Published online Aug 26, 2015. doi: 10.4330/wjc.v7.i8.490
Peer-review started: January 25, 2015
First decision: March 6, 2015
Revised: June 5, 2015
Accepted: June 18, 2015
Article in press: June 19, 2015
Published online: August 26, 2015
Processing time: 214 Days and 6.1 Hours
AIM: To evaluate the safety and efficacy of the permanent high interventricular septal pacing in a long term follow up, as alternative to right ventricular apical pacing.
METHODS: We retrospectively evaluated: (1) 244 patients (74 ± 8 years; 169 men, 75 women) implanted with a single (132 pts) or dual chamber (112 pts) pacemaker (PM) with ventricular screw-in lead placed at the right ventricular high septal parahisian site (SEPTAL pacing); (2) 22 patients with permanent pacemaker and low percentage of pacing (< 20%) (NO pacing); (3) 33 patients with high percentage (> 80%) right ventricular apical pacing (RVA). All patients had a narrow spontaneous QRS (101 ± 14 ms). We evaluated New York Heart Association (NYHA) class, quality of life (QoL), 6 min walking test (6MWT) and left ventricular function (end-diastolic volume, LV-EDV; end-systolic volume, LV-ESV; ejection fraction, LV-EF) with 2D-echocardiography.
RESULTS: Pacing parameters were stable during follow up (21 mo/patient). In SEPTAL pacing group we observed an improvement in NYHA class, QoL score and 6MWT. While LV-EDV didn’t significantly increase (104 ± 40 mL vs 100 ± 37 mL; P = 0.35), LV-ESV slightly increased (55 ± 31 mL vs 49 ± 27 mL; P = 0.05) and LV-EF slightly decreased (49% ± 11% vs 53% ± 11%; P = 0.001) but never falling < 45%. In the RVA pacing control group we observed a worsening of NYHA class and an important reduction of LV-EF (from 56% ± 6% to 43% ± 9%, P < 0.0001).
CONCLUSION: Right ventricular permanent high septal pacing is safe and effective in a long term follow up evaluation; it could be a good alternative to the conventional RVA pacing in order to avoid its deleterious effects.
Core tip: We evaluated the safety and efficacy of the permanent high interventricular septal pacing in a long term follow up, as alternative to right ventricular apical pacing. We retrospective evaluated 244 patients with a narrow QRS implanted with a single/dual chamber pacemaker with ventricular screw-in lead placed at the right ventricular high septal (parahisian) site. Contemporary we checked the clinical evolution of two control groups of patients: without ventricular stimulation and with conventional right ventricular apical stimulation. In a long term follow up we observed stability of pacing parameters and ejection fraction, and improvement in New York Heart Association class, quality of life and exercise tolerance.
- Citation: Occhetta E, Quirino G, Baduena L, Nappo R, Cavallino C, Facchini E, Pistelli P, Magnani A, Bortnik M, Francalacci G, Dell’Era G, Plebani L, Marino P. Right ventricular septal pacing: Safety and efficacy in a long term follow up. World J Cardiol 2015; 7(8): 490-498
- URL: https://www.wjgnet.com/1949-8462/full/v7/i8/490.htm
- DOI: https://dx.doi.org/10.4330/wjc.v7.i8.490
The treatment of atrioventricular block or sinus node disease is represented by artificial pacemaker implant; usually the ventricular catheter is placed in right ventricular apical (RVA) position. This therapy proved efficacious in long term follow up, granting improvement in life expectancy and quality of life (QoL). However, similarly to the negative hemodynamic and clinical effects of spontaneous left bundle branch block, new data have emerged showing negative effects of the left bundle branch block-like activation determined by RVA pacing[1-5].
Several published studies[6-10] demonstrated that more than 40% of the heart beats are paced from the right ventricular apex, an increase in the incidence of atrial fibrillation, heart failure, hospitalizations and even death is observed. When ventricular pacing is necessary permanently or for long periods of time, sites for a more physiologic pacing should be identified to avoid he occurrence of ventricular desynchronization[11-13].
A better way to pace the heart in case of intraventricular conduction delay (especially left bundle branch block) is biventricular pacing: comparing to RVA pacing, it can improve left ventricular ejection fraction and volumes and reduce mitral regurgitation and sympathetic nervous system activity[14-17]. His bundle pacing may be considered as a reliable and effective method to prevent mechanical desynchronization when intraventricular conduction is preserved and QRS is narrow[18,19]. However, it requires adjuntive skills and may be more challenging and time-consuming; it is not always applicable and higher pacing thresholds have to be accepted[20]. The right ventricular septal pacing in the parahisian area, early penetrating the His-Purkinje conduction system, produces a more physiological ventricular activation, very similar to the one that is achieved with direct His bundle pacing[21].
We aimed to evaluate feasibility, safety and long-term clinical efficacy of permanent right ventricular septal pacing in the parahisian area, performed to obtain a shorter QRS duration than that resulting from conventional right ventricular apical pacing.
From January 2001 to December 2011, we evaluated 244 patients implanted with a single or dual-chamber pacemaker with the ventricular lead positioned in the high interventricular septum (parahisian site): “SEPTAL pacing” group.
The patients were implanted at the Cardiology Clinic of Azienda Ospedaliero-Universitaria (AOU) Maggiore della Carità in Novara (School of Medicine, Study University of Piemonte Orientale, Italy) (181 patients), at the Division of Cardiology of the Ospedale Civile in Ivrea, Italy (50 patients), and at the Division of Cardiology of the Ospedale SS. Annunziata in Cosenza, Italy (22 patients).
The mean age of patients was 74 ± 8 years; 169 patients were men (69%) and 75 patients were women (31%). Inclusion criteria were: (1) permanent VVIR pacing after AV node ablation for permanent atrial fibrillation with uncontrolled (high) ventricular rate, despite negative dromothropic therapy comprising digoxin, beta-blockers and diltiazem as monotherapy or associated (51 patients; 21%); (2) permanent VVIR pacing in permanent atrial fibrillation with impaired AV conduction and low ventricular frequency (81 patients; 33%); (3) permanent DDD(R) pacemaker in patients with sinus rhythm and first and second degree AV block, symptomatic for syncope/dizziness (82 patients; 34%); and (4) permanent DDD(R) pacemaker in patients with sinus rhythm and complete AV block (30 patients; 12%).
All patients had a narrow spontaneous QRS complex (mean 101 ± 14 ms; always < 120 ms), detected at standard ECG; in patients with AV node ablation a narrow QRS was detected during junctional escape rhythm after radiofrequency (RF) AV ablation; in patients with atrial fibrillation not undergoing AV ablation, a narrow QRS was documented during 24-h Holter recording.
At the same time, we retrospectively evaluated two other “control groups” of patients (all implanted at the Cardiology Clinic of AOU Maggiore della Carità in Novara, School of Medicine, Study University of Piemonte Orientale, Italy): (1) 22 consecutive patients with ventricular apical pacing (single or dual chamber pacemakers) but percentage of permanent pacing < 20%, retrospectively detected by pacemaker telemetry, owing to the presence of spontaneous AV conduction and preserved intraventricular conduction (QRS < 120 ms): “NO pacing” control group; (2) 33 consecutive patients with a ventricular or dual-chamber pacemaker providing a high percentage of ventricular pacing (> 80%) in the apex of the right ventricle, always retrospectively detected by pacemaker memories: “RVA pacing” group.
Before the implantation procedure, all patients were planned to undergo a complete evaluation.
Following assessments were performed: (1) New York Heart Association (NYHA) functional class; (2) quality of life (QoL), evaluated with “Minnesota Living with Heart Failure” questionnaire[22]; (3) twenty-four hour Holter monitoring; (4) six-minute walking test; (5) standard 2D-echocardiogram with measurement of left ventricular end-diastolic (LV-EDV) and end-systolic (LV-ESV) volumes computed according to a biplane Simpson’s method, and left ventricular ejection fraction (LV-EF).
Clinical characteristics of the population are presented in Table 1: enrolled patients presented LV-EF values close to the lower limit of the normal range, narrow QRS with normal electrical axis and moderate compromission of functional class.
| NO pacing | RVA pacing | PH pacing | |
| Total patients | 22 patients | 33 patients | 244 patients |
| Age (yr) | 75 ± 7 | 77 ± 9 | 74 ± 8 |
| Sex | 13 M/9 F | 21 M/12 F | 169 M/ 75 F |
| NYHA class | 1.09 ± 0.29 | 1.15 ± 0.36 | 2.13 ± 0.46 |
| LV ejection fraction (%) | 57 ± 5 | 55 ± 8 | 53 ± 11 |
| LV end-dyastolic volume (cc) | 89 ± 25 | 98 ± 22 | 100 ± 37 |
| LV end-systolic volume (cc) | 38 ± 13 | 47 ± 17 | 49 ± 27 |
| Associated heart diseases | Ischemic heart disease: 6/22 (27%) Valvular heart disease: 2/22 (9%) Hypertensive heart disease: 2/22 (9%) No significant heart disease: 12/22 (55%) | Ischemic heart disease: 12/33 (37%) Valvular heart disease: 4/33 (12%) Hypertensive heart disease: 3/33 (9%) No significant heart disease: 14/33 (42%) | Ischemic heart disease: 80/244 (33%) Valvular heart disease: 29/244 (12%) Hypertensive heart disease: 90/244 (37%) No significant heart disease: 45/244 (18%) |
| Atrial fibrillation | 1 (5%) | 4 (12%) | 132 (54%) |
| Sinus rhythm | 21 (95%) | 29 (88%) | 112 (46%) |
In patients with permanent atrial fibrillation and AV node ablation, pacing leads were placed after RF ablation procedure. A quadripolar RF catheter was used to map the His bundle and an active fixation bipolar lead was placed as near as possible to the hisian dipole of the catheter. A second conventional bipolar lead was placed at the right ventricular apex. The septal and the apical leads were then connected to the “atrial” and “ventricular” pacemaker channels, respectively. The pacemaker was programmed in “DDDR” mode with “short” atrio-ventricular delay (i.e., 90 ms). Thus, if the parahisian stimulation was effective through the “atrial” channel, the following RVA pulse pacing was inhibited or delivered during the refractory period through the “ventricular” channel. While, in case of ineffective parahisian stimulation, the RVA pulse pacing ensured ventricular capture.
In patients with permanent atrial fibrillation and bradyarrhythmia (without indication to AV node ablation), a single chamber VVIR pacemaker was used and connected to the lead positioned in the parahisian area, without RVA back up lead.
In patients with sinus rhythm and advanced spontaneous AV block (first, second or third degree) a conventional atrial lead was placed in addition to the parahisian lead; both leads were connected to a DDD/DDDR pacemaker.
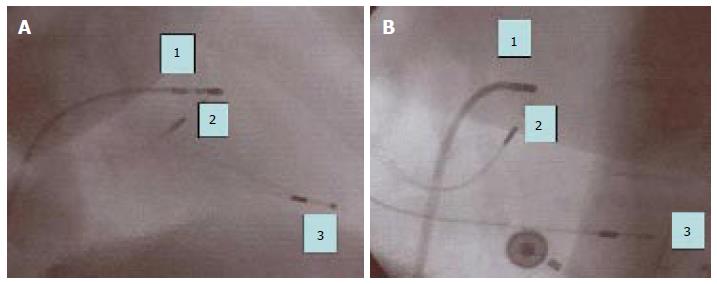
Following criteria were applied to obtain parahisian pacing[20]: (1) positioning of the tip of the screw-in lead as close as possible to the mapping dipole of the electrophysiological catheter (distance < 1 cm in left and right oblique projections) (Figure 1); (2) even if larger than the spontaneous QRS, the duration of the paced QRS had to be < 130 ms; (3) full concordance between electrical axis of the paced QRS and that of the spontaneous QRS; and (4) the pacing threshold had to be < 1 V (pacing of the muscular portion of the interventricular septum).
Control groups patients were implanted with a conventional apical right ventricular lead (and conventional atrial lead in dual chamber pacing).
Continuous variables with normal or Gaussian distribution of each group of patients were expressed in terms of average ± SD. Pre-implantation and follow-up data in the parahisian pacing group were analyzed and compared by means of the parametric Student t test for paired data. Similar parameters observed in the three groups were compared by means of the Student t test for numerically different samples with the same variance. A value of P≤ 0.05 was considered statistically significant.
The study was reviewed by our expert Biostatistic Gabriele Dell'Era, MD.
To obtain the parahisian high septal pacing we used: (1) a bipolar catheter with 1.5 mm retractable screw lead (CapsureFix 4068/5068/5076; Medtronic Inc., Minneapolis, Minnesota) in 172 patients; (2) a bipolar catheter with 1.5 mm retractable screw lead (Cristalline ICQ09B, Vitatron BV, The Netherlands) in 10 patients; (3) a bipolar catheter with 1.5 mm retractable screw lead (Tendril 1488T/1888T; St.Jude Medical, Inc. St. Paul, Minnesota) in 12 patients; (4) a bipolar catheter with 3 mm retractable screw lead (10627 Medtronic Inc., Minneapolis, Minnesota) in 5 patients (controlled clinical evaluation); and (5) a bipolar, fixed screw, steroid eluting lead (Select Secure 3830, Medtronic Inc., Minneapolis, Minnesota) in 45 patients.
The total radiological exposure time was 15 ± 9 min (range from 3 to 68 min for the first implant with Select Secure system). Electrical parameters at the parahisian site were measured in bipolar configuration.
We excluded from the analysis 14 patients (6%), in which the criteria for parahisian pacing were not met, specifically the paced QRS was > 130 ms.
For patients in analysis, the average duration of the basal QRS was 101 ± 14 ms, and 122 ± 9 ms during parahisian pacing.
We obtained an average parahisian pacing threshold of 0.6 ± 0.3 V (at 0.5 ms pulse duration), pacing impedance 736 ± 238 Ω, endocavitary potential 10.1 ± 5.3 mV; we never recorded high-amplitude “far-field” type atrial potentials from the parahisian lead.
The average follow up of the 230 patients in analysis was 21 mo/patient, with a maximum of 70 mo for the first enrolled patient and a minimum of 12 mo for the last one.
In one patient a 3 cm dislodgment of the parahisian lead was reported. However, the paced QRS appeared superimposable to that recorded at the end of the implantation.
During long-term follow-up, the duration of the QRS during parahisian pacing remained comparable to that recorded at the implantation. Electrical measurements from the parahisian position remained stable and acceptable during time: pacing threshold was 0.6 ± 0.3 V at implantation and 0.8 ± 0.5 V at follow-up, mean endocardial potential was 10.1 ± 5.3 mV at implantation and 9.1 ± 4.4 mV at follow up, pacing impedance was 736 ± 238 ohms at implantationand 540 ± 116 ohms at follow up.
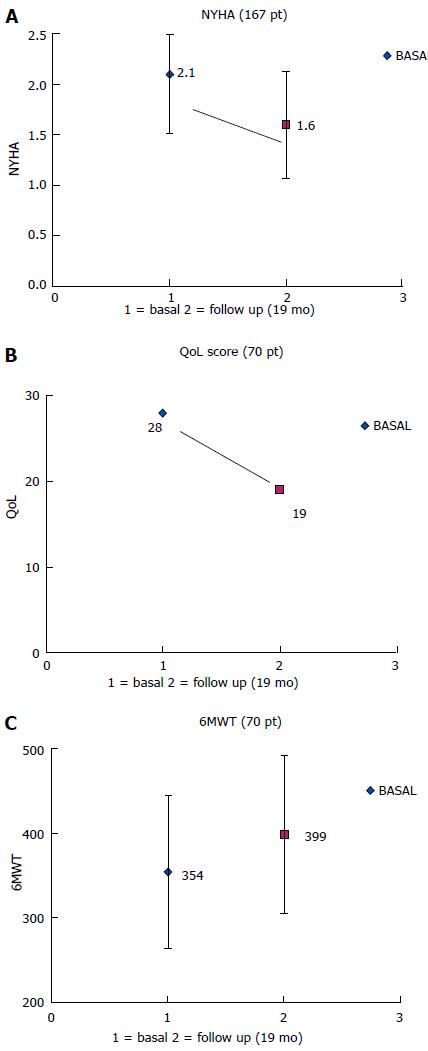
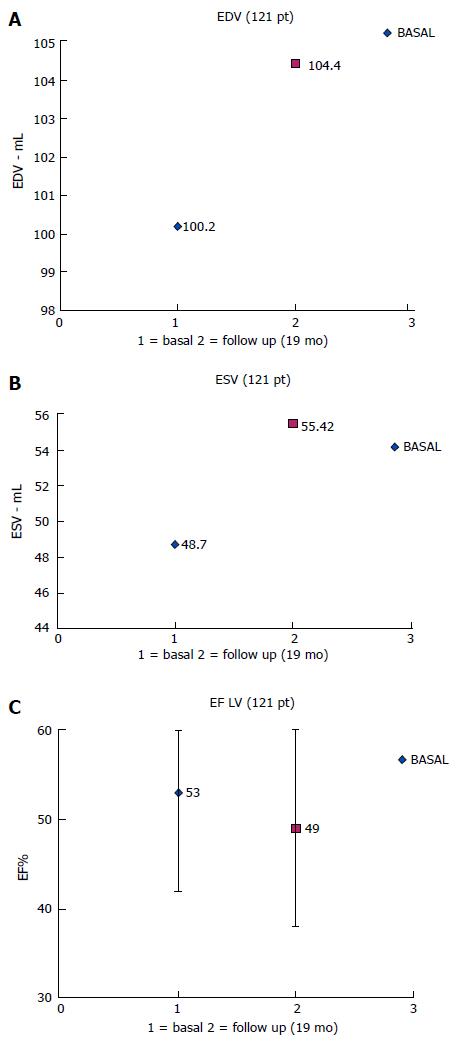
The clinical results at long-term follow-up were (Table 2): (1) In 167/230 patients (73%) we compared NYHA functional class measured before implantation and at a mean follow-up of 18 ± 16 mo: the prolonged parahisian pacing led to a significant improvement from 2.15 ± 0.51 to 1.59 ± 0.55; P < 0.001 (Figure 2); (2) The quality of life score and exercise performances (6 min walk), performed in a sub-group of 70/230 patients (30%), significantly changed after a mean follow up of 14 ± 2 mo (QoL score from 29 ± 18 to 19 ± 17, P = 0.02; 6 min walk distance from 354 ± 90 m to 400 ± 88 m, P = 0.03) (Figure 2); (3) In 121/230 patients (53%) we compared echocardiographic volumes and ejection fraction before and after parahisian pacing (Figure 3): LV-EDV went from 100 ± 37 to 104 ± 40 mL, P = 0.35; LV-ESV from 49 ± 27 to 55 ± 31 mL, P = 0.05; LV-EF from 53% ± 11% to 49% ± 11%, P = 0.01. Medium-long term evaluation of the LV-EF showed values superimposable to enrollment values, confirming that parahisian pacing can prevent deterioration of the left ventricular function.
| Basal | Parahisian pacing | P value | |
| NYHA class (167 pts) | 2.15 ± 0.51 | 1.59 ± 0.55 | < 0.001 |
| 6-min walk (m) (70 pts) | 354 ± 90 | 400 ± 88 | 0.03 |
| QoL (score) (70 pts) | 29 ± 18 | 19 ± 7 | 0.02 |
| LV-EDV (mL) (121 pts) | 100 ± 37 | 104 ± 40 | 0.35 |
| LV-ESV (mL) (121 pts) | 49 ± 27 | 55 ± 31 | 0.05 |
| LV-EF (%) (121 pts) | 53 ± 11 | 49 ± 11 | 0.01 |
In RVA-paced patients QRS duration increased significantly (average 165 ± 10 ms, with values always > 130 ms).
In the “NO pacing” control group, the NYHA functional class was good both at the baseline and during follow-up; the conduction system disease did not significantly affect the functional class, which did not change during follow-up in the absence of ventricular pacing. By contrast, in “RVA pacing” patients there was a trend toward worsening NYHA functional class, though the upper classes of overt heart failure were not reached. Thus, during follow-up the NYHA functional class was better in patients without stimulation and those on PH stimulation (no significant difference between these two groups) and worse in patients stimulated at the apex (P < 0.05 vs unstimulated patients and vs PH-stimulated patients) (Table 3). QoL scores did not significantly differ among the three groups: 21 ± 19 score in NO pacing patients; 29 ± 13 in RVA pacing patients; 19 ± 17 in PH pacing patients (P < 0.06 RVA group vs NO pacing; P < 0.07 PH group vs NO pacing). Exercise tolerance, expressed in meters walked in 6 min, was better in patients without persistent pacing (448 ± 110 m) than in PH-stimulated patients (400 ± 88 m), but the difference was not significant; on the contrary it was worse in RVA-stimulated patients (338 ± 158 m) (P < 0.05 vs both “NO pacing” and PH-stimulated patients).
| NO pacing(22 pts) | RVA pacing(33 pts) | PH pacing(167 pts) | |
| Baseline | 1.09 ± 0.29 | 1.15 ± 0.36 | 2.15 ± 0.51 |
| Follow-up | 1.22 ± 0.52 | 1.88 ± 0.99 | 1.59 ± 0.55 |
| Significance | 0.32 (ns) | P < 0.05 | P < 0.001 |
| Unchanged | Worsening | Improvement |
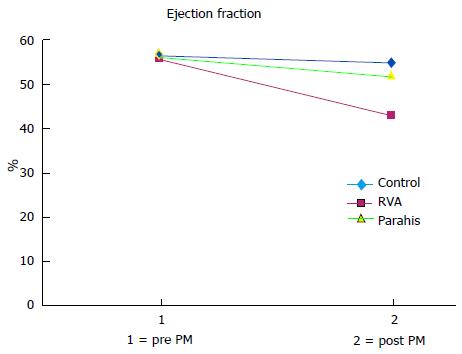
Left ventricular volumes and ejection fraction (EF) values in the controls and parahisian pacing groups of patients are shown in Table 4. In patients without significant ventricular pacing (NO pacing group), left ventricular function was almost unchanged during follow-up; indeed, no significant changes in volumes and EF were recorded (P = ns). All patients on ventricular pacing, however, presented some differences. The average end-diastolic and end-systolic volumes increased markedly in the RVA group, while in the PH group these volume increments were so modest as to be almost comparable to those observed in control patients. In RVA-paced patients, the increased left ventricular volumes led to a significant reduction in the mean EF to below normal values (post-pacing average of 43% ± 9% vs 56% ± 6% at the baseline; mean decrease of 13.2 percentage points, P < 0.0001); in PH patients, left ventricular function was fairly well preserved (post-pacing EF 49% ± 11%, vs 53% ± 11% baseline; mean change of 4 percentage points) (Figure 4).
| Basal | Follow-up | P | ||
| Contr (22 pts) | EDV (mL) | 88 ± 25 | 99 ± 46 | 0.23 (ns) |
| ESV (mL) | 38 ± 13 | 46 ± 29 | 0.11 (ns) | |
| EF (%) | 57 ± 5 | 56 ± 5 | 0.1 (ns) | |
| RVA (33 pts) | EDV (mL) | 98 ± 23 | 139 ± 31 | < 0.0001 |
| ESV (mL) | 44 ± 14 | 79 ± 22 | < 0.0001 | |
| EF (%) | 56 ± 6 | 43 ± 9 | < 0.0001 | |
| PH (121 pts) | EDV (mL) | 100 ± 37 | 104 ± 40 | 0.35 (ns) |
| ESV (mL) | 49 ± 27 | 55 ± 31 | 0.05 | |
| EF (%) | 53 ± 11 | 49 ± 11 | 0.01 |
Cardiac pacing aims at providing an adequate cardiac rhythm, restoring a physiological excito-conduction of the heart. Two elements are traditionally considered as cornerstone for “physiologic pacing”: the maineinance of a correct atrioventricular sequence and the presence of chronotropic response (via rate-responsive sensors) during exercise or stress; till recent times, dual-chamber rate-response pacemakers were considered “physiological”.
However, we know that conventional RVA pacing has the potential to induce electro-mechanichal desyncronization, causing potential harm (negative remodeling and worsening heart failure) in less than normal heart[23,24].
Therefore, a real physiological pacing must: (1) increase the cardiac frequency according to the metabolic needs; (2) keep correct atrioventricular sequence of activation; and (3) keep inter and intraventricular synchrony.
Biventricular pacing proved effective in improving quality of life and cardiac function in patients with left bundle branch block (spontaneous electromechanical desynchronization)[14,17]. However, when intraventricular conduction is preserved and an atrioventricular block occurs, pacing must be as physiological as possible[25]. His bundle pacing has already established itself as an effective alternative to biventricular pacing for these patients. Indeed, it uses the His-Purkinje system without inducing intraventricular conduction delays[18,19]. Unfortunately, direct His bundle pacing may be challenging, needs high pacing electrical output and may pose the risk of traumatic (post-screwing of the pacing lead) His bundle block[20].
In our experience, a simpler and reliable method to achieve physiological intraventricular conduction is the so-called parahisian pacing: placing the tip of the catheter in the upper muscular part of the interventricular septum, activation is granted through the myocardium, but the His-Purkinje conduction system is activated at the same time[21]. With this technique, a fairly narrow (120-130 ms) QRS with an electrical axis concordant to the non-paced QRS can be obtained[26].
We already presented data about the improvement of hemodynamic and functional parameters obtained with parahisian pacing compared to conventional right apical pacing at a short follow up in patients undergoing AV node ablation for permanent atrial fibrillation with unsatisfactory rate control despite optimal therapy[27,28].
Long-term follow-up confirms these results, showing that parahisian pacing confers a durable improvement of quality of life, functional class and exercise tolerance. The improvement is sustained over time, modifying the expected natural progression of the underlying cardiopathy by means of a preserved atrioventricular and interventricular synchrony and by rate regularization; ejection fraction was positively affected, too, avoiding deterioration usually observed in paced patients.
Therefore, parahisian pacing should be considered easy to apply, reliable and effective in preventing the detrimental remodeling caused by non-physiological right ventricular apical pacing[29]. This kind of physiological pacing may be proposed as first line in patients needing high ventricular pacing percentage, presenting with preserved intraventricular conduction and mild systolic left ventricular dysfunction[30-32].
The aim of the study was to evaluate the long term safety of septal parahisian permanent cardiac pacing and this has been definitively confirmed.
As for the long term efficacy of this pacing site, the main limitation of the study was the heterogeneity of our population: 54% of patients had atrial fibrillation (21% with concomitant AV node ablation) and VVIR pacing, 46% were in sinus rhythm with various AV block degrees and DDD(R) pacing.
This can surely affect the general prognosis, but all patients had an high percentage of ventricular pacing and a more “physiological” site of stimulation, respect to RVA pacing, could make the difference. In effect, basal NYHA functional class, higher in parahisian group than in control groups of patients, improved during the follow up; on the contrary, patients with high percentage RVA pacing had a NYHA class worsening (Table 3). Unfortunately, we could not collect definite informations about hospital readmission for heart failure and long-term mortality of our patients: this is another limitation to better establish the long term efficacy of parahisian septal permanent pacing.
The second main limit of the the study was the retrospective evaluation of patients; however, in every group (NO pacing, RVA pacing and SEPTAL pacing) the patients evaluated were consecutively enrolled and this could reproduce a real world situation.
Surely, the superiority of parahisian septal vs RVA permanent pacing should be evaluated and confirmed with a prospective multicenter study.
The usual way to treat symptomatic severe bradycardia is to implant an artificial pacemaker, with a stimulating lead in the apex of the right ventricle of the heart, providing electrical stimuli that generate the pulse. Unfortunately, that kind of stimulation can be detrimental in the long term, causing progressive heart failure in a number of patients. Alternative strategies were attempted: one of the most promising seems the placement of the stimulating lead in the upper region of the interventricular septum (parahisian site, near the division between right and left bundle branches), a position that can partly reproduce the physiological electrical activation of a normal heart. This kind of cardiac stimulation is called “septal parahisian pacing”.
The authors’ group pioneered septal parahisian stimulation; the authors think that this kind of cardiac pacing must have a wide diffusion (as an alternative to the usual way) and they provide support to their hypothesis with this paper, reporting safety and efficacy in a long term follow up.
In the past, some concerns arose about long-term safety and efficacy of septal pacing. In addition, some authors described it as difficult to perform for the traditionally trained interventional cardiologists. This paper shows that septal parahisian pacing can be easily obtained (some “tips and tricks” are provided to attempt the procedure) and that long term safety is guaranteed; in addition, better outcomes in term of exercise capacity, quality of life and cardiac function are obtained.
Patients with symptomatic severe bradycardia will benefit from a physiologic heart stimulation, if treated with septal parahisian pacing, avoiding unfavorable long term effect of the conventional electrical therapy.
Septal parahisian (PH) pacing is a kind of cardiac stimulation that uses a transvenous lead placed in the upper region of the inter-ventricular septum, near the division between right and left bundle branches, to determine “physiological” ventricular electrical depolarization. The ejection fraction of the left ventricle is the measure commonly used to quantify cardiac function, and is negatively affected by conventional cardiac artificial pacemakers in a number of patients.
Very good work has been performed by Eraldo Occhetta et al comparing the safety, efficacy and benefits of right ventricular septal pacing vs right ventricular apical pacing. Congratulation to the authors for adding valuable data for the long-term superiority of septal pacing above apical stimulation.
P- Reviewer: Lee TS, Said SAM, Teragawa H S- Editor: Song XX L- Editor: A E- Editor: Wu HL
| 1. | Prinzen FW, Peschar M. Relation between the pacing induced sequence of activation and left ventricular pump function in animals. Pacing Clin Electrophysiol. 2002;25:484-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 197] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 2. | Tantengco MV, Thomas RL, Karpawich PP. Left ventricular dysfunction after long-term right ventricular apical pacing in the young. J Am Coll Cardiol. 2001;37:2093-2100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 265] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 3. | Barold SS. Adverse effects of ventricular desynchronization induced by long-term right ventricular pacing. J Am Coll Cardiol. 2003;42:624-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Karpawich PP, Rabah R, Haas JE. Altered cardiac histology following apical right ventricular pacing in patients with congenital atrioventricular block. Pacing Clin Electrophysiol. 1999;22:1372-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 205] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 5. | Tse HF, Yu C, Wong KK, Tsang V, Leung YL, Ho WY, Lau CP. Functional abnormalities in patients with permanent right ventricular pacing: the effect of sites of electrical stimulation. J Am Coll Cardiol. 2002;40:1451-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 244] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 6. | Connolly SJ, Kerr CR, Gent M, Roberts RS, Yusuf S, Gillis AM, Sami MH, Talajic M, Tang AS, Klein GJ. Effects of physiologic pacing versus ventricular pacing on the risk of stroke and death due to cardiovascular causes. Canadian Trial of Physiologic Pacing Investigators. N Engl J Med. 2000;342:1385-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 448] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 7. | Nielsen JC, Kristensen L, Andersen HR, Mortensen PT, Pedersen OL, Pedersen AK. A randomized comparison of atrial and dual-chamber pacing in 177 consecutive patients with sick sinus syndrome: echocardiographic and clinical outcome. J Am Coll Cardiol. 2003;42:614-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 354] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 8. | Wilkoff BL, Cook JR, Epstein AE, Greene HL, Hallstrom AP, Hsia H, Kutalek SP, Sharma A. Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: the Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. JAMA. 2002;288:3115-3123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1485] [Cited by in RCA: 1465] [Article Influence: 63.7] [Reference Citation Analysis (0)] |
| 9. | Sweeney MO, Hellkamp AS, Ellenbogen KA, Greenspon AJ, Freedman RA, Lee KL, Lamas GA. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation. 2003;107:2932-2937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1195] [Cited by in RCA: 1217] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 10. | Goldenberg I, Moss AJ, Hall WJ, McNitt S, Zareba W, Andrews ML, Cannom DS. Causes and consequences of heart failure after prophylactic implantation of a defibrillator in the multicenter automatic defibrillator implantation trial II. Circulation. 2006;113:2810-2817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 173] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 11. | Manolis AS. The deleterious consequences of right ventricular apical pacing: time to seek alternate site pacing. Pacing Clin Electrophysiol. 2006;29:298-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 114] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | Gammage MD. Base over apex: does site matter for pacing the right ventricle? Europace. 2008;10:572-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Francis J, Jayesh B, Ashishkumar M, Faizal A, Mond H. Right ventricular septal pacing: has it come of age? Indian Pacing Electrophysiol J. 2010;10:69-72. [PubMed] |
| 14. | Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, Carson P, DiCarlo L, DeMets D, White BG. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140-2150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4324] [Cited by in RCA: 4152] [Article Influence: 197.7] [Reference Citation Analysis (0)] |
| 15. | Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4673] [Cited by in RCA: 4551] [Article Influence: 227.6] [Reference Citation Analysis (0)] |
| 16. | Yu CM, Lin H, Fung WH, Zhang Q, Kong SL, Sanderson JE. Comparison of acute changes in left ventricular volume, systolic and diastolic functions, and intraventricular synchronicity after biventricular and right ventricular pacing for heart failure. Am Heart J. 2003;145:E18. [PubMed] |
| 17. | Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, Estes NA, Foster E, Greenberg H, Higgins SL. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361:1329-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2231] [Cited by in RCA: 2308] [Article Influence: 144.3] [Reference Citation Analysis (0)] |
| 18. | Deshmukh P, Casavant DA, Romanyshyn M, Anderson K. Permanent, direct His-bundle pacing: a novel approach to cardiac pacing in patients with normal His-Purkinje activation. Circulation. 2000;101:869-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 544] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 19. | Zanon F, Baracca E, Aggio S, Pastore G, Boaretto G, Cardano P, Marotta T, Rigatelli G, Galasso M, Carraro M. A feasible approach for direct his-bundle pacing using a new steerable catheter to facilitate precise lead placement. J Cardiovasc Electrophysiol. 2006;17:29-33. [PubMed] |
| 20. | Zanon F, Barold SS. Direct His bundle and paraHisian cardiac pacing. Ann Noninvasive Electrocardiol. 2012;17:70-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Laske TG, Skadsberg ND, Hill AJ, Klein GJ, Iaizzo PA. Excitation of the intrinsic conduction system through his and interventricular septal pacing. Pacing Clin Electrophysiol. 2006;29:397-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Rector TS, Kubo SH, Cohn JH. Patients’ self-assessment of their heart failure: content, reliability, and validity of a new measure, the Minnesota Living with Heart Failure questionnaire. Heart Fail. 1987;3:198-209. |
| 23. | Kindermann M, Hennen B, Jung J, Geisel J, Böhm M, Fröhlig G. Biventricular versus conventional right ventricular stimulation for patients with standard pacing indication and left ventricular dysfunction: the Homburg Biventricular Pacing Evaluation (HOBIPACE). J Am Coll Cardiol. 2006;47:1927-1937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 183] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 24. | Doshi RN, Daoud EG, Fellows C, Turk K, Duran A, Hamdan MH, Pires LA. Left ventricular-based cardiac stimulation post AV nodal ablation evaluation (the PAVE study). J Cardiovasc Electrophysiol. 2005;16:1160-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 442] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 25. | Lieberman R, Grenz D, Mond HG, Gammage MD. Selective site pacing: defining and reaching the selected site. Pacing Clin Electrophysiol. 2004;27:883-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 82] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 26. | Kronborg MB, Mortensen PT, Gerdes JC, Jensen HK, Nielsen JC. His and para-His pacing in AV block: feasibility and electrocardiographic findings. J Interv Card Electrophysiol. 2011;31:255-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 27. | Occhetta E, Bortnik M, Magnani A, Francalacci G, Piccinino C, Plebani L, Marino P. Prevention of ventricular desynchronization by permanent para-Hisian pacing after atrioventricular node ablation in chronic atrial fibrillation: a crossover, blinded, randomized study versus apical right ventricular pacing. J Am Coll Cardiol. 2006;47:1938-1945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 218] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 28. | Victor F, Mabo P, Mansour H, Pavin D, Kabalu G, de Place C, Leclercq C, Daubert JC. A randomized comparison of permanent septal versus apical right ventricular pacing: short-term results. J Cardiovasc Electrophysiol. 2006;17:238-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 103] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 29. | Lustgarten DL, Calame S, Crespo EM, Calame J, Lobel R, Spector PS. Electrical resynchronization induced by direct His-bundle pacing. Heart Rhythm. 2010;7:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 113] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 30. | Occhetta E, Bortnik M, Marino P. Future easy and physiological cardiac pacing. World J Cardiol. 2011;3:32-39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Shimony A, Eisenberg MJ, Filion KB, Amit G. Beneficial effects of right ventricular non-apical vs. apical pacing: a systematic review and meta-analysis of randomized-controlled trials. Europace. 2012;14:81-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 171] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 32. | Hillock RJ, Mond HG. Pacing the right ventricular outflow tract septum: time to embrace the future. Europace. 2012;14:28-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |