Published online May 26, 2015. doi: 10.4330/wjc.v7.i5.238
Peer-review started: September 28, 2014
First decision: December 17, 2014
Revised: January 12, 2015
Accepted: January 30, 2015
Article in press: February 2, 2015
Published online: May 26, 2015
Processing time: 234 Days and 21.3 Hours
New uses of cardiovascular drugs with proven experience are emerging, including for treating cancer. Quinazoline is a compound made up of two fused six member simple aromatic rings, benzene and pyrimidine rings, with several biological effects. Cardiologists first used quinazoline-based α1-adrenoceptor antagonists prazosin, doxazosin, and terazosin; currently available data support their use as safe, well tolerated, and effective add-on therapy in uncontrolled hypertension with additional favourable metabolic effects. Recent findings highlight the anticancer effects of quinazoline-based α1-adrenoceptor antagonists, indicating that they may have a significant role in uncontrolled hypertensive cancer patients without signs of ischemia.
Core tip: New uses of cardiovascular drugs with proven experience and without high cost have been emerging, including to have anticancer abilities by targeting human ether-a-go-go-related gene K(+) channels, epidermal growth factor receptors, vascular endothelial growth factor receptors, as well as to overcome cancer multidrug resistance. Quinazoline-based α1-adrenoceptor antagonists (doxazosin, prazosin, and terazosin) exhibit anticancer abilities and emerging findings indicate that these drugs may have a significant role in uncontrolled hypertensive cancer patients without signs of ischemia.
- Citation: Patanè S. Insights into cardio-oncology: Polypharmacology of quinazoline-based α1-adrenoceptor antagonists. World J Cardiol 2015; 7(5): 238-242
- URL: https://www.wjgnet.com/1949-8462/full/v7/i5/238.htm
- DOI: https://dx.doi.org/10.4330/wjc.v7.i5.238
Despite the tremendous efforts, the medicine field has not yet come to absolute conclusions in oncology and the emerging scenario of the onco-cardiovascular patients is emerging[1]. New targeted anticancer therapies have not proven to be free from cardiovascular side effects while old anticancer therapies have shown delayed serious consequences in long-term cancer survivors[1,2]. Moreover, the heavy burden of concomitant problems and diseases requires changes in setting to prevent serious diseases such as infective endocarditis or in perioperative oncosurgery[3-12]. The progress in cancer biology and treatment has led to a new frontier: the cardio-oncology[1-27]. New uses of cardiovascular drugs with proven experience have been emerging[1,4,5-9,27-32], including to have anticancer abilities by targeting human ether-a-go-go-related gene K(+) (HERG) channels[5], epidermal growth factor (EGF) receptors[9], vascular endothelial growth factor (VEGF) receptors, as well as to overcome cancer multidrug resistance (MDR)[4,26,29,33,34]. These old cardiovascular drugs do not have high cost, however, there was a lack of noninferiority randomized, controlled trials[33], comparing them with new anticancer therapies.
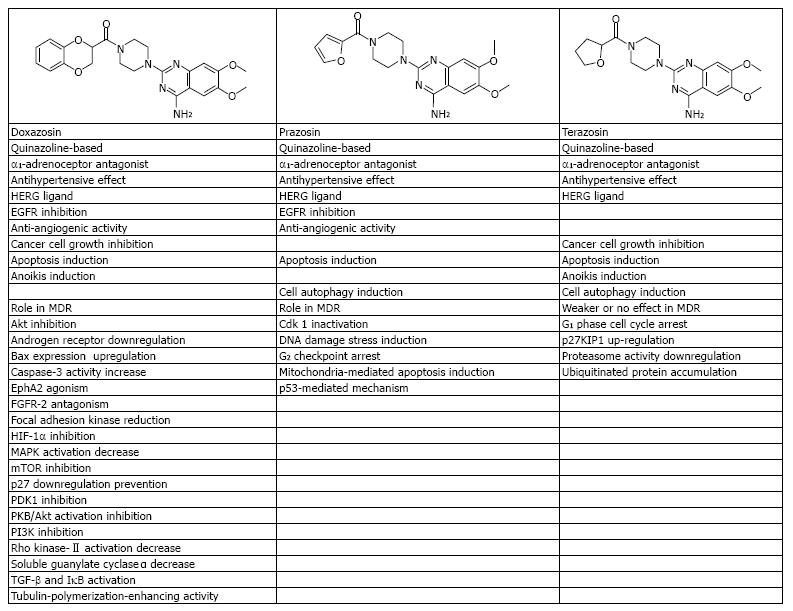
Quinazoline is a compound made up of two fused six member simple aromatic rings, benzene and pyrimidine rings[35]. The search for quinazoline-based substances as cardiovascular agents begun after pharmacological identification of quinazoline compounds having a glycine amide or β-alanine amide residue in the 3rd position that display a hypotensive activity. Other quinazoline derivatives have also demonstrated significant anticancer activities[26,35-39] and new molecules have been synthesized as gefitinib, erlotinib, afatinib, and lapatinib[26,36]. Cardiologists first used quinazoline-based α1-adrenoceptor antagonists, including prazosin, doxazosin, and terazosin[26] (Figure 1). Currently available data have supported the use of these antagonists as safe, well tolerated, and effective add-on therapy in uncontrolled hypertension with additional favorable metabolic effects[37] and without association with an increased risk of heart failure[26,37-39]. New data suggest that adverse cardiac outcome of doxazosin is only among patients with moderate-to-severe ischemia on myocardial perfusion imaging[26,40]. Furthermore, it has been reported that the β-plus α1-blocker pretreatment (propranolol + prazosin) has led to better severity reduction of postresuscitation myocardial tissue injury and myocardial dysfunction with better neurologic function and prolonged duration of survival than propranolol treatment alone[41]. This latter finding will require certainly further evaluation.
Research has suggested several anticancer mechanisms of doxazosin, including upregulation of Bax expression, transforming growth factor (TGF)-β and IκB activation[42], focal adhesion kinase reduction[43], inhibition of protein kinase B/Akt activation[44], and death receptor mediated apoptosis induction[45,46]. Doxazosin is known to be a HERG ligand, EGFR inhibitor[47], VEGF-mediated angiogenic response antagonist[48], and fibroblast growth factor receptor-2 antagonist[48,49]. Several signalling pathways are also inhibited from doxazosin VEGF antagonism including PI3K, Akt, 3-phosphoinositide-dependent protein kinase 1, mammalian target of rapamycin, and hypoxia-inducible factor 1α[49]. In addition, doxazosin is also an agonist of receptor tyrosine kinase triggering ephrin type-A receptor 2 internalization which, in turn, suppresses haptotactic and chemotactic migration of prostate cancer, breast cancer, and glioma cells[26,50]. Notably, a tubulin polymerization-enhancing activity of doxazosin has been found[51]. A doxazosin derivative, DZ-50, impairs tumour growth and metastasis via anoikis[52]; similarly, doxazosin induces changes in morphology consistent with anoikis in both benign and cancerous prostatic cells and increased caspase-3 activity[43]. Moreover, doxazosin significantly decreases benign prostatic hyperplasia-induced mitogen-activated protein kinase kinase and Rho kinase-II activation and decreases expression of soluble guanylate cyclase α[53] also leading to prostate cancer cell growth inhibition[54] Doxazosin also downregulates expression of androgen receptor[54], prevents p27 downregulation[55] and may partly reverse P-glycoprotein/MDR1-mediated cancer multidrug resistance (CMDR) and the transport of anticancer drugs[56].
Terazosin, another quinazoline-based antihypertensive α1-adrenoceptor antagonist[57], is also an HERG ligand[58], a cancer cell growth inhibitor[59], and an apoptosis and anoikis inductor[58,60]. Terazosin induces cell death which is associated with G1 phase cell cycle arrest, upregulation of cyclin-dependent kinase inhibitor 1B (p27KIP1)[60], accumulation of ubiquitinated proteins and downregulation of proteasome activity[46]. Terazosin seems to have weaker or no effects regarding CMDR[55].
Prazosin, another quinazoline-based and antihypertensive α1-adrenoceptor antagonist[60], is also an HERG ligand[58] and EGFR inhibitor[61]. Prazosin induces autophagic cell death via a p53-mediated mechanism[62] and cell apoptosis through the induction of DNA damage stress, leading to cyclin-dependent kinase 1 inactivation and G2 checkpoint arrest triggering mitochondria-mediated apoptosis induction[62]. In addition, prazosin exhibits an anti-angiogenic activity[63] and its role in MDR modulation has also been suggested[55,64]. These emerging findings indicate that the quinazoline-based antihypertensive α1-adrenoceptor antagonists may have a significant role in uncontrolled hypertensive cancer patients without signs of ischemia[26,29,38,40].
P- Reviewer: Fang Y, Rigante D S- Editor: Ji FF L- Editor: Wang TQ E- Editor: Lu YJ
| 1. | Patanè S. The Emerging Scenario of the Onco-Cardiovascular Patients. J Cardiol Therapy. 2014;1:141-149. [DOI] [Full Text] |
| 2. | Kupeli S. Risks and diagnosis of coronary artery disease in Hodgkin lymphoma survivors. World J Cardiol. 2014;6:555-561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Patanè S. Breast cancer treatment cardioprotective strategies: the King is naked. Available from: http: //www.jaha.ahajournals.org/content /3/ 2/e000665/reply. |
| 4. | Patanè S. Cancer multidrug resistance-targeted therapy in both cancer and cardiovascular system with cardiovascular drugs. Int J Cardiol. 2014;176:1306-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Patanè S. HERG-targeted therapy in both cancer and cardiovascular system with cardiovascular drugs. Int J Cardiol. 2014;176:1082-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Vasti C, Hertig CM. Neuregulin-1/erbB activities with focus on the susceptibility of the heart to anthracyclines. World J Cardiol. 2014;6:653-662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 7. | Patanè S. Cardiotoxicity: anthracyclines and long term cancer survivors. Int J Cardiol. 2014;176:1326-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Arora M, Kaul D, Sharma YP. Blood cellular mutant LXR-α protein stability governs initiation of coronary heart disease. World J Cardiol. 2013;5:305-312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Patanè S. ERBB1/EGFR and ERBB2 (HER2/neu)--targeted therapies in cancer and cardiovascular system with cardiovascular drugs. Int J Cardiol. 2014;176:1301-1303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Patanè S. A challenge in cardiology: the oncosurgery. Int J Cardiol. 2014;174:411-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Patanè S. Is there a need for bacterial endocarditis prophylaxis in patients undergoing urological procedures? J Cardiovasc Transl Res. 2014;7:369-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Patanè S. Is there a need for bacterial endocarditis prophylaxis in patients undergoing gastrointestinal endoscopy? J Cardiovasc Transl Res. 2014;7:372-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Patanè S. Cardiotoxicity: Trastuzumab and cancer survivors. Int J Cardiol. 2014;177:554-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Patanè S. Cardiotoxicity: cisplatin and long-term cancer survivors. Int J Cardiol. 2014;175:201-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 15. | Pugliatti P, Donato R, Di Bella G, Carerj S, Patanè S. Contrast-enhancing right atrial thrombus in cancer patient. Int J Cardiol. 2014;173:e35-e37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Pugliatti P, Donato R, Zito C, Carerj S, Patanè S. Cardioinhibitory vasovagal syncope in a cancer patient. Int J Cardiol. 2014;174:e64-e65. [PubMed] |
| 17. | Pugliatti P, De Gregorio C, Patanè S. The chance finding of echocardiographic complications of infective endocarditis. Int J Cardiol. 2012;161:e50-e51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Calvagna GM, Patanè S. Transvenous pacemaker lead extraction in infective endocarditis. Int J Cardiol. 2014;176:511-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Pugliatti P, Recupero A, Zito C, Patanè S. The chance finding of an atrial septal defect in a cancer patient. Int J Cardiol. 2014;177:e68-e69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Patanè S, Marte F. Prostate-specific antigen and acute myocardial infarction: a possible new intriguing scenario. Int J Cardiol. 2009;134:e147-e149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Patanè S. Prostate-specific antigen kallikrein and the heart. World J Cardiol. 2009;1:23-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Patanè S, Marte F. Prostate-specific antigen kallikrein: from prostate cancer to cardiovascular system. Eur Heart J. 2009;30:1169-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Patanè S, Marte F. Prostate-specific antigen kallikrein and acute myocardial infarction: where we are. Where are we going? Int J Cardiol. 2011;146:e20-e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Patanè S. Insights into Cardio-oncology: Adrenergic receptor signaling and pathways in breast cancer. Curr Med Res Opin. 2014;26:1-2. [PubMed] |
| 25. | Patanè S. Heart failure and breast cancer: emerging controversies regarding some cardioprotective strategies. J Card Fail. 2014;20:456-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Patanè S. Is There a Role for Quinazoline-Based α (1)-Adrenoceptor Antagonists in Cardio-Oncology? Cardiovasc Drugs Ther. 2014;28:587-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | La Rocca R, Ferrari-Toninelli G, Patanè S. Widened QRS interval and left ventricular systolic depression after propafenone and promazine exposure. Int J Cardiol. 2014;177:57-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Patanè S. Ebola: Is there a hope from treatment with cardiovascular drugs? Int J Cardiol. 2014;177:524-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Dueñas-González A, García-López P, Herrera LA, Medina-Franco JL, González-Fierro A, Candelaria M. The prince and the pauper. A tale of anticancer targeted agents. Mol Cancer. 2008;7:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Patanè S. M3 muscarinic acetylcholine receptor in cardiology and oncology. Int J Cardiol. 2014;177:646-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 31. | Patanè S. Regulator of G-protein signaling 2 (RGS2) in cardiology and oncology. Int J Cardiol. 2015;179:63-65. [PubMed] |
| 32. | Patanè S. Insights into cardio-oncology: The patient’s heavy cancer journey among doubts, controversies and pitfalls. The role of the cardiologist. Int J Cardiol. 2014;178C:175-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Mailankody S, Prasad V. Comparative effectiveness questions in oncology. N Engl J Med. 2014;370:1478-1481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 34. | Haines I. The war on cancer: time for a new terminology. Lancet. 2014;383:1883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Selvam TP, Kumar PV, Vijayaraj P. Quinazoline Marketed drugs. Research in Pharmacy. 2011;1:1-21 Available from: http: //www.researchinpharmacy.com/view/article /3/1/1. |
| 36. | Roskoski R. ErbB/HER protein-tyrosine kinases: Structures and small molecule inhibitors. Pharmacol Res. 2014;87:42-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 159] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 37. | Chapman N, Chen CY, Fujita T, Hobbs FD, Kim SJ, Staessen JA, Tanomsup S, Wang JG, Williams B. Time to re-appraise the role of alpha-1 adrenoceptor antagonists in the management of hypertension? J Hypertens. 2010;28:1796-1803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 38. | Chapman N, Chang CL, Dahlöf B, Sever PS, Wedel H, Poulter NR. Effect of doxazosin gastrointestinal therapeutic system as third-line antihypertensive therapy on blood pressure and lipids in the Anglo-Scandinavian Cardiac Outcomes Trial. Circulation. 2008;118:42-48. [PubMed] [DOI] [Full Text] |
| 39. | Einhorn PT, Davis BR, Massie BM, Cushman WC, Piller LB, Simpson LM, Levy D, Nwachuku CE, Black HR. The Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Heart Failure Validation Study: diagnosis and prognosis. Am Heart J. 2007;153:42-53. [PubMed] |
| 40. | Wolak T, Toledano R, Novack V, Sharon A, Shalev A, Wolak A. Doxazosin to treat hypertension: it’s time to take it personally--a retrospective analysis of 19, 495 patients. J Hypertens. 2014;32:1132-1137; discussion 1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 41. | Yang M, Hu X, Lu X, Wu X, Xu J, Yang Z, Qian J, Sun S, Cahoon J, Tang W. The effects of α- and β-adrenergic blocking agents on postresuscitation myocardial dysfunction and myocardial tissue injury in a rat model of cardiac arrest. Transl Res. 2015;165:589-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 42. | Partin JV, Anglin IE, Kyprianou N. Quinazoline-based alpha 1-adrenoceptor antagonists induce prostate cancer cell apoptosis via TGF-beta signalling and I kappa B alpha induction. Br J Cancer. 2003;88:1615-1621. [PubMed] |
| 43. | Walden PD, Globina Y, Nieder A. Induction of anoikis by doxazosin in prostate cancer cells is associated with activation of caspase-3 and a reduction of focal adhesion kinase. Urol Res. 2004;32:261-265. [PubMed] |
| 44. | Shaw YJ, Yang YT, Garrison JB, Kyprianou N, Chen CS. Pharmacological exploitation of the alpha1-adrenoreceptor antagonist doxazosin to develop a novel class of antitumor agents that block intracellular protein kinase B/Akt activation. J Med Chem. 2004;47:4453-4462. [PubMed] |
| 45. | Garrison JB, Kyprianou N. Doxazosin induces apoptosis of benign and malignant prostate cells via a death receptor-mediated pathway. Cancer Res. 2006;66:464-472. [PubMed] |
| 46. | Shujue L, Wenzheng W, Weidong J, Yeping L, Lili O, Guohua Z, Wenqi W. Terazosin Suppress Human Prostatic Cancer PC3 Cell Viability via Proteasome Inhibition. Biol Med. 2014;6:203. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 47. | Bilbro J, Mart M, Kyprianou N. Therapeutic value of quinazoline-based compounds in prostate cancer. Anticancer Res. 2013;33:4695-4700. [PubMed] |
| 48. | Hui H, Fernando MA, Heaney AP. The alpha1-adrenergic receptor antagonist doxazosin inhibits EGFR and NF-kappaB signalling to induce breast cancer cell apoptosis. Eur J Cancer. 2008;44:160-166. [PubMed] |
| 49. | Park MS, Kim BR, Dong SM, Lee SH, Kim DY, Rho SB. The antihypertension drug doxazosin inhibits tumor growth and angiogenesis by decreasing VEGFR-2/Akt/mTOR signaling and VEGF and HIF-1α expression. Oncotarget. 2014;5:4935-4944. [PubMed] |
| 50. | Petty A, Myshkin E, Qin H, Guo H, Miao H, Tochtrop GP, Hsieh JT, Page P, Liu L, Lindner DJ. A small molecule agonist of EphA2 receptor tyrosine kinase inhibits tumor cell migration in vitro and prostate cancer metastasis in vivo. PLoS One. 2012;7:e42120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 51. | Kintscher U, Wakino S, Kim S, Jackson SM, Fleck E, Hsueh WA, Law RE. Doxazosin inhibits retinoblastoma protein phosphorylation and G(1)--& gt; S transition in human coronary smooth muscle cells. Arterioscler Thromb Vasc Biol. 2000;20:1216-1224. [PubMed] |
| 52. | Hensley PJ, Desiniotis A, Wang C, Stromberg A, Chen CS, Kyprianou N. Novel pharmacologic targeting of tight junctions and focal adhesions in prostate cancer cells. PLoS One. 2014;9:e86238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 53. | Liu CM, Fan YC, Lo YC, Wu BN, Yeh JL, Chen IJ. Cyclic guanosine monophosphate-enhancing reduces androgenic extracellular regulated protein kinases-phosphorylation/Rho kinase II-activation in benign prostate hyperplasia. Int J Urol. 2014;21:87-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 54. | Liu CM, Lo YC, Tai MH, Wu BN, Wu WJ, Chou YH, Chai CY, Huang CH, Chen IJ. Piperazine-designed alpha 1A/alpha 1D-adrenoceptor blocker KMUP-1 and doxazosin provide down-regulation of androgen receptor and PSA in prostatic LNCaP cells growth and specifically in xenografts. Prostate. 2009;69:610-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 55. | Takara K, Sakaeda T, Kakumoto M, Tanigawara Y, Kobayashi H, Okumura K, Ohnishi N, Yokoyama T. Effects of alpha-adrenoceptor antagonist doxazosin on MDR1-mediated multidrug resistance and transcellular transport. Oncol Res. 2009;17:527-533. [PubMed] |
| 56. | Xu K, Wang X, Ling PM, Tsao SW, Wong YC. The alpha1-adrenoceptor antagonist terazosin induces prostate cancer cell death through a p53 and Rb independent pathway. Oncol Rep. 2003;10:1555-1560. [PubMed] |
| 57. | Thomas D, Wimmer AB, Wu K, Hammerling BC, Ficker EK, Kuryshev YA, Kiehn J, Katus HA, Schoels W, Karle CA. Inhibition of human ether-a-go-go-related gene potassium channels by alpha 1-adrenoceptor antagonists prazosin, doxazosin, and terazosin. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:462-472. [PubMed] |
| 58. | Alberti C. Apoptosis induction by quinazoline-derived alpha1-blockers in prostate cancer cells: biomolecular implications and clinical relevance. Eur Rev Med Pharmacol Sci. 2007;11:59-64. [PubMed] |
| 59. | Kyprianou N, Benning CM. Suppression of human prostate cancer cell growth by alpha1-adrenoceptor antagonists doxazosin and terazosin via induction of apoptosis. Cancer Res. 2000;60:4550-4555. [PubMed] |
| 60. | Papadopoulos G, Vlachodimitropoulos D, Kyroudi A, Kouloukoussa M, Perrea D, Mitropoulos D. Terazosin treatment induces caspase-3 expression in the rat ventral prostate. J Clin Med Res. 2013;5:127-131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 61. | Han C, Bowen WC, Michalopoulos GK, Wu T. Alpha-1 adrenergic receptor transactivates signal transducer and activator of transcription-3 (Stat3) through activation of Src and epidermal growth factor receptor (EGFR) in hepatocytes. J Cell Physiol. 2008;216:486-497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 62. | Yang YF, Wu CC, Chen WP, Chen YL, Su MJ. Prazosin induces p53-mediated autophagic cell death in H9C2 cells. Naunyn Schmiedebergs Arch Pharmacol. 2011;384:209-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 63. | Lin SC, Chueh SC, Hsiao CJ, Li TK, Chen TH, Liao CH, Lyu PC, Guh JH. Prazosin displays anticancer activity against human prostate cancers: targeting DNA and cell cycle. Neoplasia. 2007;9:830-839. [PubMed] |
| 64. | Liao CH, Guh JH, Chueh SC, Yu HJ. Anti-angiogenic effects and mechanism of prazosin. Prostate. 2011;71:976-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |