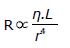Published online Apr 26, 2015. doi: 10.4330/wjc.v7.i4.204
Peer-review started: December 20, 2014
First decision: January 8, 2015
Revised: January 22, 2015
Accepted: February 10, 2015
Article in press: February 12, 2015
Published online: April 26, 2015
Processing time: 121 Days and 11.6 Hours
The function of the heart is to contract and pump oxygenated blood to the body and deoxygenated blood to the lungs. To achieve this goal, a normal human heart must beat regularly and continuously for one’s entire life. Heartbeats originate from the rhythmic pacing discharge from the sinoatrial (SA) node within the heart itself. In the absence of extrinsic neural or hormonal influences, the SA node pacing rate would be about 100 beats per minute. Heart rate and cardiac output, however, must vary in response to the needs of the body’s cells for oxygen and nutrients under varying conditions. In order to respond rapidly to the changing requirements of the body’s tissues, the heart rate and contractility are regulated by the nervous system, hormones, and other factors. Here we review how the cardiovascular system is controlled and influenced by not only a unique intrinsic system, but is also heavily influenced by the autonomic nervous system as well as the endocrine system.
Core tip: The function of the heart is to contract and pump oxygenated blood to the body and deoxygenated blood to the lungs. To achieve this goal, a normal human heart must contract regularly and continuously, and respond to the changing requirements of the body’s tissues. Here we review how the cardiovascular system is controlled and influenced by not only a unique intrinsic system, but is also heavily influenced by the autonomic nervous system as well as the endocrine system.
- Citation: Gordan R, Gwathmey JK, Xie LH. Autonomic and endocrine control of cardiovascular function. World J Cardiol 2015; 7(4): 204-214
- URL: https://www.wjgnet.com/1949-8462/full/v7/i4/204.htm
- DOI: https://dx.doi.org/10.4330/wjc.v7.i4.204
The cardiovascular system is a closed system connecting a pump to blood vessels (i.e., arteries, capillaries, veins). The heart serves as the pump that moves blood through blood vessels thereby providing the needed oxygen and nutrients to the body. The heart consists of four chambers: right atrium, right ventricle, left atrium and left ventricle. The right atrium receives oxygen-poor blood from the systemic veins; this blood then moves across the tricuspid valve to the right ventricle. From the right ventricle the de-oxygenated blood is pumped pass semilunar valves out through the pulmonary arteries to the lungs. In the lungs, the blood becomes oxygenated and returns to the left atrium via the pulmonary veins. This oxygen-rich blood next moves across the mitral valve to the left ventricle and is pumped out across semilunar valves to the systemic arteries and to body tissues. To achieve this goal, a normal human heart must beat regularly and continuously for one’s entire life. Autorhythmic cardiac cells initiate and distribute impulses (action potentials) throughout the heart. The intrinsic conduction system coordinates heart electrical activity. This electrical activity in the heart corresponds to electrocardiogram (ECG) wave tracings. On a normal ECG recording, the P wave reflects atrial depolarization followed by atrial contraction. The QRS wave reflects ventricular depolarization followed by ventricular contraction and the T wave reflects ventricular repolarization and ventricular relaxation.
In the intrinsic conduction system, heartbeats originate from the rhythmic pacing discharge from the sinoatrial node (SA node) within the heart itself. The SA node, located in the right atrium, is a part of the intrinsic conduction (or nervous) system found in the heart. This conduction system in order of rate of depolarization starts with the SA node or pacemaker and results in atrial depolarization and atrial contraction, the internodal pathway, the AV node (where the impulse is delayed), AV bundle, the left and right branches of the bundle of His and lastly the Purkinje fibers, both of which result in ventricular depolarization and contraction. All of the components of the intrinsic conduction system contain autorhythmic cells that spontaneously depolarize. In the absence of extrinsic neural or hormonal influences, the SA node pacing rate would be about 100 beats per minute (bpm). The heart rate and cardiac output, however, must vary in response to the needs of the body’s cells for oxygen and nutrients under varying conditions. In order to respond rapidly to changing requirements of the body’s tissues, the heart rate and contractility are regulated by the autonomic nervous system, hormones, and other factors.
The autonomic nervous system (ANS) is the component of the peripheral nervous system that controls cardiac muscle contraction, visceral activities, and glandular functions of the body. Specifically the ANS can regulate heart rate, blood pressure, rate of respiration, body temperature, sweating, gastrointestinal motility and secretion, as well as other visceral activities that maintain homeostasis[1-4]. The ANS functions continuously without conscious effort. The ANS, however, is controlled by centers located in the spinal cord, brain stem, and hypothalamus.
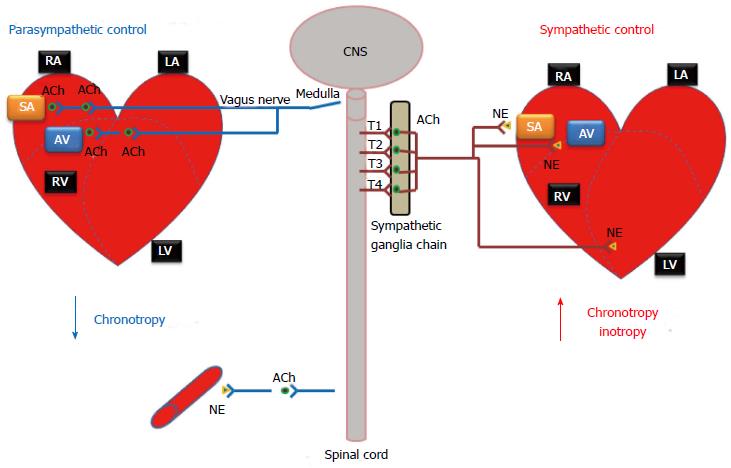
The ANS has two interacting systems: the sympathetic and parasympathetic systems. As illustrated in Figure 1, sympathetic and parasympathetic neurons exert antagonistic effects on the heart. The sympathetic system prepares the body for energy expenditure, emergency or stressful situations, i.e., fight or flight. Conversely, the parasympathetic system is most active under restful conditions. The parasympathetic counteracts the sympathetic system after a stressful event and restores the body to a restful state. The sympathetic nervous system releases norepinephrine (NE) while the parasympathetic nervous system releases acetylcholine (ACh). Sympathetic stimulation increases heart rate and myocardial contractility. During exercise, emotional excitement, or under various pathological conditions (e.g., heart failure)[5], the sympathetic nervous system is activated. The stimulation of the sympathetic nervous system causes pupil dilatation, bronchiole dilatation, blood vessel constriction, sweat secretion, inhibits peristalsis, increases renin secretion by the kidneys, as well as can induce reproductive organ contraction and secretion. In contrast, parasympathetic stimulation decreases heart rate and constricts the pupils. It also increases secretion of the eye glands, increases peristalsis, increases secretion of salivary and pancreatic glands, and constricts bronchioles. Most organs receive innervations from both systems, which usually exert opposing actions. However, this is not always the case. Some systems do not have a response to parasympathetic stimulation. For example, most blood vessels lack parasympathetic innervations and their diameter is regulated by sympathetic nervous system input, so that they have a constant state of sympathetic tone. It is a decrease in sympathetic stimulation or tone that allows vasodilatation. During rest, sleep, or emotional tranquility, the parasympathetic nervous system predominates and controls the heart rate at a resting rate of 60-75 bpm. At any given time, the effect of the ANS on the heart is the net balance between the opposing actions of the sympathetic and parasympathetic systems.
Unlike the somatic nervous system, where a single neuron originating in the spinal cord typically connects the central nervous system and a skeletal muscle via a neuromuscular junction, both sympathetic and parasympathetic pathways are composed of a two-neuron chain: a preganglionic neuron and a postganglionic neuron. The neurotransmitter between the preganglionic and postganglionic neurons is acetylcholine, the same as that in neuromuscular junctions. Messages from these systems are conveyed as electrical impulses that travel along axons and cross synaptic clefts (using chemical neurotransmitter).
In the sympathetic system (thoracolumbar division), these nerves originate from the thoracolumbar region of the spinal cord (T1-L2) and radiate out towards the target organs. In contrast, the nerves of the parasympathetic system originate within the midbrain, pons and medulla oblongata of the brain stem and part of these fibers originate in the sacral region (S2-S4 sacral spinal nerves) of the spinal cord. While sympathetic nerves utilize a short preganglionic neuron followed by a relatively long postganglionic neuron, parasympathetic nerves (e.g., the vagus nerve, which carries about 75 percent of all parasympathetic fibers) have a much longer preganglionic neuron, followed by a short postganglionic neuron.
The sympathetic nervous system is the component of the ANS that is responsible for controlling the human body’s reaction to situations of stress or emergency (otherwise known as the “fight-or-flight” response), while the parasympathetic nervous system is generally responsible for basal organ system function.
Cardiac sympathetic preganglionic nerves (typically all myelinated) emerge from the upper thoracic segments of the spinal cord (T1-T4). After traveling a short distance, preganglionic fibers leave the spinal nerves through branches called white rami and enter sympathetic ganglia. The cardiac sympathetic neurons form the sympathetic chain ganglia located along the side of the viscera column (i.e., paravertebral ganglia). These ganglia comprise the sympatheric trunks with their connecting fibers. The postganglionic fibers, extend to the viscera, such as the heart. In general, sympathetic preganglionic neurons are shorter than sympathetic postganglionic neurons (Figure 1).
Sympathetic neurotransmitters: Neurotransmitters are chemical substances released into the synaptic cleft from nerve terminals in response to action potentials. They transmit signals from a neuron to a target cell across a synapse, e.g., acetylcholine for neuromuscular junctions. While the preganglionic neurons of both the sympathetic and parasympathetic system secret acetylcholine (ACh) which is why they are referred to as cholinergic, the majority of the postganglionic endings of the sympathetic nervous system release NE, which resembles epinephrine (i.e., adrenalin). Thus, these sympathetic postganglionic fibers are commonly called adrenergic fibers.
Sympathetic receptors: There are two types of adrenergic receptors: β and α. In the cardiovascular system there are β1, β2, α1, and α2 adrenergic receptors (Table 1).
| Heart | Vessels | |||||
| Receptor | Function | Receptor | Function | |||
| Inotropy | Chronotropy | Dromotropy | ||||
| Norepinephrine | α1 | + | + | + | α1 | Vasoconstriction |
| β1 | + | + | + | β1 | Vasoconstriction | |
| β2 | + | + | + | β2 | Vasodilation | |
| Acetylcholine | M2 | - | - | - | M2 | Vasodilation |
β1 adrenergic receptors are expressed in the heart (in the SA node, AV node, and on atrial and ventricular cardiomyocytes). The activation of β1 receptors increases heart rate (via the SA node), increases contractility as result of increased intracellular calcium concentrations and increased calcium release by the sarcoplasmic reticulum (SR), and increased AV node conduction velocity. Additionally, activation of this receptor also induces renin release by the kidneys to help maintain blood pressure, plasma sodium levels and blood volume.
β2 adrenergic receptors are mainly expressed in vascular smooth muscle, skeletal muscle, and in the coronary circulation. Their activation elicits vasodilatation, which, in turn increases blood perfusion to target organs (especially the liver, heart, and skeletal muscle). These receptors are not innervated and thus are primarily stimulated by circulating epinephrine. There are also some low expressions of β2 receptors in cardiomyocytes.
α1 adrenergic receptors are expressed in vascular smooth muscle proximal to sympathetic nerve terminals. Their activation elicits vasoconstriction. There are also some low expressions of α1 receptors in cardiomyocytes.
α2 adrenergic receptors are expressed in vascular smooth muscle distal from sympathetic nerve terminals. Their activation also elicits vasoconstriction.
Sympathetic nervous system control and heart function: Stimulation by the sympathetic nervous system results in the following effects on the heart (Table 1): Positive chronotropic effect (increase in heart rate): The sinoatrial (SA) node is the predominate pacemaker of the heart. It is located within the upper posterior wall of the right atrium, and is responsible for maintaining a sinus rhythm of between 60 and 100 beats per minute. This rate is constantly being affected by innervations from both the sympathetic and parasympathetic nervous systems. Stimulation by the sympathetic system nerves results in an increase of heart rate, as occurs during the “fight-or-flight” response.
Positive inotropic effect (increase of contractility): Myocardial contractility represents the ability of the heart to produce force during contraction. It is determined by the incremental degrees of binding between myosin (thick) and actin (thin) filaments, which in turn depends on the concentration of calcium ions (Ca2+) in the cytosol of the cardiomyocyte. Stimulation by the sympathetic nervous system causes an elevation in intracellular (Ca2+) and thus an increase in contraction of both the atria and ventricles.
Positive dromotropic effect (enhancement of conduction): Stimulation by the sympathetic nervous system also enhances the conductivity of the electrical signal. For example, it increases AV conduction velocity.
As previously mentioned, the parasympathetic nervous system is responsible for the unconscious regulation of the body’s systems, most notably, salivation, lacrimation, urination, digestion, and defecation (acronym SLUDD). Importantly, the parasympathetic nervous system plays an antagonistic role in regulating heart function.
The parasympathetic system has preganglionic neurons (craniosacral division) that arise from neurons in the mid-brain, pons and medulla oblongata. The cell bodies of parasympathetic preganglionic neurons are located in the homologous motor nuclei of the cranial nerves. Parasympathetic preganglionic fibers associated with parts of the head are carried by the oculomotor, facial, and glossopharygeal nerves. The fibers that innervate organs of the thorax and upper abdomen are parts of the vagus nerve which as previously mentioned carries approximately 75% of all parasympathetic nerve fibers passing to the heart, the lungs, the stomach, and many other visceral organs. Preganglionic fibers arising from the sacral region of the spinal cord make up parts of S2-S4 sacral spinal nerves and carry impulses to viscera in the pelvic cavity. The short postganglionic neurons reside near effector organs, e.g., lacrimal gland, salivary glands, heart, trachea, lung, liver, gallbladder, spleen, pancreas, intestines, kidney, and urinary bladder, etc. Unlike the sympathetic system, most parasympathetic preganglionic fibers reach the target organs and form the peripheral ganglia in the wall of the organ. The preganglionic fibers synapse within the ganglion, and then short postganglionic fibers leave the ganglia to the target organ. Thus, in the parasympathetic system, preganglionic neurons are generally longer than postganglionic neurons (Figure 1).
Parasympathetic neurotransmitters: Acetylcholine is the predominant neurotransmitter from the parasympathetic nervous system, in both the preganglionic and postganglionic neurons. Although excitatory in skeletal muscle by binding to nicotinic receptors and inducing the opening of ligand gated sodium channels, acetylcholine inhibits the contraction of cardiomyocytes by activating muscarinic receptors (M2). These parasympathetic postganglionic fibers are commonly called cholinergic fibers because they secrete acetylcholine at their nerve endings.
Acetylcholine is synthesized by choline acetlytransferase in cholinergic neurons by combining choline and acetyl-COA molecules. Once assembled in synaptic vesicles near the end of the axon, the entry of calcium causes the vesicles to fuse with the membrane of the neuron and to release acetylcholine into the synaptic cleft (the space between the neuron and post-synaptic membrane or effector cell). Acetylcholine diffuses across the synaptic cleft and binds to receptors on the post-synaptic membrane increasing the permeability to sodium causing depolarization of the membrane and propagation of the impulse. This chemical transmission is much slower than the electrical “all or none” transmission of the action potential seen in the intrinsic nervous system of the heart. To regulate the function of these neurons (and thus, the muscles they control), acetylcholinesterase is present in the synaptic cleft. It serves to hydrolyze the acetylcholine molecule by breaking it down into choline and acetate, which are then both taken back up by the neuron, to be again synthesized into acetylcholine.
Parasympathetic receptors: The parasympathetic postganglionic fibers are cholinergic. Acetylcholine can bind to two types of cholinergic receptors called nicotinic receptors and muscarinic receptors. Muscarinic receptors are located in the membranes of effector cells at the end of postganglionic parasympathetic nerves and at the ends of cholinergic sympathetic fibers. Responses from these receptors are excitatory and relatively slow. The nicotinic receptors are located at synapses between pre- and post-ganglionic neurons of the sympathetic and parasympathetic pathways. Nicotinic receptors in contrast to muscarinic receptors produce rapid, excitatory responses. Neuromuscular junctions found in skeletal muscle fibers are nicotinic.
In relation to the cardiovascular system the parasympathetic nervous system has two different kinds of muscarinic receptors: the M2 and M3 receptors (Table 1).
The M2 receptors are expressed in the heart; abundant in nodal and atrial tissue, but sparse in the ventricles. The binding of acetylcholine to M2 receptors serves to slow heart rate till it reaches normal sinus rhythm. This is achieved by slowing the rate of depolarization, as well as by reducing the conduction velocity through the atrioventricular node. Additionally, the activation of M2 receptors reduces the contractility of atrial cardiomyocytes, thus reducing, in part, the overall cardiac output of the heart as a result of reduced atrial kick, smaller stroke volume, and slower heart rate. Cardiac output is determined by heart rate and stroke volume (CO = HR x SV).
The M3 receptors are mainly expressed in vascular endothelium. The predominate effect of M3 receptor activation is dilatation of the vessels, by stimulating nitric oxide production by vascular endothelial cells[6]. M3 receptors impact afterload and vascular resistance which can again alter cardiac output and blood pressure.
Parasympathetic nervous system control and heart function: As mentioned earlier, parasympathetic activity produces effects that are, in general, opposite to those of sympathetic activation. However, in contrast to sympathetic activity, the parasympathetic nervous system has little effect on myocardial contractility.
Negative chronotropic effect (decrease in heart rate): The vagus nerve directly innervates the sinoatrial node; when activated, it serves to lower the heart rate, thus exhibiting a negative chronotropic effect.
Negative inotropic effect (decrease in myocardial contractility): Currently, it is a matter of debate whether parasympathetic stimulation can exhibit negative inotropic effects, as the vagus nerve does not directly innervate cardiomyocytes other than that of the sinoatrial and atrioventricular nodes, however, recent in vivo studies may suggest otherwise, at least in the atrium.
Negative dromotropic effect (decrease conduction velocity): Stimulation of the parasympathetic system serves to inhibit AV node conduction velocity.
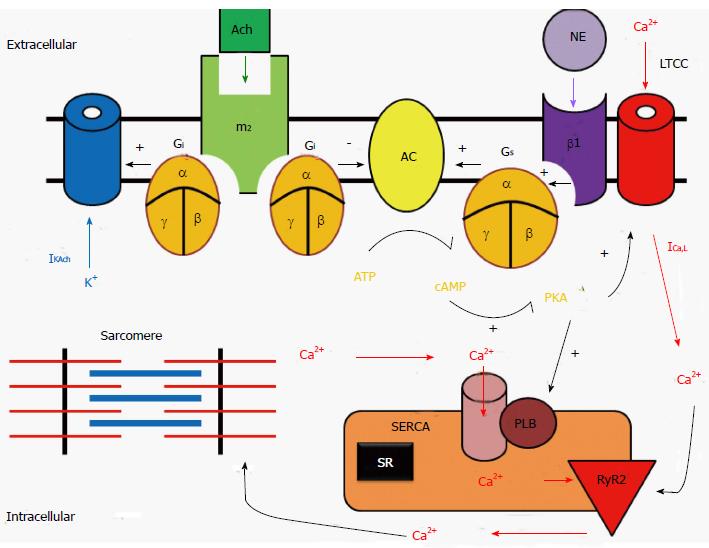
Most sympathetic and parasympathetic receptors are known to be G protein-coupled receptors (GPCRs). In the heart, the G-protein-cAMP-PKA signaling pathway mediates the catecholaminergic control on heart rate and contractility.
Signaling pathway of sympathetic stimulation: The sympathetic stimulation-induced effects in the heart result from activation of β1-adrenoceptors, which are GPCRs (Figure 2). The sympathetic neurotransmitter NE (as well as other catecholamines) bind to β1 receptors and activate stimulatory G proteins (Gs) by causing a conformational change within the Gs, so that the disassociated αs subunit can then bind to and activate adenylyl cyclase (AC). The activation of this enzyme then catalyzes the conversion of ATP into cyclic adenosine monophosphate (cAMP). This second messenger may then activate a myriad of other pathways, ion channels, transcription factors, or enzymes. With regards to the cardiovascular system, the most important enzyme that cAMP activates is protein kinase A (PKA). PKA, which in turn, phosphorylates multiple target proteins, such as L-type Ca channels (LTCC), the SR Ca handling protein phospholamban, and contractile machinery such as troponin C, I and T. Additionally, cAMP binds directly to ion channels responsible for the funny current (If), thus increasing the heart rate[7].
Signaling pathway of parasympathetic stimulation: The parasympathetic stimulation-induced effects in the heart result from activation of muscarinic (M2) receptors, which are also GPCRs by acetylcholine (Figure 2). The parasympathetic neurotransmitter ACh binds to M2 receptors thereby activating inhibitory G proteins (Gi) by causing a conformational change within the Gi subunit, so that the disassociated αi subunit can then bind to and inhibits AC. Since M2 receptors are negatively coupled to AC and thus reduce cAMP formation, M2 receptors act to inhibit PKA activity and exert an opposite effect on ion channels, Ca2+ handling proteins, and contractile machinery, compared to sympathetic stimulation.
Autorhythmic cells: Regulation of pacemaking current and heart rate: The funny current (If) is thought to be the pace making current in the SA node. It is a non-selective cation channel that can inwardly conduct both sodium and potassium ions. As the membrane potential becomes increasingly hyperpolarized during phase 3 and 4 of the action potential, If increases inward potassium and sodium currents, which causes the phase 4 diastolic depolarization. If channels are activated by direct binding of cAMP[7].
In addition to the funny current, one of the other driving mechanisms behind the automaticity of the pacemaking cells within the SA node is the calcium clock[8]. As the SR fills with calcium, the probability of spontaneous calcium release increases; in contrast, when the SR calcium stores are depleted, the probability of spontaneous calcium release is reduced. Increased Ca2+ entry also increases automaticity because of the effect of [Ca2+]i on the transient inward current carried by the sodium-calcium exchange current (INCX). When these pacemaking mechanisms depolarize the resting membrane potential and reach the threshold voltage, which induces the opening of L-type Ca channel (LTCC), an action potential is fired.
On the other hand, M2 receptor stimulation opens muscarinic potassium channels (KACh)[9]. These channels are opened by M2 receptors binding to ACh and produce a hyperpolarizing current that opposes the inward pacemaker current. Therefore, the parasympathetic stimulation increases outward K+ permeability, slowing the heart rate and reducing automaticity.
Cardiomyocytes: Regulation of cellular Ca2+ handling and cardiac contraction: Excitation-contraction coupling in cardiomyocytes is dependent on calcium-induced calcium release, whereby an action potential initiates an increase in cellular calcium through opening of the LTCC on the cellular membrane. This creates a positive feedback loop by activating the ryanodine receptors of the SR causing the release of an even greater amount of calcium. This calcium then binds to troponin C, moving the tropomyosin complex off the actin active site, so that the myosin head can bind to the actin filament. Hydrolysis of ATP then causes the myosin head to pull the actin filament toward the center of the sarcomere. Free intracellular calcium is then resequestered into the SR via the SR ATPase pump (SERCA), or is pumped from the cell via the sodium-calcium exchanger on the cellular membrane. Finally, the troponin complex returns the actin filament to its binding sites to tropomyosin.
Sympathetic stimulation leads to the elevation of cAMP levels and the activation of PKA, which phosphorylates the α1 subunits of the LTCCs. This increases the opening probability of LTCCs and the inward Ca2+ current, and thus enhances the force of cardiac contraction. In addition, PKA phosphorylates phospholamban, thus relieving its inhibition of SERCA, which in turn facilitates Ca2+ uptake by the SR and increases the amount of Ca2+ (i.e., SR Ca2+ content) available for release by the action potential. Furthermore, activation of β1-adrenoceptors also increases the Ca2+ sensitivity of the contractile machinery, mediated by phosphorylation of troponin C. Taken together, the net result of sympathetic stimulation is to elevate cardiac function and steepen both contraction and relaxation.
Since M2 receptors are negatively coupled to AC and thus reduce cAMP formation, they act to decrease the open probability of LTCCs and reduce Ca2+ current. In opposition to sympathetic stimulation, parasympathetic stimulation reduces the activity of Ca2+ handling proteins in cardiomyocytes.
Autonomic regulation of vascular function: In contrast to the heart, most vessels (arteries and veins) only receive sympathetic innervation, while capillaries receive no innervation. These sympathetic nerve fibers tonically release norepinephrine, which activates α1-adrenergic and β2-adrenergic receptors on blood vessels thereby providing basal vascular tone. Since there is greater α1-adrenergic than β2-adrenergic receptor distribution in the arteries, activation of sympathetic nerves causes vasoconstriction and increases the systemic vascular resistance primarily viaα1 receptor activation. On the other hand, modified sympathetic nerve endings in the adrenal medulla release circulating epinephrine, which also binds to α1 and β2-adrenergic receptors in vessels. However, β-adrenergic receptors show greater affinity for epinephrine than for norepinephrine. Therefore, circulating epinephrine at low concentrations activates only β1-adrenergic (mainly in the heart) and β2-adrenergic (mainly in vessels) receptors, which increase cardiac output and cause vasodilation, respectively. It should be noted that vessels at different locations may react differently to sympathetic stimulation. For example, during the “fight or flight” response the sympathetic nervous system causes vasodilation in skeletal muscle, but vasoconstriction in the skin.
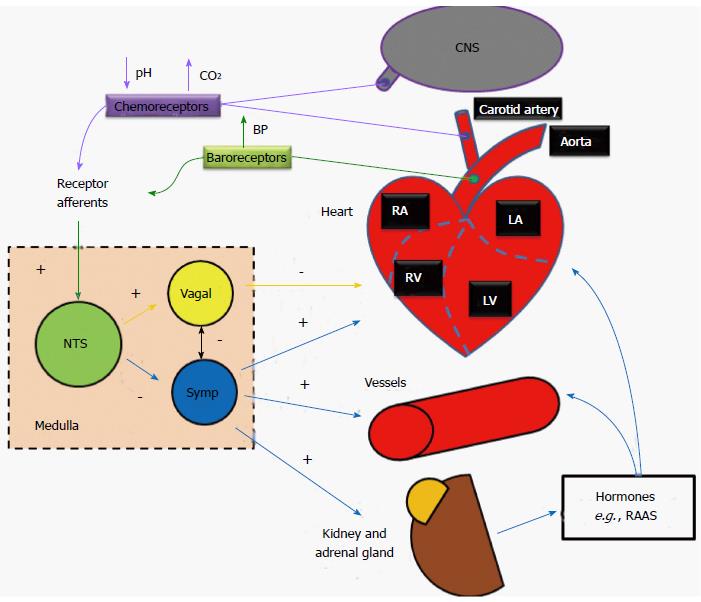
In the human body, the ANS is organized as functional reflex arcs (Figure 3). Sensory signals from receptors distributed in certain parts of the body are relayed via afferent autonomic pathways to the central nervous system (i.e., spinal cord, brain stem, or hypothalamus), the impulses are then integrated and transmitted via efferent pathways to the visceral organs to control their activities. The following reflexes play major roles in regulating cardiovascular functions.
Baroreceptor reflex: Baroreceptors located within the aortic arch and the carotid sinuses detect increases in blood pressure. These mechanoreceptors are activated when distended, and subsequently send action potentials to the rostral ventrolateral medulla (RVLM; located in the medulla oblongata of the brainstem) which further propagates signals, through the autonomic nervous system, adjusting total peripheral resistance through vasodilatation (sympathetic inhibition), and reducing cardiac output through negative inotropic and chronotropic regulation of the heart (parasympathetic activation). Conversely, baroreceptors within the venae cavae and pulmonary veins are activated when blood pressure drops. This feedback results in the release of antidiuretic hormone from cell bodies in the hypothalamus into the bloodstream from the nerve endings in the posterior lobe of the pituitary gland. The renin-angiotensin-aldosterone system is also activated. The subsequent increase in blood plasma volume then results in increased blood pressure. The final baroreceptor reflex involves the stretch receptors located within the atria; like the mechanoreceptors in the aortic arch and carotid sinuses, the receptors are activated when distended (as the atria become filled with blood), however, unlike the other mechanoreceptors, upon activation, the receptors in the atria increase the heart rate through increased sympathetic activation (first to the medulla, then subsequently to the SA node), thus increasing cardiac output and alleviating the increased blood volume-caused pressure in the atria[10].
Chemoreceptor reflex: Peripheral chemoreceptors located in the carotid and aortic bodies monitor oxygen and carbon dioxide content as well as the pH of the blood. Central chemoreceptors are located on the ventrolateral medullary surface in the central nervous system and are sensitive to the surrounding pH and CO2 levels. During hypovolemia or severe blood loss, blood oxygen content drops and/or pH is decreased (more acidic), and levels of carbon dioxide are likely increased, action potentials are sent along the glossopharyngeal or vagus nerves (the former for the carotid receptors, the latter for the aortic) to the medullary center, where parasympathetic stimulation is decreased, resulting in an increase in heart rate (and thus an increase in gas exchange as well as respiration). Additionally, sympathetic stimulation is increased, resulting in further increases to heart rate, as well as stroke volume, which in turn results in an even greater restoration of cardiac output.
Cardiovascular autonomic dysfunction and heart rate variability: It has been known that sympathetic stress/dominance occurs during heart failure or after myocardial infarction, and may trigger lethal arrhythmias. This sympathovagal imbalance is reflected by reduced heart rate variability (HRV). HRV is determined from ECG and has currently been used clinically as both a diagnostic as well as a prognostic factor for assessing cardiovascular autonomic dysfunction including cardiac autonomic neuropathy. Please refer a recent review article for specific HRV indicators and their interpretations[11].
In addition to the ANS, cardiovascular function is also influenced by numerous endocrine hormones. Released from the adrenal gland, epinephrine and dopamine (and ultimately, norepinephrine) are all involved in the initiation of the “fight-or-flight” response, while vasopressin, renin, angiotensin, aldosterone, and atrial-natriuretic peptide are all involved in water reabsorption for the purpose of blood pressure regulation.
An important exception to the usual arrangement in sympathetic fibers is the set of preganglionic fibers that pass through the sympathetic ganglia and extend to the medulla of the adrenal glands. These fibers terminate on special hormone secreting cells, i.e., chromaffin cells, that release norepinephrine (20%) and epinephrine (80%) when stimulated. Epinephrine and norepinephrine are the two main catecholamines that can activate or deactivate sympathetic receptors within the cardiovascular system. Another neurotransmitter dopamine that has limited actions in the autonomic nervous system may excite or inhibit depending on the receptors. Dopamine can be converted into norepinephrine and thus can increase heart rate and blood pressure. Epinephrine is produced (from phenylalanine and tyrosine) and released from chromaffin cells in the adrenal medulla of the adrenal glands. It can stimulate all major adrenergic receptors, including α1, α2, β1, and β2 receptors. Epinephrine at low concentrations is β2-selective, producing vasodilatation, while at high concentrations it also stimulates α1, α2, and β1 receptors, producing vasoconstriction (mediated by α1 and α2 receptors), and increases heart rate and contractility (mediated by β1 receptor). Blood pressure is regulated through a system of vasoconstriction and vasodilatation (i.e., vascular resistance). The change in vessel resistance is proportional to the length (L) of the vessel and the viscosity (η) of the blood and inversely proportional to the radius of the vessel to the fourth power (r4). It is clear from this relationship that vessel diameter controlled by the sympathetic nervous system can have a tremendous impact on blood pressure regulation via small changes in vessel diameter.
Math 4
Importantly, epinephrine serves to initiate the fight or flight response system by boosting the oxygen and glucose supplies to the brain and skeletal muscle through increased cardiac output and vasodilatation.
Vasopressin (antidiuretic hormone) is released during hypovolemic shock as a homeostatic attempt to increase blood pressure and maintain organ perfusion. Vasopressin serves to regulate water retention and vasoconstriction. Vasopressin is produced and released from the parvocellular neurosecretory neurons. It is synthesized in the hypothalamus, and then stored in the posterior pituitary gland, until it is secreted in response to a reduction in plasma volume, an increase in plasma osmolarity, or an increase in cholecystokinin[12]. Within the kidney, vasopressin causes water retention by increasing water permeability of the distal tubule and collecting duct cells, by inserting Aquaporin-2 channels, thus resulting in the inner medullary collecting duct becoming more permeable to urea. Within the cardiovascular system, vasopressin is a vasoconstrictor which increases arterial blood pressure. An increase in blood volume results in increased cardiac output and improved cardiovascular function.
There are three hormones produced in the kidneys: calcitriol, thrombopoietin and renin. Of these three, only renin is involved in cardiovascular reflexes and the regulation of blood pressure. Calcitrol works in conjunction with parathyroid hormone to increase the absorption of calcium and phosphate from the gastrointestinal tract[13]. Abnormal calcium metabolism in the cardiovascular system can result in medial arterial calcification and increased vascular stiffness, plaque formation and rupture. Thrombopoietin is made by the proximal convoluted tubule cells, and is responsible for stimulating the production of megakaryocytes of the bone marrow to eventually produce platelets[14]. Low numbers of platelets can lead to hemorrhage and anemic states. Anemia is known to result in high output heart failure.
In the kidney renin is released from the juxtaglomeruler cells, and activates the renin-angiotensin system. The renin-angiotensin-aldosterone system can play both physiological and pathological roles in the cardiovascular system. Angiotensin is known to be involved in heart failure. A main stay in the treatment of heart failure is the use of angiotensin converting enzyme inhibitors.
Renin-angiotensin-aldosterone system: The renin-angiotensin-aldosterone system serves to regulate blood pressure and fluid balance during for example instances of hypovolemia or blood loss. There are three mechanisms by which this system can be activated: baroreceptors with the carotid sinus can detect decreases in blood pressure, a decrease in sodium chloride concentration and/or a decreased rate of blood flow through the macula densa. Once a decrease in blood volume is detected, renin is released by the kidney and cleaves angiotensinogen (produced in the liver) into angiotensin I. Angiotensin Iis further converted to angiotensin II by the angiotensin converting enzyme (which is produced in the capillary beds of the lungs). Angiotensin II then acts upon the proximal tubules to increase sodium reabsorption, thus helping to retain water while maintaining the glomerular filtration rate and blood pressure. It also serves to constrict the renal arteries, as well as the afferent and efferent arterioles. Through contraction of the mesangial cells, it can also decrease the filtration rate of the kidneys. Angiotensin II also increases the sensitivity to tubuloglomerular feedback by increasing the afferent arterioles responsiveness in the macula densa. It can also reduce medullary blood flow. Finally, it causes the adrenal cortex to release aldosterone, which causes sodium retention and potassium excretion.
Angiotensin II has three major effects on the cardiovascular system: it is a potent vasoconstrictor, causing a direct increase in systemic blood pressure; it also exhibits prothrombotic effects, stimulating platelet aggregation and causing the production of PAI-1 and PAI-2[15]; finally, it acts as a Gq stimulator when released in an autocrine-paracrine fashion from cardiomyocytes, causing cell growth through protein kinase C during myocardial hypertrophy.
There are two major hormones produced by the heart. The first, atrial-natriuretic peptide (ANP), is produced by atrial cardiomyocytes, and serves to reduce blood pressure through several mechanisms.
ANP is produced, stored, and released by atrial myocytes (while also being produced in the ventricles, brain, suprarenal glands, and renal glands). There are five major causes for ANP release: distention of the atria, β-adrenergic stimulation, hypernatremia, increases in angiotensin II, and increases in endothelin[16]. Upon the vasculature, atrial-natriuretic peptide blocks catecholamines, while in the heart, it inhibits hypertrophy by blocking norepinephrine-stimulated protein synthesis. It is also believed to exhibit cardioprotective properties related to its ability to block cardiac fibrosis following ischemia-reperfusion injuries[17].
The other major hormone, brain-natriuretic peptide (BNP), is produced by ventricular cardiomyocytes, and works in a similar fashion to ANP. BNP is secreted by the ventricles of the heart in response to excessive stretching of ventricular myocytes and its level is typically increased in patients with left ventricular dysfunction. Therefore, clinically BNP levels are used to monitor heart function. Elevated levels of BNP are thought to be indicative of poor left ventricular function and heart failure.
Endothelin-1: Endothelin-1 is a potent vasoconstrictor that is produced by endothelial cells. There are four endothelin receptors, which are mainly expressed in vascular smooth muscles, each with varying actions upon activation. Activation of ETA results in smooth muscle vasoconstriction; ETB causes the release of nitric oxide from endothelial cells, thus resulting in vasodilatation; while activation of ETB2 causes vasoconstriction. ETA receptors also function like G-protein coupled receptors in ventricular cardiomyocytes[18,19]. The effects of ETC activation are currently unknown[20]. Endothelin-1 may play a role in cardiac hypertrophy via intracellular alkalinization.
Thyroxin: Thyroxin (T4) is a hormone produced by the follicular cells of the thyroid gland. While it acts on nearly every cell type within the human body, one of its most important functions is to increase the effect of epinephrine. Through this permissive relationship, thyroxin increases the number of β1 receptors and is thus indirectly responsible for increasing cardiac output (in both an inotropic and chronotropic manner) and increasing respiration rates. It is directly responsible for increasing basal metabolic rates by increasing protein and carbohydrate metabolism[21]. Clinical increases in thyroxin are associated with the occurrence of atrial fibrillation, a common cardiac arrhythmia. Elevated heart rates from thyroxin induced atrial fibrillation or other arrhythmias can result in myocardial decompensation and heart failure if not returned to normal sinus rhythm.
In conclusion, the heart is not simply an isolated actor. The cardiovascular system responds to not only acute but also chronic changes in blood pressure and homeostasis. Body homeostasis and survival are therefore the main functions of the cardiovascular system. Factors actively influencing the cardiovascular system range from the central nervous system including the brain and spinal cord to the peripheral nervous system with fibers being transported through spinal nerves to the glands, e.g., adrenals, vasculature and even to the urinary system (kidneys). The cardiovascular system is controlled and influenced by not only a unique intrinsic conduction system, but is also heavily influenced by the autonomic nervous system as well as the endocrine system.
P- Reviewer: Kolettis TM, Lazzeri C, O-Uchi J S- Editor: Tian YL L- Editor: A E- Editor: Wu HL
| 1. | Boron W, Boulpaep E. Medical physiology: a cellular and molecular approach. 2nd ed. Philadelphia, PA: Elsevier Saunders 2011; . |
| 2. | Gwathmey JK, Briggs GM, Allen PD. Heart Failure: Basic Science and Clinical Aspects. New York: Marcel Dekker Inc 1994; 282-283. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (2)] |
| 3. | Mann DL, Zipes DP, Libby P, Bonow RO. Braunwald’s Heart Disease: Textbook of Cardiovascular Medicine. 10th ed. Philadelphia, Pennsylvania: Elsevier - Health Sciences Division 2014; . |
| 4. | Rhoadesand RA, Bell DR. Medical Physiology: Principles for Clinical Medicine. 3rd Ed. Philadelphia, Pennsylvania: Lippincott Williams and Wilkins, Wolters Kluwer Health 2009; . |
| 5. | Kishi T. Heart failure as an autonomic nervous system dysfunction. J Cardiol. 2012;59:117-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 164] [Article Influence: 12.6] [Reference Citation Analysis (1)] |
| 6. | Brodde OE, Michel MC. Adrenergic and muscarinic receptors in the human heart. Pharmacol Rev. 1999;51:651-690. [PubMed] |
| 7. | DiFrancesco D, Tortora P. Direct activation of cardiac pacemaker channels by intracellular cyclic AMP. Nature. 1991;351:145-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 584] [Cited by in RCA: 600] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 8. | Lakatta EG, DiFrancesco D. What keeps us ticking: a funny current, a calcium clock, or both? J Mol Cell Cardiol. 2009;47:157-170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 241] [Cited by in RCA: 216] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 9. | Krapivinsky G, Gordon EA, Wickman K, Velimirović B, Krapivinsky L, Clapham DE. The G-protein-gated atrial K+ channel IKACh is a heteromultimer of two inwardly rectifying K(+)-channel proteins. Nature. 1995;374:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 661] [Cited by in RCA: 670] [Article Influence: 22.3] [Reference Citation Analysis (1)] |
| 10. | Hakumäki MO. Seventy years of the Bainbridge reflex. Acta Physiol Scand. 1987;130:177-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 39] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Metelka R. Heart rate variability--current diagnosis of the cardiac autonomic neuropathy. A review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2014;158:327-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (2)] |
| 12. | Salata RA, Jarrett DB, Verbalis JG, Robinson AG. Vasopressin stimulation of adrenocorticotropin hormone (ACTH) in humans. In vivo bioassay of corticotropin-releasing factor (CRF) which provides evidence for CRF mediation of the diurnal rhythm of ACTH. J Clin Invest. 1988;81:766-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 57] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Voet D, Voet JG. Biochemistry. Volume one. Biomolecules, mechanisms of enzyme action, and metabolism, 3rd ed. New York: John Wiley & Sons 2004; 663-664. |
| 14. | Kaushansky K. Lineage-specific hematopoietic growth factors. N Engl J Med. 2006;354:2034-2045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 320] [Article Influence: 16.8] [Reference Citation Analysis (2)] |
| 15. | Skurk T, Lee YM, Hauner H. Angiotensin II and its metabolites stimulate PAI-1 protein release from human adipocytes in primary culture. Hypertension. 2001;37:1336-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 98] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 16. | de Bold AJ. Atrial natriuretic factor: a hormone produced by the heart. Science. 1985;230:767-770. [PubMed] |
| 17. | Kasama S, Furuya M, Toyama T, Ichikawa S, Kurabayashi M. Effect of atrial natriuretic peptide on left ventricular remodelling in patients with acute myocardial infarction. Eur Heart J. 2008;29:1485-1494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 18. | James AF, Xie LH, Fujitani Y, Hayashi S, Horie M. Inhibition of the cardiac protein kinase A-dependent chloride conductance by endothelin-1. Nature. 1994;370:297-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 19. | Xie LH, Horie M, James AF, Watanuki M, Sasayama S. Endothelin-1 inhibits L-type Ca currents enhanced by isoproterenol in guinea-pig ventricular myocytes. Pflugers Arch. 1996;431:533-539. [PubMed] |
| 20. | Boron WF, Boulpaep EL. Medical physiology: a cellular and molecular approach. 2nd ed. Philadelphia, PA: Elsevier Saunders 2011; . |
| 21. | Popovic WJ, Brown JE, Adamson JW. The influence of thyroid hormones on in vitro erythropoiesis. Mediation by a receptor with beta adrenergic properties. J Clin Invest. 1977;60:907-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 1.1] [Reference Citation Analysis (1)] |