Published online Apr 26, 2015. doi: 10.4330/wjc.v7.i4.224
Peer-review started: November 11, 2014
First decision: December 26, 2014
Revised: February 7, 2015
Accepted: February 10, 2015
Article in press: February 12, 2015
Published online: April 26, 2015
Processing time: 160 Days and 4.4 Hours
Pannus formation is a rare complication and occurs almost exclusively in mechanical prosthetic valves. It consists of fibrous tissue that covers the surface of the prosthesis either concentrically or eccentrically, resulting in valve dysfunction. The pathophysiology seems to be associated to a chronic inflammatory process that explains the late and insidious clinical presentation. This diagnosis should be considered in patients with high transvalvular gradients on transthoracic echo, and workup should be completed with fluoroscopy and transesophageal echocardiography. Treatment is always surgical and recurrence is rare. We present a case of pannus formation in a prosthetic aortic valve and a review of the literature regarding this disorder.
Core tip: Pannus is an infrequent complication that mainly affects mechanical prosthetic valves. Its diagnosis requires clinical suspicion and the association of fluoroscopy, transthoracic and transesophageal echocardiography. The case presented is a characteristic example of pannus, given its clinical presentation (progressive dyspnea), the steps followed to reach diagnosis and the surgical resolution. Suspecting this disorder and making an accurate diagnosis is of paramount importance, to implement adequate treatment and to avoid prolonging the natural course of the disease and its repercussion on the left ventricle and the quality of life of affected patients.
- Citation: Soumoulou JB, Cianciulli TF, Zappi A, Cozzarin A, Saccheri MC, Lax JA, Guidoin R, Zhang Z. Limitations of multimodality imaging in the diagnosis of pannus formation in prosthetic aortic valve and review of the literature. World J Cardiol 2015; 7(4): 224-229
- URL: https://www.wjgnet.com/1949-8462/full/v7/i4/224.htm
- DOI: https://dx.doi.org/10.4330/wjc.v7.i4.224
Mechanical prosthetic valve dysfunction caused by pannus or thrombosis is an unusual but serious complication of heart valve replacement. Thrombotic complications are most common early postoperatively, whereas pannus occurs later, especially in bileaflet valves in the aortic position. Pannus formation consists of fibrous tissue usually covering the circumference of a prosthetic valve, and causing valve dysfunction[1]. The incidence of this rare complication is 1.6%-2% in the different series published[2,3] and occurs almost exclusively in mechanical prostheses. Its most frequent location varies according to the authors, but in most series prostheses in the aortic position were affected more often[4] than those in mitral position[3,5].
The patient is a 55-year-old man with multiple cardiovascular risk factors (type 2 diabetes, hypertension, past history of smoking, obesity, dyslipidemia, and family history of cardiovascular disease) and intermittent claudication at 200 meters. Also, in 1998 he underwent aortic valve replacement with a mechanical #23 St. Jude valve, and coronary artery bypass grafting (CABG) with three grafts (left internal mammary artery to the left anterior descending artery and saphenous vein graft to the right coronary artery and circumflex coronary artery). In 2009 he began experiencing dyspnea in FC II [New York Heart Association (NYHA) classification], that progressed to FC IV. Upon clinical consultation, a meso-telesystolic murmur radiating to the neck was detected, as well as a pulsus tardus et parvus and an apical beat in the anterior axillary line. No other relevant findings were reported.
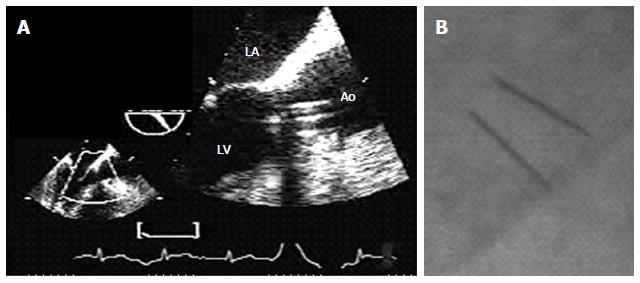
His chest X-ray showed a cardiothoracic index slightly above 0.5, an old posterior infarction (R/S > 1 in lead V2) was seen on ECG, and routine laboratory results were within normal values. Transthoracic echocardiography (TTE) and two-dimensional transesophageal echocardiography (TEE) showed a bileaflet mechanical prosthesis in aortic position with normal opening of both discs (Figure 1A), with severely increased mechanical aortic valve gradients (peak instantaneous gradient: 97 mmHg, mean gradient: 58 mmHg) and decreased effective prosthetic area (0.67 cm2). No detectable image suggestive of pannus or thrombus was seen in the left ventricular (LV) outflow tract. There was infero-posterior akinesis and mild LV dysfunction (EF: 40%). Fluoroscopy revealed normal opening of both tilting discs (Figure 1B). Cardiac multiple detector computed tomography (MDCT) did not showed any soft tissue mass on the ventricular side of the prosthetic aortic. Coronary angiography showed a severe lesion in the venous graft to the right coronary artery, without significant lesions in the other grafts or native arteries.
In spite of absence of any tissue mass on the ventricular side of the prosthetic aortic and absence of limitation of motion of the tilting disc to allow suggest pannus, given the clinical suspicion of prosthetic valve obstruction due to pannus formation, the decision was made to replace the aortic prosthesis with a new mechanical valve (ATS # 23) and perform CABG (venous graft to the right coronary artery); there were no postoperative complications. On pathological examination (Figure 2), the explanted specimen exhibited fibrous tissue with a smooth, annular surface, in contact with the ventricular surface of the mechanical prosthesis, consistent with pannus. Histological examination confirmed the diagnosis (Figure 3).
Pannus formation consists of fibrous tissue usually covering the circumference of a prosthetic valve, and causing valve dysfunction[1]. The incidence of this rare complication is 1.6%-2% in the different series published[2,3] and occurs almost exclusively in mechanical prostheses. Its most frequent location varies according to the authors, but in most series (Table 1) prostheses in the aortic position were affected more often[4] than those in mitral position[3,5].
| Ref. | Year | Total number/No. of re-interventions | Pannus(n) | Location of pannus | Type of valve affected | Time tore-intervention (mo) | Follow-up (yr) | |||
| Aortic | Mitral | Combined | Biologic | Mechanical | ||||||
| Vitale et al[5] | 1997 | 1878/87 | 66 | 0 (0) | 66 (100) | 0 (0) | 0 (0) | 66 (100) | 51.5 ± 41.41 | 0.26-20.1 |
| Deviri et al[9] | 1998 | ND/100 | 51 | ND | ND | ND | 0 (0) | 51 (100) | 48 (1.5-144)1 | 13 |
| Barbetseas et al[4] | 1998 | ND/23 | 10 | 7 (70) | 3 (30) | 0 (0) | 0 (0) | 23 (100) | 178 ± 522 | ND |
| Rizzoli et al[3] | 1999 | 2680/334 | 44 | 13 (30) | 27 (61) | 4 (9) | 0 (0) | 44 (100) | 1563 | 27 |
| Girard et al[18] | 2001 | ND/92 | 27 | 27 (100) | 0 (0) | 0 (0) | 1 (4) | 26 (96) | Vmec 156 ± 983 | ND |
| Vbio 84 ± 483 | ||||||||||
| Roudaut et al[10] | 2003 | 17250/126 | 26 | ND | ND | ND | 0 (0) | 26 (100) | ND | 23 |
| Teshima et al[2] | 2003 | 615/12 | 12 | 12 (100) | 0 (0) | 0 (0) | 0 (0) | 12 (100) | 83 ± 522 | 19 |
| Toker et al[19] | 2006 | 63 | 45 | ND | ND | ND | 0 (0) | 45 (100) | 58.9 ± 56.11 | ND |
Pathology studies of valves explanted due to pannus formation have shown that it consists of fibrous tissue ingrowth, with a generally smooth surface and a ring-like shape covering the valve surface. Pannus formation may be an isolated finding or associated to various degrees of thrombosis[2,5,6]. According to the type of growth, pannus may be classified as concentric or eccentric[7,8], the latter being more frequent[5]. However, the morphology of pannus could be associated to the type of prosthetic valve affected, which would explain the higher frequency of the eccentric type on single-disc valves[5], while the concentric type is more common in bi-leaflet valves[2].
On histological examination, pannus consists of a structure of collagen fibers interspersed with small vessels and capillaries surrounded by giant cells, especially around and over suture stitches[5]. Pannus can be systematically divided into three layers and one core[2]. From the surface in contact with blood flow, the three layers towards the prosthetic material are: the lumen (which consists of endothelial cells, is found in the surface of the pannus), the internal lamina media (is composed of myofibroblasts) and the external lamina media (is composed of collagen and elastic fibers).
The core is located between the prosthetic tissue and the pannus, and consists mainly of a chronic inflammatory infiltrate comprising macrophages, lymphocytes, giant cells, plasmocytes and mastocytes[2].
The pathophysiology of this disorder is not yet completely understood. After implantation of a prosthetic valve, two inflammatory events occur. The first involves replacement of the damaged myocardium around the valve ring by a scar formed by nonspecialized connective tissue. The second event involves a foreign body-like inflammatory response to the presence of the prosthetic material. Prolonged exposure to the non-degradable prosthetic material is a persistent stimulus for inflammatory cells such as macrophages (which cluster as giant cells) and for proliferation of fibroblasts; both phenomena are characteristically seen in chronic inflammation. The presence of giant cells should be construed as a severe reaction, in which the foreign material is not well tolerated and hence is a target for phagocytosis. This explanation allows to infer that the presence of pannus in only one valve surface represents an early stage of a chronic progressive inflammatory disorder[5].
The clinical presentation of pannus is variable; in most cases signs and symptoms of the disease occur as a consequence of prosthetic valve obstruction. The most common symptom is dyspnea, which may occur as a manifestation of obstruction in valves implanted both in aortic and in mitral valve position. Other less common clinical presentations are: low cardiac output syndrome, shock, embolization, chest pain, absence of a valve click on auscultation, exercise intolerance, cardiorespiratory arrest, and in many cases, patients may be asymptomatic[4,9,10]. In a study[4] that assessed the clinical characteristics of patients with pannus formation vs patients with thrombosis, the first group had a longer delay in appearance of symptoms, a longer duration of symptoms at the time of re-intervention, a greater time interval between the first and second surgical intervention and a higher rate of adherence to anticoagulation treatment.
The imaging techniques that are used most often for the diagnosis of this disorder are fluoroscopy, TTE and/or TEE HYPERLINK\l “Cia05”[11], although currently other techniques offer promising results, such as three-dimensional echocardiography and multislice Angio-CT[12-14].
For mechanical prostheses, the use of fluoroscopy is simple and allows to clearly identify the valve ring, cage, ball, the tilting disc/discs and the opening and closure angles; however, in biologic valves its use remains limited[1,11,15-17]. Specifically, in the case of pannus formation, fluoroscopy allows to detect absent motion of the disc/s; a frequent finding in pannus as well as in prosthetic valve thrombosis, albeit more frequent in the latter. In patients with normal leaflet motion, in whom high gradients are found and possible causes are prosthesis-patient mismatch, pannus or thrombosis, the echocardiographic findings shall define the final diagnosis[11]. In a study by Girard et al[18] that assessed 16 patients before their second aortic valve replacement, 63% of patients with a post-operative diagnosis of pannus had an abnormal fluoroscopy.
Pannus is suspected in patients who exhibit high gradients on echocardiography. Once structural failure and patient-prosthesis mismatch are ruled out, the only two differential diagnoses that remain to be defined are: pannus or thrombus. Since the advent of thrombolytics as an option for the treatment of valve thrombosis, making an accurate diagnosis has become of utmost importance, since such patients could benefit from the use of thrombolytics, and thus avoid the need for surgical reintervention. Although TTE is most useful in the initial approach to the diagnosis of pannus and thrombus, its usefulness to assess disc/s motion or the etiology of valve obstruction remains limited. However, where TTE fails, TEE appears as a more sensitive and specific method at the time of assessing the etiology of prosthetic valve obstruction. Thus, TEE has allowed to determine certain characteristics associated with pannus, such as: preserved prosthetic disc motion and evidence of a hyper-reflective mass of decreased length and motion, associated to the prosthetic valve[4]. Currently available diagnostic tools including TTE and 2D-TEE are insufficient to detect pannus formation, and detection rate is so poor that a preoperative diagnosis is almost impossible. Real-time three-dimensional transesophageal echocardiography may provide data to the diagnosis of pannus formation.
The treatment of pannus formation is surgical re-intervention to perform a new valve replacement. Occasionally, when pannus does not make contact with the prosthetic ring, the fibrotic tissue could be resected without replacing the prosthetic valve, but certain authors suggest that this surgical option is associated to a greater recurrence of pannus formation. All series agree in that the time to re-intervention is prolonged (Table 1). During follow-up of 63 patients with an obstructed mitral or aortic prosthetic valve, or both (pannus in 71.4% of cases), of whom 100% underwent valve replacement, in-hospital mortality was 20.6%. The main cause of death was low cardiac output syndrome and the only predictor of high mortality on multivariate analysis was LV systolic impairment[19]. In the series by Vitale et al[5] which included 87 patients with obstructive mitral disease, of whom 75.8% had pannus either alone or associated to thrombus, 100% of patients underwent valve replacement (mechanical valve in 88.8%, biologic valve in 11.8%) with a 30-d mortality of 12.5%.
Recurrence is a finding of low prevalence and high mortality, and occurred predominantly in patients who underwent pannus resection without valve replacement[5,10].
Pannus is an infrequent complication that mainly affects mechanical prosthetic valves. Its diagnosis requires clinical suspicion and the association of fluoroscopy + TTE/TEE. Currently, the treatment of choice is a new valve replacement and prognosis depends mainly on LV function. The case presented is a characteristic example of pannus, given its clinical presentation (progressive dyspnea), the steps followed to reach diagnosis and the surgical resolution. Suspecting this disorder and making an accurate diagnosis is of paramount importance, to implement adequate treatment and to avoid prolonging the natural course of the disease and its repercussion on the LV and the quality of life of affected patients.
A 55-year-old man with a mechanical aortic prosthetic valve presented with clinical suspicion of prosthetic valve obstruction due to pannus formation.
The patient began experiencing dyspnea in FC II (NYHA) that progressed to FC IV. A meso-telesystolic murmur radiating to the neck was detected, as well as a pulsus tardus et parvus, with severely increased mechanical aortic valve gradients and decreased effective prosthetic area. No detectable image suggestive of pannus or thrombus was seen in the left ventricular outflow tract in multimodality imaging.
Mechanical prosthetic valve dysfunction caused by pannus or thrombosis.
Fluoroscopy, transthoracic echocardiography, two-dimensional transesophageal echordiography and cardiac multiple detector computed tomography, failed to diagnose pannus formation.
Given the clinical suspicion of prosthetic valve obstruction due to pannus formation, the decision was made to replace the aortic prosthesis with a new mechanical valve (ATS # 23). On pathological examination, the explanted specimen exhibited fibrous tissue with a smooth, annular surface, in contact with the ventricular surface of the mechanical prosthesis, consistent with pannus. Histological examination confirmed the diagnosis.
The case presented is a characteristic example of pannus, given its clinical presentation (progressive dyspnea), the steps followed to reach diagnosis and the surgical resolution.
Multimodality imaging in the diagnosis of pannus formation may have limitations. Suspecting this disorder and making an accurate diagnosis is of paramount importance, to implement adequate treatment and to avoid prolonging the natural course of the disease and its repercussion on the left ventricle and the quality of life of affected patients.
Authors have made good and fluent review of pannus formation and presented a clinical case ignored by transesophageal echocardiography and fluoroscopy.
P- Reviewer: Biyik I, Caceres-Loriga F, Miyasaka Y, Schoenhagen P S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Aoyagi S, Nishimi M, Kawano H, Tayama E, Fukunaga S, Hayashida N, Akashi H, Kawara T. Obstruction of St Jude Medical valves in the aortic position: significance of a combination of cineradiography and echocardiography. J Thorac Cardiovasc Surg. 2000;120:142-147. [PubMed] |
| 2. | Teshima H, Hayashida N, Yano H, Nishimi M, Tayama E, Fukunaga S, Akashi H, Kawara T, Aoyagi S. Obstruction of St Jude Medical valves in the aortic position: histology and immunohistochemistry of pannus. J Thorac Cardiovasc Surg. 2003;126:401-407. [PubMed] |
| 3. | Rizzoli G, Guglielmi C, Toscano G, Pistorio V, Vendramin I, Bottio T, Thiene G, Casarotto D. Reoperations for acute prosthetic thrombosis and pannus: an assessment of rates, relationship and risk. Eur J Cardiothorac Surg. 1999;16:74-80. [PubMed] |
| 4. | Barbetseas J, Nagueh SF, Pitsavos C, Toutouzas PK, Quiñones MA, Zoghbi WA. Differentiating thrombus from pannus formation in obstructed mechanical prosthetic valves: an evaluation of clinical, transthoracic and transesophageal echocardiographic parameters. J Am Coll Cardiol. 1998;32:1410-1417. [PubMed] |
| 5. | Vitale N, Renzulli A, Agozzino L, Pollice A, Tedesco N, de Luca Tupputi Schinosa L, Cotrufo M. Obstruction of mechanical mitral prostheses: analysis of pathologic findings. Ann Thorac Surg. 1997;63:1101-1106. [PubMed] |
| 6. | Aoyagi S, Nishimi M, Tayama E, Fukunaga S, Hayashida N, Akashi H, Kawara T. Obstruction of St Jude medical valves in the aortic position: a consideration for pathogenic mechanism of prosthetic valve obstruction. Cardiovasc Surg. 2002;10:339-344. [PubMed] |
| 7. | Cianciulli TF, Saccheri MC, Lax JA, Guidoin R, Zhang Z, Guerra JE, Prezioso HA, Vidal LA. Intermittent acute aortic regurgitation of a mechanical bileaflet aortic valve prosthesis: diagnosis and clinical implications. Eur J Echocardiogr. 2009;10:446-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Ozkan M, Gündüz S, Yildiz M, Duran NE. Diagnosis of the prosthetic heart valve pannus formation with real-time three-dimensional transoesophageal echocardiography. Eur J Echocardiogr. 2010;11:E17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Deviri E, Sareli P, Wisenbaugh T, Cronje SL. Obstruction of mechanical heart valve prostheses: clinical aspects and surgical management. J Am Coll Cardiol. 1991;17:646-650. [PubMed] |
| 10. | Roudaut R, Roques X, Lafitte S, Choukroun E, Laborde N, Madona F, Deville C, Baudet E. Surgery for prosthetic valve obstruction. A single center study of 136 patients. Eur J Cardiothorac Surg. 2003;24:868-872. [PubMed] |
| 11. | Cianciulli TE, Lax JA, Beck MA, Cerruti FE, Gigena GE, Saccheri MC, Fernández E, Dorelle AN, Leguizamón JH, Prezioso HA. Cinefluoroscopic assessment of mechanical disc prostheses: its value as a complementary method to echocardiography. J Heart Valve Dis. 2005;14:664-673. [PubMed] |
| 12. | Symersky P, Budde RP, de Mol BA, Prokop M. Comparison of multidetector-row computed tomography to echocardiography and fluoroscopy for evaluation of patients with mechanical prosthetic valve obstruction. Am J Cardiol. 2009;104:1128-1134. [PubMed] |
| 13. | Kassi M, Garg N, Chang SM. Utility of cardiac computed tomography for assessment of prosthetic aortic valve dysfunction with pannus formation. Methodist Debakey Cardiovasc J. 2013;9:174-175. [PubMed] |
| 14. | Sugeng L, Shernan SK, Weinert L, Shook D, Raman J, Jeevanandam V, DuPont F, Fox J, Mor-Avi V, Lang RM. Real-time three-dimensional transesophageal echocardiography in valve disease: comparison with surgical findings and evaluation of prosthetic valves. J Am Soc Echocardiogr. 2008;21:1347-1354. [PubMed] |
| 15. | White AF, Dinsmore RE, Buckley MJ. Cineradiographic evaluation of prosthetic cardiac valves. Circulation. 1973;48:882-889. [PubMed] |
| 16. | Mehlman DJ. A guide to the radiographic identification of prosthetic heart valves: an addendum. Circulation. 1984;69:102-105. [PubMed] |
| 17. | Mehlman DJ. A pictorial and radiographic guide for identification of prosthetic heart valve devices. Prog Cardiovasc Dis. 1988;30:441-464. [PubMed] |
| 18. | Girard SE, Miller FA, Orszulak TA, Mullany CJ, Montgomery S, Edwards WD, Tazelaar HD, Malouf JF, Tajik AJ. Reoperation for prosthetic aortic valve obstruction in the era of echocardiography: trends in diagnostic testing and comparison with surgical findings. J Am Coll Cardiol. 2001;37:579-584. [PubMed] |
| 19. | Toker ME, Eren E, Balkanay M, Kirali K, Yanartaş M, Calişkan A, Güler M, Yakut C. Multivariate analysis for operative mortality in obstructive prosthetic valve dysfunction due to pannus and thrombus formation. Int Heart J. 2006;47:237-245. [PubMed] |