] [Cited by in RCA: 1356] [Article Influence: 54.2] [Reference Citation Analysis (0)] |
| 65. | Melikian N, Kearney MT, Thomas MR, De Bruyne B, Shah AM, MacCarthy PA. A simple thermodilution technique to assess coronary endothelium-dependent microvascular function in humans: validation and comparison with coronary flow reserve. Eur Heart J. 2007;28:2188-2194. [PubMed] |
| 66. | Fujii K, Kawasaki D, Oka K, Akahori H, Fukunaga M, Sawada H, Masutani M, Lee-Kawabata M, Tsujino T, Ohyanagi M. Endothelium-dependent coronary vasomotor response and neointimal coverage of zotarolimus-eluting stents 3 months after implantation. Heart. 2011;97:977-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 67. | Zeiher AM, Krause T, Schächinger V, Minners J, Moser E. Impaired endothelium-dependent vasodilation of coronary resistance vessels is associated with exercise-induced myocardial ischemia. Circulation. 1995;91:2345-2352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 202] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 68. | Hasdai D, Gibbons RJ, Holmes DR, Higano ST, Lerman A. Coronary endothelial dysfunction in humans is associated with myocardial perfusion defects. Circulation. 1997;96:3390-3395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 252] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 69. | Halcox JP, Schenke WH, Zalos G, Mincemoyer R, Prasad A, Waclawiw MA, Nour KR, Quyyumi AA. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106:653-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1032] [Cited by in RCA: 1014] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
| 70. | Fichtlscherer S, Breuer S, Zeiher AM. Prognostic value of systemic endothelial dysfunction in patients with acute coronary syndromes: further evidence for the existence of the “vulnerable” patient. Circulation. 2004;110:1926-1932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 188] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 71. | Shechter M, Matetzky S, Arad M, Feinberg MS, Freimark D. Vascular endothelial function predicts mortality risk in patients with advanced ischaemic chronic heart failure. Eur J Heart Fail. 2009;11:588-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 72. | Hollenberg SM, Klein LW, Parrillo JE, Scherer M, Burns D, Tamburro P, Oberoi M, Johnson MR, Costanzo MR. Coronary endothelial dysfunction after heart transplantation predicts allograft vasculopathy and cardiac death. Circulation. 2001;104:3091-3096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 119] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 73. | Gutiérrez E, Flammer AJ, Lerman LO, Elízaga J, Lerman A, Fernández-Avilés F. Endothelial dysfunction over the course of coronary artery disease. Eur Heart J. 2013;34:3175-3181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 243] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 74. | Fuke S, Maekawa K, Kawamoto K, Saito H, Sato T, Hioka T, Ohe T. Impaired endothelial vasomotor function after sirolimus-eluting stent implantation. Circ J. 2007;71:220-225. [PubMed] |
| 75. | Brugaletta S, Heo JH, Garcia-Garcia HM, Farooq V, van Geuns RJ, de Bruyne B, Dudek D, Smits PC, Koolen J, McClean D. Endothelial-dependent vasomotion in a coronary segment treated by ABSORB everolimus-eluting bioresorbable vascular scaffold system is related to plaque composition at the time of bioresorption of the polymer: indirect finding of vascular reparative therapy? Eur Heart J. 2012;33:1325-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 124] [Article Influence: 9.5] [Reference Citation Analysis (0)] |