Published online Jul 26, 2015. doi: 10.4330/wjc.v7.i7.383
Peer-review started: April 26, 2015
First decision: May 13, 2015
Revised: May 28, 2015
Accepted: June 15, 2015
Article in press: June 16, 2015
Published online: July 26, 2015
Processing time: 101 Days and 14.3 Hours
Aspirin is the mainstay in prophylaxis of cardiovascular diseases. Impaired aspirin antiplatelet effects are associated with enhanced incidence of cardiovascular events. Comedication with non-opioid analgesic drugs has been described to interfere with aspirin, resulting in impaired aspirin antiplatelet effects. Additionally, non-opioid analgesic medication has been shown to enhance the risk of cardiovascular events and death. Pain is very frequent and many patients rely on analgesic drugs to control pain. Therefore effective analgesic options without increased risk of cardiovascular events are desirable. This review focuses on commonly used non-opioid analgesics, interactions with aspirin medication and impact on cardiovascular risk.
Core tip: Aspirin is the mainstay in prophylaxis of cardiovascular diseases. Impaired aspirin antiplatelet effects are associated with enhanced incidence of cardiovascular events. Comedication with non-opioid analgesic drugs has been described to interfere with aspirin, resulting in impaired aspirin antiplatelet effects. Additionally, non-opioid analgesic medication has been shown to enhance the risk of cardiovascular events and death. Pain is very frequent and many patients rely on analgesic drugs to control pain. Therefore effective analgesic options without increased risk of cardiovascular events are desirable. This review focuses on commonly used non-opioid analgesics, interactions with aspirin medication and impact on cardiovascular risk.
- Citation: Polzin A, Hohlfeld T, Kelm M, Zeus T. Impairment of aspirin antiplatelet effects by non-opioid analgesic medication. World J Cardiol 2015; 7(7): 383-391
- URL: https://www.wjgnet.com/1949-8462/full/v7/i7/383.htm
- DOI: https://dx.doi.org/10.4330/wjc.v7.i7.383
Approximately 20% of European adults suffer of acute or chronic pain and rely on analgesic drugs[1,2]. The incidence of pain and usage of non-opioid analgesics is even higher in patients with cardiovascular diseases. Forty percent of patients with coronary artery disease reported intake of non-opioid analgesic drugs[3]. This is not surprising, as the incidence of pain correlates with increasing age[4] and cardiovascular diseases are morbidities of middle to older age patients[5].
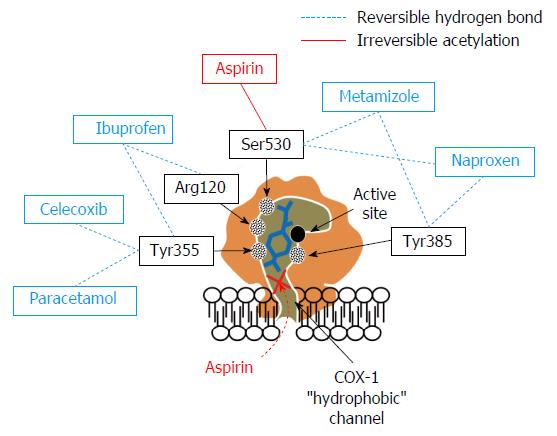
Aspirin (acetylsalicylic acid; ASA) is essential secondary prevention of cardio-and cerebrovascular events[6]. It inhibits cyclooxygenase (COX)-1 by irreversible acetylating serine 530 near the active site. This hampers conversion of arachidonic acid to thromboxane (TX) A2 for the life span of the affected platelet[7]. Aspirin has been shown to reduce the incidence of death, myocardial infarction and stroke[8-10]. However, during the last decade substantial inter-individual variation in pharmacodynamic response to aspirin has been described. This is called high on-treatment platelet reactivity (HTPR) (formerly known as “aspirin resistance”). Patients with HTPR have an increased incidence of death, myocardial infarction and stroke[11]. Many potential mechanisms including non-compliance[12,13], impaired absorption[14], genetic polymorphisms[15] increased turnover rate, enteric coating of aspirin[16,17] and COX-1 independent pathways may cause this HTPR[18]. Besides that, non-opioid analgesic medication may impair aspirin antiplatelet effects. In contrast to above mentioned internal factors, this drug-drug interaction is avoidable. Therefore special attention should be paid to this interaction leading to impaired aspirin antiplatelet effects. This review focuses on (1) mechanisms-; (2) laboratory-; and (3) clinical evidence of the aspirin drug-drug interaction with commonly used non-opioid analgesics.
Non-steroidal anti-inflammatory drugs (NSAIDs) are among the most frequently used drugs in the world[19]. They are available on prescription as well as over the counter. In the United States, 70 million NSAID prescriptions- and 30 billion over the counter sales per year were registered[20]. Approximately 83% of United States - American adults use NSAID to relief pain at least once a year, 29% once a week and 15% daily[21]. In Australia, 55% of people consume NSAIDs at least once per month[22].
The term “NSAID” subsumes a variety of drugs with different chemical structures, pharmacokinetics, pharmacodynamics and mechanism of action but similar effects[23]. The main common feature is prevention of prostaglandin formation by inhibition of COX isoforms. This results in desirable anti-inflammatory and analgesic effects due to COX-2 inhibition in inflamed tissues[24]. On the other hand, inhibition of COX-1 in the gastric mucosa impairs maintenance of the mucosal barrier. This results in an increased risk of gastrointestinal events[25]. Additionally NSAIDs may worsen renal function[26] and affect platelets[27]. Based on the above mentioned differences in pharmacokinetics and pharmacodynamics between different NSAIDs, a considerable variability in analgesic as well as anti-inflammatory and antiplatelet effects is not surprising[23,27]. NSAIDs may also impair cardiovascular prognosis[28,29], possibly by inhibition of prostaglandin synthesis in the vasculature resulting in an increase in blood pressure and disturbed endothelial control of thrombogenesis. This aspect must not be confounded with the pharmacodynamic interaction of non-opioid analgesics with aspirin, which is discussed here. However, it is possible that this interaction contributes to the overall cardiovascular risk of NSAIDs. In the following, we will discuss the most commonly used NSAIDs with respect to their potential to interfere with platelet inhibition by low dose aspirin.
Ibuprofen was the first propionic acid derivative NSAID. Worldwide more than 100 million patients consumed ibuprofen and it is available in more than 100 countries[30]. Ibuprofen forms hydrogen bonds to arginine 120 and tyrosine 355 near the active site of the COX[27]. In-vitro analysis revealed, that ibuprofen inhibits platelet aggregation of human platelets[27]. In healthy individuals, ibuprofen intake led to inhibition of thromboxane formation[31] and platelet aggregation ex-vivo. As ibuprofen inhibited COX transiently, platelet function returned to normal within 4 to 6 h[32]. In-vitro coincubation with aspirin completely abrogated platelet inhibition and thromboxane formation by aspirin. This has been shown ex-vivo in healthy individuals as well with multiple studies demonstrating hampered aspirin antiplatelet effects in ibuprofen co-treated healthy subjects[33-37]. Catella-Lawson et al[33] reported that controlled order of intake with single dose ibuprofen (400 mg) two hours after aspirin intake preserves aspirin antiplatelet effects in healthy individuals. However ibuprofen medication three times per day inhibits aspirin antiplatelet effects independently of the above mentioned order of intake. This finding may be consistent even in lower doses of ibuprofen (150 mg)[36]. None of patients on aspirin for secondary prophylaxis of a cerebrovascular event with ibuprofen co-treatment had adequate aspirin induced inhibition of platelet aggregation. Additionally, 72% of patients experienced recurrent ischemic events. After termination of analgesic medication, aspirin antiplatelet effects restored[32]. In patients with cardiovascular diseases, different studies detected increased incidence of death and recurrent myocardial infarction in aspirin and ibuprofen comedicated patients[38-40]. This finding was confirmed in two meta-analyses, investigating the risk of death and cardiovascular events in patients at increased risk of vascular disease on ibuprofen medication[29,41].
Naproxen is a propionic acid derivative NSAID like ibuprofen. However, its’ pharmacokinetics are different. Plasma half-life of ibuprofen is about two hours, whereas the plasma half-life of naproxen is approximately 12 h[42]. Naproxen forms hydrogen bonds to tyrosine 385 and serine 530 in the active site of COX[27]. This leads to dose-dependent, reversible inhibition of platelet activation in-vitro. However, increasing concentrations of arachidonic acid can overcome this COX-inhibition. Additionally, ASA administration after pre-incubation with naproxen prevents ASA antiplatelet effects[43]. In healthy individuals, naproxen co-treatment with aspirin impairs aspirin antiplatelet effects as well[32,35,37]. This effect was consistent in over the counter doses as well as prescription doses[44]. However, data of clinical studies are contradictory. Some studies described an increased incidence of cardiovascular events in naproxen treated patients[32,45]. However others described a beneficial effect on the incidence of adverse events[39,40,46,47]. Two meta-analyses described no significant increase of vascular events and death in naproxen medicated patients[29,41]. The reasons for these inconstant results are unclear. Naproxen inhibits aspirin antiplatelet effects in-vitro similar to ibuprofen[27]. However, Capone et al[48] described permanent functionally relevant inhibition of ex-vivo platelet function in healthy individuals with 500 mg naproxen twice a day. Therefore, most probably the increased plasma half-life and therefore longer lasting reversible inhibition of platelets by naproxen may be responsible for the protective, respectively less harmful effects of naproxen in patients with cardiovascular diseases. The importance of naproxen’s potential to interfere with the antiplatelet action of aspirin is presently not clear. Different authors recommended preferring the use of naproxen in patients with increased cardiovascular risk[49,50].
Diclofenac is a heteroaryl acetic acid NSAID. It is commonly prescribed to alleviate acute and chronic pain[51]. Like other NSAIDs, diclofenac inhibits COX enzymes (COX-2 > COX-1). There may be additional mechanisms of action inducing its anti-inflammatory, antipyretic and analgesic effects, which are not completely understood. Besides affection of arachidonic acid uptake and release, activation of nitric oxide-cGMP antinociceptive pathway, inhibition of thromboxane prostanoid receptor and lipoxygenase enzymes, it may also inhibit peroxisome proliferator activated receptor gamma, block acid-sensing ion channels, alter interleukin-6 production and inhibit substrate P and N-methyl-D-aspartate receptor hyperalgesia[51].
In-vitro incubation of human platelets with diclofenac inhibited thromboxane formation and platelet aggregation[27]. This was reproducible ex-vivo after diclofenac treatment of healthy volunteers[31,52]. Additionally, platelet function was inhibited after diclofenac - treatment in patients[53,54]. However, there seems to be no interaction with aspirin treatment. ASA antiplatelet effects including inhibition of thromboxane formation were preserved in-vitro after pre-incubation with diclofenac[27]. Additionally, diclofenac treatment in healthy individuals on aspirin revealed sufficient pharmacodynamic response to aspirin as well[33,34]. This may be explained by molecular docking analyses. Diclofenac did not form any hydrogen bond interactions in the hydrophobic active channel of COX. Therefore it appears not to interfere with the ASA induced acetylation of serine 530, preserving aspirin antiplatelet effects despite of diclofenac co-treatment[27].
In aspirin and analgesic co-treated patients with coronary artery disease, MacDonald et al[38] described improved outcome of diclofenac co-treated patients in comparison to ibuprofen comedicated patients. In contrast, in a study including 83667 patients after myocardial infarction, diclofenac co-treatment was associated with the highest risk of recurrent myocardial infarction and death during 90 d[39] as well as in one- and five year follow-up[40]. These findings were confirmed in two meta-analysis investigating death and cardiovascular events in patients with analgesic medication. Both reported an increased risk in diclofenac treated patients as well[29,41].
Anti-inflammatory and analgesic effects of COX- inhibitors are largely mediated by prevention of COX-2 induced prostaglandin formation in inflamed tissues[24]. Impairment of the gastric mucosal barrier resulting in increased incidence of gastrointestinal events, and affection of platelets are mostly caused by COX-1 inhibition[25,27]. Therefore, during the 90’s NSAIDs with COX-2 selectivity were developed[55].
Conflicting data has been reported regarding impairment of aspirin antiplatelet effects by COX-2 inhibitors. Despite COX-2 selectivity, celecoxib was shown to form a hydrogen bond in the hydrophobic channel of COX-1 with tyrosine 355[27]. This goes in line with in-vitro experiments, demonstrating inhibition of thromboxane formation and platelet aggregation by celecoxib incubation. In ASA and celecoxib co-incubated platelets, ASA antiplatelet effects were inhibited[27]. Celecoxib administration in dogs interfered with the ability of aspirin to inhibit platelet aggregation[56]. In contrast, no impact on platelet function was observed in healthy individuals on celecoxib treatment[35]. Additionally, different groups reported that COX-2 inhibiting co-treatment in aspirin treated healthy individuals did not impair the pharmacodynamic response to aspirin[33,35,37,57]. Regardless, multiple studies reported increased risk of cardiovascular events and death in patients receiving COX-2 inhibitors independently of concomitant aspirin intake[28,58-60].
Paracetamol is an aniline derivative. It is one of the most widely used antipyretic and analgesic drugs worldwide, especially as the risk of gastrointestinal bleeding events are lower in comparison to NSAIDs[61]. It is available over the counter in many countries[62]. However, in supra-therapeutic doses depletion of endogenous glutathione occurs, resulting in paracetamol metabolism shunting to toxic pathways causing severe, even fatal, hepatotoxicity[63,64]. To date, the mechanisms of analgesia by paracetamol remain unclear despite of extensive investigations[65]. A plethora of mechanisms have been postulated including activation of the endocannabinoid pathway[66,67], inhibition of the nitric oxide synthase[68,69], and indirect activation of descending serotonergic pathways[70-72]. Additionally, inhibition of cyclooxygenases in a direct-[73-75], or indirect (by converting to their oxidized, inactive form[76]) way has been described. Current opinion suggests that paracetamol performs its analgesic actions by multiple mechanisms predominantly in the central nervous system[65].
In-vitro addition of paracetamol to human platelets was reported to inhibit collagen, epinephrine and arachidonic acid induced platelet aggregation and TX formation[77]. Accordingly, in healthy individuals a reduced arachidonic acid-induced TX formation one hour after single dose of paracetamol was shown. However an effect on platelet aggregation was observed in only one of five investigated individuals[77]. Munsterhjelm et al[78] detected an inhibition of platelet aggregation in healthy individuals 10 min after ingestion of paracetamol. Already 90 min after intake of paracetamol, platelet aggregation was restored. Additionally, a combination of paracetamol and diclofenac exhibits an additive effect on platelet inhibition. In comparison to diclofenac treatment in healthy individuals alone, addition of paracetamol preserves inhibition of platelet aggregation and TX formation 90 min after intake. Nevertheless, platelet function normalized after 24 h[52]. In patients, a single dose of paracetamol reduced arachidonic acid induced TX formation, but did not inhibit platelet aggregation in patients[54]. Molecular modelling and docking analyses revealed that paracetamol forms only one single hydrogen-bond to arginine 120 in the hydrophobic channel of COX-1[27]. No aspirin interaction resulting in inhibition of aspirin antiplatelet effects was seen, suggesting that one hydrogen-bond might not be sufficient to induce impairment of aspirin antiplatelet effects[27]. These findings were supported by the results of Catella-Lawson et al[33] and Rao et al[79], both did not observe altered aspirin antiplatelet effects in presence of paracetamol, either. However an increased incidence of first cardiovascular event in patients with frequent use of paracetamol was observed[80]. Potential reasons for this observation may be a dose dependent risk of renal insufficiency of paracetamol[81] which is a predictor of cardiovascular events[82]. Secondly, an increased blood pressure in patients with paracetamol usage has been described[83-86]. Also, an impairment of endothelial function by depletion of glutathione is thinkable to induce this enhanced risk of cardiovascular events[87].
Dipyrone is a pyrazolinone analgesic with favorable analgesic, spasmolytic and antipyretic effects. Gastrointestinal complications are rare in comparison to NSAIDs like ibuprofen or diclofenac[88]. Due to the risk of agranulocytosis, it has been withdrawn in many countries including the United States. Nevertheless, it is extensively used in Central- and South America and freely available over the counter in Mexico. Therefore despite of the withdrawal by the Food and Drug Administration, there is a wide spread use in the United States as well[89]. Moreover, it is freely available and the most used analgesic in Eastern European countries like Bulgaria[90]. Guidelines of the European Society of Cardiology do not recommend the use of NSAIDs in patients with cardiovascular diseases[91,92]. This may be one of the reasons why dipyrone daily doses tripled during the last decade in European countries like Germany[93]. The exact mechanism of its analgesic effects is complex and not completely understood, yet. Besides COX inhibition, an activation of opioidergic- and cannabinoid system in combination with inhibition of central COX-3 appears to contribute to its analgesic effects. Comedication with opioids causes superadditive analgesic effects. It inhibits both prostaglandin dependent and - independent pathways of fever and exhibits its spasmolytic effects by inhibition of intracellular calcium release[94]. Dipyrone inhibits all COX isoforms. It reversibly binds near the active site of COX, forming hydrogen bonds to tyrosin 385 and serine 530[27]. Dipyrone sterically hinders aspirin access to the active site and serine 530 of COX-1. Plasma half-life of dipyrone is about 2.5 h. Therefore it is 7.5 fold longer available than aspirin with a plasma half-life of only 20 min[95]. In-vitro experiments revealed that dipyrone active metabolite impairs ASA induced inhibition of microsomal platelet COX. The active metabolite of dipyrone in therapeutically relevant (low micromolar) concentrations showed little inhibition of platelet aggregation and TX formation. However, it prevented ASA dependent inhibition of platelet aggregation and thromboxane formation caused by arachidonic acid as well as collagen. The effect was reproducible in terms of microsomal platelet COX activity and p-selectin expression as well[96]. Increasing ASA concentrations in-vitro overcame this effect[97]. Additionally, previous incubation with ASA before addition of dipyrone preserves ASA antiplatelet effects[98].
In healthy individuals, aspirin intake sufficiently inhibits platelet aggregation, seven days of additional dipyrone intake completely blunts aspirin antiplatelet effects. This effect was reversible within three days of continued aspirin administration after termination of dipyrone intake. However, multiple daily doses of dipyrone were not tested[97]. Interestingly, Börgermann et al[99] reported that there was no impaired pharmacodynamic response to aspirin in dipyrone treated healthy individuals. However the duration of aspirin and dipyrone co-treatment was only two days. As aspirin antiplatelet effects are irreversible and persist for the remainder of the affected platelets life-span, it is not surprising, that no relevant differences were observed after two days of additional dipyrone treatment. A partial inhibition of aspirin antiplatelet effects has been described after four days of concomitant intake and complete inhibition after seven days[100]. Furthermore, it has been shown that aspirin intake prior to dipyrone preserves aspirin antiplatelet effects, whereas dipyrone intake prior to aspirin completely blunts aspirin antiplatelet effects measured by platelet aggregation in healthy individuals[97].
In patients with coronary artery disease, residual platelet reactivity despite of aspirin was detected in 50% of dipyrone comedicated patients[101]. Residual platelet TX formation in patients with coronary artery disease correlated with the concentration of dipyrone metabolites. Additionally, in dipyrone treated patients after cardiac surgery, the incidence of HTPR to aspirin nearly tripled postoperatively[99]. The impact of this in-vitro and ex-vivo effects on clinical outcome has not been investigated yet.
The optimal analgesic regimen in patients with pain is challenging. Considering laboratory and clinical data, naproxen and paracetamol seem to display the most favourable benefit/risk ratio. However, increased incidence of adverse events has been described with these analgesics as well. Alternatively, aspirin would be a possible alternative to relieve pain and inhibit platelet function. Yet it is well known, that analgesic doses of aspirin increase the risk of gastrointestinal complications (Figure 1 and Table 1). If medication with non-opioid analgesics is considered indispensable, a strict order of intake, with aspirin medication at least two hours prior to analgesic medication is advisable. However, the optimal analgesic and antiplatelet regimen in patients with increased risk of cardiovascular disease is still unknown and requires further investigation.
| Substance | Plateletinhibition | Aspirin interaction | Benefits | Risks |
| Aspirin | Irreversible | - | Analgesic, antipyretic, anti-inflammatory | Bleeding events |
| Reduction of cardiovascular events and death | ||||
| Ibuprofen | Reversible (half-life 1-4 h) | Yes | Analgesic, antipyretic, anti-inflammatory | Death |
| Cardiovascular events | ||||
| Bleeding events | ||||
| Naproxen | Reversible (half-life 12-24 h) | Yes | Analgesic, antipyretic, anti-inflammatory | Bleeding events |
| Reduction of cardiovascular events (?) | ||||
| Diclofenac | Reversible (half-life 1-2 h) | No | Analgesic, antipyretic, anti-inflammatory | Death |
| Cardiovascular events | ||||
| Bleeding events | ||||
| Celecoxib | Reversible (half-life 8-13 h) | Yes | Analgesic, antipyretic, anti-inflammatory | Death |
| Less gastrointestinal events | Cardiovascular events | |||
| Paracetamol | Reversible (half-life 1-4 h) | No | Analgesic, antipyretic | Cardiovascular events |
| Less gastrointestinal events | ||||
| Dipyrone | Reversible (half-life 2-4 h) | Yes | Analgesic, antipyretic, anti-inflammatory | Risk of death and cardiovascular events not investigated |
| Less gastrointestinal events |
P- Reviewer: Al-Mohammad A, Iacoviello M, Nunez-Gil IJ, Satoh H S- Editor: Ji FF L- Editor: A E- Editor: Wang CH
| 1. | Reid KJ, Harker J, Bala MM, Truyers C, Kellen E, Bekkering GE, Kleijnen J. Epidemiology of chronic non-cancer pain in Europe: narrative review of prevalence, pain treatments and pain impact. Curr Med Res Opin. 2011;27:449-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 330] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 2. | van Hecke O, Torrance N, Smith BH. Chronic pain epidemiology and its clinical relevance. Br J Anaesth. 2013;111:13-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 404] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 3. | Gislason GH, Jacobsen S, Rasmussen JN, Rasmussen S, Buch P, Friberg J, Schramm TK, Abildstrom SZ, Køber L, Madsen M. Risk of death or reinfarction associated with the use of selective cyclooxygenase-2 inhibitors and nonselective nonsteroidal antiinflammatory drugs after acute myocardial infarction. Circulation. 2006;113:2906-2913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 284] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 4. | Raftery MN, Sarma K, Murphy AW, De la Harpe D, Normand C, McGuire BE. Chronic pain in the Republic of Ireland--community prevalence, psychosocial profile and predictors of pain-related disability: results from the Prevalence, Impact and Cost of Chronic Pain (PRIME) study, part 1. Pain. 2011;152:1096-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 121] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 5. | Trzos E, Uznańska B, Rechciński T, Krzemińska-Pakuła M, Bugała M, Kurpesa M. Myocardial infarction in young people. Cardiol J. 2009;16:307-311. [PubMed] |
| 6. | Halvorsen S, Andreotti F, ten Berg JM, Cattaneo M, Coccheri S, Marchioli R, Morais J, Verheugt FW, De Caterina R. Aspirin therapy in primary cardiovascular disease prevention: a position paper of the European Society of Cardiology working group on thrombosis. J Am Coll Cardiol. 2014;64:319-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 126] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 7. | Pamukcu B. A review of aspirin resistance; definition, possible mechanisms, detection with platelet function tests, and its clinical outcomes. J Thromb Thrombolysis. 2007;23:213-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Collaborative overview of randomised trials of antiplatelet therapy--I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. Antiplatelet Trialists’ Collaboration. BMJ. 1994;308:81-106. [PubMed] |
| 9. | Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71-86. [PubMed] |
| 10. | Berger JS, Brown DL, Becker RC. Low-dose aspirin in patients with stable cardiovascular disease: a meta-analysis. Am J Med. 2008;121:43-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 131] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 11. | Krasopoulos G, Brister SJ, Beattie WS, Buchanan MR. Aspirin “resistance” and risk of cardiovascular morbidity: systematic review and meta-analysis. BMJ. 2008;336:195-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 513] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 12. | Schwartz KA, Schwartz DE, Barber K, Reeves M, De Franco AC. Non-compliance is the predominant cause of aspirin resistance in chronic coronary arterial disease patients. J Transl Med. 2008;6:46. [PubMed] |
| 13. | Ho PM, Spertus JA, Masoudi FA, Reid KJ, Peterson ED, Magid DJ, Krumholz HM, Rumsfeld JS. Impact of medication therapy discontinuation on mortality after myocardial infarction. Arch Intern Med. 2006;166:1842-1847. [PubMed] |
| 14. | Benedek IH, Joshi AS, Pieniaszek HJ, King SY, Kornhauser DM. Variability in the pharmacokinetics and pharmacodynamics of low dose aspirin in healthy male volunteers. J Clin Pharmacol. 1995;35:1181-1186. [PubMed] |
| 15. | Li M, Shi J, Fu L, Wang H, Zhou B, Wu X. Genetic polymorphism of MMP family and coronary disease susceptibility: a meta-analysis. Gene. 2012;495:36-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Mahoney FI, Barthel DW. Functional evaluation: the barthel index. Md State Med J. 1965;14:61-65. [PubMed] |
| 17. | Cox D, Maree AO, Dooley M, Conroy R, Byrne MF, Fitzgerald DJ. Effect of enteric coating on antiplatelet activity of low-dose aspirin in healthy volunteers. Stroke. 2006;37:2153-2158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 153] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 18. | Linden MD, Tran H, Woods R, Tonkin A. High platelet reactivity and antiplatelet therapy resistance. Semin Thromb Hemost. 2012;38:200-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Rollason V, Samer CF, Daali Y, Desmeules JA. Prediction by pharmacogenetics of safety and efficacy of non-steroidal anti- inflammatory drugs: a review. Curr Drug Metab. 2014;15:326-343. [PubMed] |
| 20. | Green GA. Understanding NSAIDs: from aspirin to COX-2. Clin Cornerstone. 2001;3:50-60. [PubMed] |
| 21. | Wilcox CM, Cryer B, Triadafilopoulos G. Patterns of use and public perception of over-the-counter pain relievers: focus on nonsteroidal antiinflammatory drugs. J Rheumatol. 2005;32:2218-2224. [PubMed] |
| 22. | Stosic R, Dunagan F, Palmer H, Fowler T, Adams I. Responsible self-medication: perceived risks and benefits of over-the-counter analgesic use. Int J Pharm Pract. 2011;19:236-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 23. | Sharma S, Prasad A, Anand KS. Nonsteroidal anti-inflammatory drugs in the management of pain and inflammation: a basis for drug selection. Am J Ther. 1999;6:3-11. [PubMed] |
| 24. | Cashman JN. The mechanisms of action of NSAIDs in analgesia. Drugs. 1996;52 Suppl 5:13-23. [PubMed] |
| 25. | Simon LS, Weaver AL, Graham DY, Kivitz AJ, Lipsky PE, Hubbard RC, Isakson PC, Verburg KM, Yu SS, Zhao WW. Anti-inflammatory and upper gastrointestinal effects of celecoxib in rheumatoid arthritis: a randomized controlled trial. JAMA. 1999;282:1921-1928. [PubMed] |
| 26. | Whelton A. Nephrotoxicity of nonsteroidal anti-inflammatory drugs: physiologic foundations and clinical implications. Am J Med. 1999;106:13S-24S. [PubMed] |
| 27. | Saxena A, Balaramnavar VM, Hohlfeld T, Saxena AK. Drug/drug interaction of common NSAIDs with antiplatelet effect of aspirin in human platelets. Eur J Pharmacol. 2013;721:215-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (1)] |
| 28. | Kearney PM, Baigent C, Godwin J, Halls H, Emberson JR, Patrono C. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ. 2006;332:1302-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1001] [Cited by in RCA: 943] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 29. | Trelle S, Reichenbach S, Wandel S, Hildebrand P, Tschannen B, Villiger PM, Egger M, Jüni P. Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ. 2011;342:c7086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 800] [Cited by in RCA: 761] [Article Influence: 54.4] [Reference Citation Analysis (0)] |
| 30. | Busson M. Update on ibuprofen: review article. J Int Med Res. 1986;14:53-62. [PubMed] |
| 31. | Raineri-Gerber I, von Felten A. Inhibition of thrombocyte function by non-steroidal anti-rheumatic agents: a comparative study between diclofenac, acemetacin, mefenamic acid and ibuprofen. Schweiz Med Wochenschr. 1991;121:783-787. [PubMed] |
| 32. | Gengo FM, Rubin L, Robson M, Rainka M, Gengo MF, Mager DE, Bates V. Effects of ibuprofen on the magnitude and duration of aspirin’s inhibition of platelet aggregation: clinical consequences in stroke prophylaxis. J Clin Pharmacol. 2008;48:117-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 33. | Catella-Lawson F, Reilly MP, Kapoor SC, Cucchiara AJ, DeMarco S, Tournier B, Vyas SN, FitzGerald GA. Cyclooxygenase inhibitors and the antiplatelet effects of aspirin. N Engl J Med. 2001;345:1809-1817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1020] [Cited by in RCA: 927] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 34. | Schuijt MP, Huntjens-Fleuren HW, de Metz M, Vollaard EJ. The interaction of ibuprofen and diclofenac with aspirin in healthy volunteers. Br J Pharmacol. 2009;157:931-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 35. | Gladding PA, Webster MW, Farrell HB, Zeng IS, Park R, Ruijne N. The antiplatelet effect of six non-steroidal anti-inflammatory drugs and their pharmacodynamic interaction with aspirin in healthy volunteers. Am J Cardiol. 2008;101:1060-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 111] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 36. | Awa K, Satoh H, Hori S, Sawada Y. Prediction of time-dependent interaction of aspirin with ibuprofen using a pharmacokinetic/pharmacodynamic model. J Clin Pharm Ther. 2012;37:469-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 37. | Meek IL, Vonkeman HE, Kasemier J, Movig KL, van de Laar MA. Interference of NSAIDs with the thrombocyte inhibitory effect of aspirin: a placebo-controlled, ex vivo, serial placebo-controlled serial crossover study. Eur J Clin Pharmacol. 2013;69:365-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 38. | MacDonald TM, Wei L. Effect of ibuprofen on cardioprotective effect of aspirin. Lancet. 2003;361:573-574. [PubMed] |
| 39. | Schjerning Olsen AM, Fosbøl EL, Lindhardsen J, Folke F, Charlot M, Selmer C, Lamberts M, Bjerring Olesen J, Køber L, Hansen PR. Duration of treatment with nonsteroidal anti-inflammatory drugs and impact on risk of death and recurrent myocardial infarction in patients with prior myocardial infarction: a nationwide cohort study. Circulation. 2011;123:2226-2235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 215] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 40. | Olsen AM, Fosbøl EL, Lindhardsen J, Folke F, Charlot M, Selmer C, Bjerring Olesen J, Lamberts M, Ruwald MH, Køber L. Long-term cardiovascular risk of nonsteroidal anti-inflammatory drug use according to time passed after first-time myocardial infarction: a nationwide cohort study. Circulation. 2012;126:1955-1963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 41. | Bhala N, Emberson J, Merhi A, Abramson S, Arber N, Baron JA, Bombardier C, Cannon C, Farkouh ME, FitzGerald GA. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet. 2013;382:769-779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1255] [Cited by in RCA: 1202] [Article Influence: 100.2] [Reference Citation Analysis (0)] |
| 42. | Gilaman A, Goodman LS, Rall TW, Murad F. The Pharmacological Basis of Therapeutics. New York: MacMillan Publishing Company 1985; 701-702. |
| 43. | Capone ML, Sciulli MG, Tacconelli S, Grana M, Ricciotti E, Renda G, Di Gregorio P, Merciaro G, Patrignani P. Pharmacodynamic interaction of naproxen with low-dose aspirin in healthy subjects. J Am Coll Cardiol. 2005;45:1295-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 199] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 44. | Schiff M, Hochberg MC, Oldenhof J, Brune K. Platelet inhibitory effects of OTC doses of naproxen sodium compared with prescription dose naproxen sodium and low-dose aspirin. Curr Med Res Opin. 2009;25:2471-2477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 45. | Cardiovascular and cerebrovascular events in the randomized, controlled Alzheimer’s Disease Anti-Inflammatory Prevention Trial (ADAPT). PLoS Clin Trials. 2006;1:e33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 143] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 46. | Kimmel SE, Berlin JA, Reilly M, Jaskowiak J, Kishel L, Chittams J, Strom BL. The effects of nonselective non-aspirin non-steroidal anti-inflammatory medications on the risk of nonfatal myocardial infarction and their interaction with aspirin. J Am Coll Cardiol. 2004;43:985-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 80] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 47. | Bombardier C, Laine L, Reicin A, Shapiro D, Burgos-Vargas R, Davis B, Day R, Ferraz MB, Hawkey CJ, Hochberg MC. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group. N Engl J Med. 2000;343:1520-1528, 2 p following 1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2752] [Cited by in RCA: 2534] [Article Influence: 101.4] [Reference Citation Analysis (0)] |
| 48. | Capone ML, Tacconelli S, Sciulli MG, Grana M, Ricciotti E, Minuz P, Di Gregorio P, Merciaro G, Patrono C, Patrignani P. Clinical pharmacology of platelet, monocyte, and vascular cyclooxygenase inhibition by naproxen and low-dose aspirin in healthy subjects. Circulation. 2004;109:1468-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 176] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 49. | Soubrier M, Rosenbaum D, Tatar Z, Lahaye C, Dubost JJ, Mathieu S. Vascular effects of nonsteroidal antiinflammatory drugs. Joint Bone Spine. 2013;80:358-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 50. | McGettigan P, Henry D. Cardiovascular risk with non-steroidal anti-inflammatory drugs: systematic review of population-based controlled observational studies. PLoS Med. 2011;8:e1001098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 437] [Cited by in RCA: 368] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 51. | Gan TJ. Diclofenac: an update on its mechanism of action and safety profile. Curr Med Res Opin. 2010;26:1715-1731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 362] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 52. | Munsterhjelm E, Niemi TT, Syrjälä MT, Ylikorkala O, Rosenberg PH. Propacetamol augments inhibition of platelet function by diclofenac in volunteers. Br J Anaesth. 2003;91:357-362. [PubMed] |
| 53. | Scharbert G, Gebhardt K, Sow Z, Duris M, Deusch E, Kozek-Langenecker S. Point-of-care platelet function tests: detection of platelet inhibition induced by nonopioid analgesic drugs. Blood Coagul Fibrinolysis. 2007;18:775-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 54. | Silvanto M, Munsterhjelm E, Savolainen S, Tiainen P, Niemi T, Ylikorkala O, Scheinin H, Olkkola KT. Effect of 3 g of intravenous paracetamol on post-operative analgesia, platelet function and liver enzymes in patients undergoing tonsillectomy under local anaesthesia. Acta Anaesthesiol Scand. 2007;51:1147-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 55. | Amer M, Bead VR, Bathon J, Blumenthal RS, Edwards DN. Use of nonsteroidal anti-inflammatory drugs in patients with cardiovascular disease: a cautionary tale. Cardiol Rev. 2010;18:204-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 56. | Rimon G, Sidhu RS, Lauver DA, Lee JY, Sharma NP, Yuan C, Frieler RA, Trievel RC, Lucchesi BR, Smith WL. Coxibs interfere with the action of aspirin by binding tightly to one monomer of cyclooxygenase-1. Proc Natl Acad Sci USA. 2010;107:28-33. [PubMed] |
| 57. | Wilner KD, Rushing M, Walden C, Adler R, Eskra J, Noveck R, Vargas R. Celecoxib does not affect the antiplatelet activity of aspirin in healthy volunteers. J Clin Pharmacol. 2002;42:1027-1030. [PubMed] |
| 58. | Sawicki PT, Bender R, Selke GW, Klauber J, Gutschmidt S. [Assessment of the number of cardio- and cerebrovascular events due to rofecoxib (Vioxx) in Germany between 2001 and 2004]. Med Klin (Munich). 2006;101:191-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 59. | Solomon SD, McMurray JJ, Pfeffer MA, Wittes J, Fowler R, Finn P, Anderson WF, Zauber A, Hawk E, Bertagnolli M. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352:1071-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1534] [Cited by in RCA: 1457] [Article Influence: 72.9] [Reference Citation Analysis (0)] |
| 60. | Bresalier RS, Sandler RS, Quan H, Bolognese JA, Oxenius B, Horgan K, Lines C, Riddell R, Morton D, Lanas A. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med. 2005;352:1092-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1849] [Cited by in RCA: 1725] [Article Influence: 86.3] [Reference Citation Analysis (0)] |
| 61. | Lanza FL, Codispoti JR, Nelson EB. An endoscopic comparison of gastroduodenal injury with over-the-counter doses of ketoprofen and acetaminophen. Am J Gastroenterol. 1998;93:1051-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 62. | Black M. Acetaminophen hepatotoxicity. Annu Rev Med. 1984;35:577-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 176] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 63. | Toussaint K, Yang XC, Zielinski MA, Reigle KL, Sacavage SD, Nagar S, Raffa RB. What do we (not) know about how paracetamol (acetaminophen) works? J Clin Pharm Ther. 2010;35:617-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 64. | Spooner JB, Harvey JG. The history and usage of paracetamol. J Int Med Res. 1976;4:1-6. [PubMed] |
| 65. | Smith HS. Potential analgesic mechanisms of acetaminophen. Pain Physician. 2009;12:269-280. [PubMed] |
| 66. | Högestätt ED, Jönsson BA, Ermund A, Andersson DA, Björk H, Alexander JP, Cravatt BF, Basbaum AI, Zygmunt PM. Conversion of acetaminophen to the bioactive N-acylphenolamine AM404 via fatty acid amide hydrolase-dependent arachidonic acid conjugation in the nervous system. J Biol Chem. 2005;280:31405-31412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 304] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 67. | Ottani A, Leone S, Sandrini M, Ferrari A, Bertolini A. The analgesic activity of paracetamol is prevented by the blockade of cannabinoid CB1 receptors. Eur J Pharmacol. 2006;531:280-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 172] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 68. | Bujalska M. Effect of cyclooxygenase and NO synthase inhibitors administered centrally on antinociceptive action of acetaminophen (Part II). Pol J Pharmacol. 2003;55:1001-1011. [PubMed] |
| 69. | Ryu YS, Lee JH, Seok JH, Hong JH, Lee YS, Lim JH, Kim YM, Hur GM. Acetaminophen inhibits iNOS gene expression in RAW 264.7 macrophages: differential regulation of NF-kappaB by acetaminophen and salicylates. Biochem Biophys Res Commun. 2000;272:758-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 70. | Libert F, Bonnefont J, Bourinet E, Doucet E, Alloui A, Hamon M, Nargeot J, Eschalier A. Acetaminophen: a central analgesic drug that involves a spinal tropisetron-sensitive, non-5-HT(3) receptor-mediated effect. Mol Pharmacol. 2004;66:728-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 478] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 71. | Pickering G, Loriot MA, Libert F, Eschalier A, Beaune P, Dubray C. Analgesic effect of acetaminophen in humans: first evidence of a central serotonergic mechanism. Clin Pharmacol Ther. 2006;79:371-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 157] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 72. | Pickering G, Estève V, Loriot MA, Eschalier A, Dubray C. Acetaminophen reinforces descending inhibitory pain pathways. Clin Pharmacol Ther. 2008;84:47-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 93] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 73. | Ayoub SS, Colville-Nash PR, Willoughby DA, Botting RM. The involvement of a cyclooxygenase 1 gene-derived protein in the antinociceptive action of paracetamol in mice. Eur J Pharmacol. 2006;538:57-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 74. | Hinz B, Cheremina O, Brune K. Acetaminophen (paracetamol) is a selective cyclooxygenase-2 inhibitor in man. FASEB J. 2008;22:383-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 296] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 75. | Chandrasekharan NV, Dai H, Roos KL, Evanson NK, Tomsik J, Elton TS, Simmons DL. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression. Proc Natl Acad Sci USA. 2002;99:13926-13931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1314] [Cited by in RCA: 1199] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 76. | Ouellet M, Percival MD. Mechanism of acetaminophen inhibition of cyclooxygenase isoforms. Arch Biochem Biophys. 2001;387:273-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 117] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 77. | Lages B, Weiss HJ. Inhibition of human platelet function in vitro and ex vivo by acetaminophen. Thromb Res. 1989;53:603-613. [PubMed] |
| 78. | Munsterhjelm E, Munsterhjelm NM, Niemi TT, Ylikorkala O, Neuvonen PJ, Rosenberg PH. Dose-dependent inhibition of platelet function by acetaminophen in healthy volunteers. Anesthesiology. 2005;103:712-717. [PubMed] |
| 79. | Rao GH, Reddy KR, White JG. Effect of acetaminophen and salicylate on aspirin-induced inhibition of human platelet cyclo-oxygenase. Prostaglandins Leukot Med. 1982;9:109-115. [PubMed] |
| 80. | Chan AT, Manson JE, Albert CM, Chae CU, Rexrode KM, Curhan GC, Rimm EB, Willett WC, Fuchs CS. Nonsteroidal antiinflammatory drugs, acetaminophen, and the risk of cardiovascular events. Circulation. 2006;113:1578-1587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 215] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 81. | Perneger TV, Whelton PK, Klag MJ. Risk of kidney failure associated with the use of acetaminophen, aspirin, and nonsteroidal antiinflammatory drugs. N Engl J Med. 1994;331:1675-1679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 273] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 82. | Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7995] [Cited by in RCA: 8524] [Article Influence: 405.9] [Reference Citation Analysis (0)] |
| 83. | Forman JP, Stampfer MJ, Curhan GC. Non-narcotic analgesic dose and risk of incident hypertension in US women. Hypertension. 2005;46:500-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 131] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 84. | Chalmers JP, West MJ, Wing LM, Bune AJ, Graham JR. Effects of indomethacin, sulindac, naproxen, aspirin, and paracetamol in treated hypertensive patients. Clin Exp Hypertens A. 1984;6:1077-1093. [PubMed] |
| 85. | Curhan GC, Willett WC, Rosner B, Stampfer MJ. Frequency of analgesic use and risk of hypertension in younger women. Arch Intern Med. 2002;162:2204-2208. [PubMed] |
| 86. | Dedier J, Stampfer MJ, Hankinson SE, Willett WC, Speizer FE, Curhan GC. Nonnarcotic analgesic use and the risk of hypertension in US women. Hypertension. 2002;40:604-608; discussion 601-603. [PubMed] |
| 87. | Prasad A, Andrews NP, Padder FA, Husain M, Quyyumi AA. Glutathione reverses endothelial dysfunction and improves nitric oxide bioavailability. J Am Coll Cardiol. 1999;34:507-514. [PubMed] |
| 88. | Laporte JR, Carné X, Vidal X, Moreno V, Juan J. Upper gastrointestinal bleeding in relation to previous use of analgesics and non-steroidal anti-inflammatory drugs. Catalan Countries Study on Upper Gastrointestinal Bleeding. Lancet. 1991;337:85-89. [PubMed] |
| 89. | Garcia S, Canionero M, Lopes G. Dipyrone-induced granulocytopenia: a case for awareness. Pharmacotherapy. 2006;26:440-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 90. | Nikolova I, Petkova V, Tencheva J, Benbasa N, Voinikov J, Danchev N. Metamizole: A Review Profile of a Well-Known “Forgotten” Drug. Part II: Clinical Profile. Bio Biotechnol Eq. 2014;27:3605-3619. [RCA] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 91. | McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787-1847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3411] [Cited by in RCA: 3554] [Article Influence: 273.4] [Reference Citation Analysis (0)] |
| 92. | Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, Bugiardini R, Crea F, Cuisset T, Di Mario C. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949-3003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2772] [Cited by in RCA: 2994] [Article Influence: 249.5] [Reference Citation Analysis (0)] |
| 93. | Schwabe UP. Arzneiverordnungs-Report 2014: Aktuelle Daten, Kosten, Trends Und Kommentare. Germany: Springer Verlag 2014; . |
| 94. | Jasiecka A, Maślanka T, Jaroszewski JJ. Pharmacological characteristics of metamizole. Pol J Vet Sci. 2014;17:207-214. [PubMed] |
| 95. | Nagelschmitz J, Blunck M, Kraetzschmar J, Ludwig M, Wensing G, Hohlfeld T. Pharmacokinetics and pharmacodynamics of acetylsalicylic acid after intravenous and oral administration to healthy volunteers. Clin Pharmacol. 2014;6:51-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 96. | Hohlfeld T, Zimmermann N, Weber AA, Jessen G, Weber H, Schrör K, Höltje HD, Ebel R. Pyrazolinone analgesics prevent the antiplatelet effect of aspirin and preserve human platelet thromboxane synthesis. J Thromb Haemost. 2008;6:166-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 97. | Polzin A, Richter S, Schrör K, Rassaf T, Merx MW, Kelm M, Hohlfeld T, Zeus T. Prevention of dipyrone (metamizole) induced inhibition of aspirin antiplatelet effects. Thromb Haemost. 2015;114:87-95. [PubMed] |
| 98. | Papp J, Sandor B, Vamos Z, Botor D, Toth A, Rabai M, Kenyeres P, Cseplo P, Juricskay I, Mezosi E. Antiplatelet effect of acetylsalicylic acid, metamizole and their combination - in vitro and in vivo comparisons. Clin Hemorheol Microcirc. 2014;56:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 99. | Börgermann J, Kanashnik A, Sossdorf M, Gummert J, Lösche W. Individual variability of response and non-response to acetyl salicylic acid after cardiac surgery. Platelets. 2010;21:610-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 100. | Hohlfeld T, Saxena A, Schrör K. High on treatment platelet reactivity against aspirin by non-steroidal anti-inflammatory drugs--pharmacological mechanisms and clinical relevance. Thromb Haemost. 2013;109:825-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 101. | Polzin A, Zeus T, Schrör K, Kelm M, Hohlfeld T. Dipyrone (metamizole) can nullify the antiplatelet effect of aspirin in patients with coronary artery disease. J Am Coll Cardiol. 2013;62:1725-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |