Published online Mar 26, 2015. doi: 10.4330/wjc.v7.i3.150
Peer-review started: October 29, 2014
First decision: November 27, 2014
Revised: January 14, 2015
Accepted: January 30, 2015
Article in press: February 2, 2015
Published online: March 26, 2015
Processing time: 135 Days and 17.4 Hours
AIM: To investigate long-term efficacy of two different devices more than five years after percutaneous atrial septal defect (ASD) closure in adults.
METHODS: All patients who underwent percutaneous closure of an ASD in the St. Antonius Hospital, Nieuwegein, The Netherlands, between February 1998 and December 2006 were included. Percutaneous closure took place under general anaesthesia and transesophageal echocardiographic monitoring. Transthoracic echocardiography (TTE) was performed 24 h post-procedure to visualize the device position and to look for residual shunting using color Doppler. All complications were registered. All patients were invited for an outpatient visit and contrast TTE more than 5-years after closure. Efficacy was based on the presence of a residual right-to-left shunt (RLS), graded as minimal, moderate or severe. The presence of a residual left-to-right shunt (LRS) was diagnosed using color Doppler, and was not graded. Descriptive statistics were used for patients’ characteristics. Univariate analysis was used to identify predictors for residual shunting.
RESULTS: In total, 104 patients (mean age 45.5 ± 17.1 years) underwent percutaneous ASD closure using an Amplatzer device (ASO) in 76 patients and a Cardioseal/Starflex device (CS/SF) in 28 patients. The mean follow-up was 6.4 ± 3.4 years. Device migration occurred in 4 patients of whom two cases occurred during the index hospitalization (1 ASO, 1 CS/SF). The other 2 cases of device migration occurred during the first 6 mo of follow-up (2 CS/SF). The recurrent thrombo-embolic event rate was similar in both groups: 0.4% per follow-up year. More than 12 mo post-ASD closure and latest follow-up, new-onset supraventricular tachyarrhythmia’s occurred in 3.9% and 0% for the ASO and CS/SF group, respectively. The RLS rate at latest follow-up was 17.4% (minimal 10.9%, moderate 2.2%, severe 4.3%) and 45.5% (minimal 27.3%, moderate 18.2%, severe 0%) for the ASO- and CS/SF groups, respectively. There was no residual LRS in both groups.
CONCLUSION: Percutaneous ASD closure has good long-term safety and efficacy profiles. The residual RLS rate seems to be high more than 5 years after closure, especially in the CS/SF. Residual LRS was not observed.
Core tip: Several atrial septal defect (ASD) closing devices have been proven safe and effective for percutaneous ASD closure. We evaluated long-term (i.e., more than 5-year of follow-up) efficacy of two different devices used in adults. Percutaneous ASD closure seems to be relatively safe using the Amplatzer device. Though, the right-to-left shunt (RLS) rate is high, a residual left-to-right shunt was absent at latest follow up. The Cardioseal/Starflex device appears to be associated with a higher complication- and residual RLS rate. The importance of a residual RLS is unclear. Therefore, long-term follow up might be necessary.
- Citation: Snijder RJ, Suttorp MJ, Berg JMT, Post MC. Percutaneous closure of secundum type atrial septal defects: More than 5-year follow-up. World J Cardiol 2015; 7(3): 150-156
- URL: https://www.wjgnet.com/1949-8462/full/v7/i3/150.htm
- DOI: https://dx.doi.org/10.4330/wjc.v7.i3.150
An atrial septal defect (ASD) is a common cardiac defect and accounts for one third of all congenital heart diseases detected in adults[1,2]. The diagnosis in adults is often made when complications of the shunt occur, such as pulmonary hypertension, heart failure, arrhythmias, or paradoxical embolism. Most of these complications might be prevented by closure.
Since the first description of the transcatheter closure device for an ASD in 1976 by King et al[3], percutaneous ASD closure has been practiced and described extensively. Other studies evaluated various ASD closure devices and showed good mid-term (up to 2 years) safety and efficacy profiles[4-7]. However, long-term (i.e., more than 5-year after closure) safety and efficacy data on percutaneous closure devices has not been available yet. We report long-term efficacy and safety of two types of ASD closure devices.
All patients who underwent a percutaneous closure of an ASD in the St. Antonius Hospital, Nieuwegein, The Netherlands, between February 1998 and December 2006 were included in this study. All patients were invited for an outpatient visit and transthoracic echocardiography (TTE).
As reported earlier, percutaneous closure took place under general anaesthesia and transesophageal echocardiographic (TEE) monitoring according to standard techniques[4]. TTE was performed 24 h post-procedure to visualize the device position and to look for residual shunting using color Doppler.
Follow-up information was obtained at the outpatient clinic or by a telephone interview. All complications were documented, and divided into major and minor as described by Khairy et al[8]. Major complications included procedure related events such as haemorrhage requiring blood transfusion, occurrence of cardiac tamponade, need for procedure-related surgical intervention, massive fatal pulmonary emboli, occurrence of new thrombo-embolic events and death[8].
New-onset supraventricular tachycardia’s were diagnosed by routine ECG when patients visited the outpatient clinic or when patients visited the emergency department because of symptoms.
The efficacy of the ASD closure was based on the presence of residual shunting using contrast TTE (cTTE) with Valsalva manoeuvre, and color Doppler. A residual right-to-left shunt (RLS) was present if microbubbles appeared in the left atrium. Opacification of the left ventricle and shunt grade were classified as minimal (maximum of 30 micro-bubbles in left ventricle), moderate (between 30 and 100 bubbles in left ventricle), and large (> 100 micro-bubbles in the left ventricle). This division was based on the maximum number of microbubbles counted in one still frame, as previously reported[9]. The presence of a left-to-right shunt (LRS) was based color Doppler imaging at the atrial septum. The LRS was not graded.
Descriptive statistics were used for patients’ characteristics. Continuous variables with normal distribution are presented as mean ± SD or median with range if normal distribution was absent. Univariate analysis was used to identify predictors for residual shunting. All statistical analyses were performed using SPSS software (version 22.0 for Windows).
Percutaneous ASD closure was performed in 104 consecutive patients (75% women; mean age, 45.5 ± 17.1 years). Baseline characteristics, risk factors, co-morbidity and indication for closure are summarized in Table 1.
| Number | 104 |
| Age (yr) | 45.5 ± 17.1 |
| Female, n (%) | 78 (75.0) |
| Weight (kg) | 73.1 ± 15.1 |
| Risk factors and co-morbidities (%) | |
| Arterial hypertension | 18.4 |
| Hypercholesterolemia | 3.9 |
| Diabetes | 1.9 |
| Smoking | 18.4 |
| CAD | 4.9 |
| History of SVT | 26.2 |
| Antithrombotic treatment, n (%) | |
| None | 59 (56.7) |
| Aspirin | 20 (19.2) |
| Dipyridamol | 1 (1.0) |
| Oral anticoagulants | 21 (20.2) |
| Unknown | 3 (2.9) |
| Indication for closure, n (%) | |
| RV volume overload | 72 (69.2) |
| Cryptogenic TIA/stroke | 21 (20.2) |
| Asymptomatic | 11 (10.6) |
| RVSP + CVP (mmHg)1 | 34.6 ± 10.5 |
| ASD diameter (mm)2 | 18.3 ± 6.3 |
| Follow up (yr) | 6.4 ± 3.4 |
Device implantation was initially uneventful in 102 patients (98.1%). In 76 patients (73.1%) an Amplatzer (ASO), and in 28 patients (26.9%) a Cardioseal/Starflex (CS/SF) was used for closure. In total, 4 major complications occurred within the first 6 mo. Two patients (1.9%, 1 ASO, 1 CS/SF) suffered from embolization of the device during the index hospitalization and two (1.9%, 2 CS/SF) within the first 6 mo after closure. All underwent surgical device extraction; the ASD was closed using a patch during the same operation. All patients recovered well. Procedural characteristics are shown in Table 2.
| Devices | |
| Amplatzer | 76 (73.1) |
| Diameter, mm1 | 25 (12-38) |
| Cardioseal/starflex | 28 (26.9) |
| Diameter, mm1 | 33 (20-40) |
| General anaesthesia | 104 (100) |
| TEE guiding | 104 (100) |
| In-hospital complications | |
| Device embolization | 2 (1.9) |
| New-onset SVT | 3 (2.9) |
| Allergic reaction | 1 (1.0) |
| Fever | 1 (1.0) |
| Groin hematoma | 1 (1.0) |
| Tamponade | 1 (1.0) |
| Shunt by TTE2 | |
| Color Doppler | 9 (11.4) |
| Hospital stay, d1 | 2 (2-7) |
Between 6- and 12 mo, another two patients (1.9%, 2 CS/SF) underwent surgical extraction of the device and the ASD was closed with a patch during the same operation. One patient had a large residual shunt, which could not be closed with a second device. The other patient needed rhythm surgery, therefore, device extraction was performed.
Within the first 12-mo, recurrent thrombo-embolic events occurred in 1 patient (0.9%, 1 CS/SF). This 58-year-old patient suffered a transient ischemic attack (TIA). Because of a history of supraventricular tachyarrhythmia’s (SVT) the patient was already using oral anticoagulation. cTTE showed no residual RLS.
New-onset SVT’s occurred in 6.6% of the ASO group and in 17.9% of the CS/SF group.
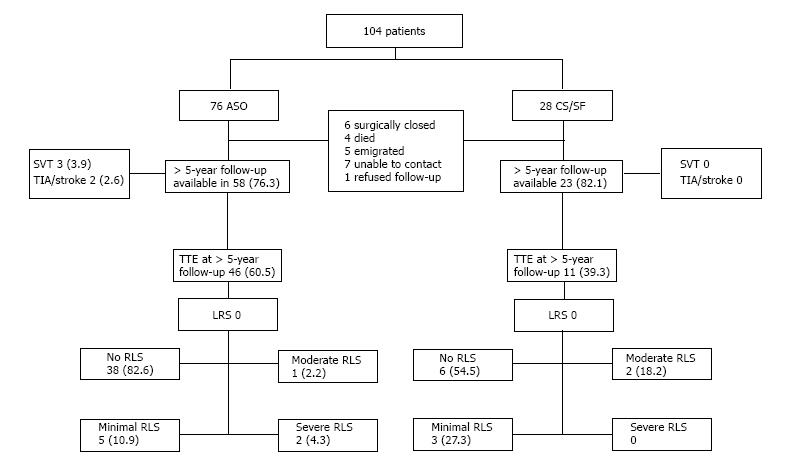
Contrast TTE was performed in 57 patients (54.8%, 46 ASO and 11 CF/SF). Median follow-up time for the ASO group was 6.6 years (5.0-11.1 years) and the CS/SF group 9.9 years (6.3-13.4 years). Though, cTTE could only be performed in 57 patients, follow-up information was available in a total of 81 patients.
Long-term follow-up data could not be retrieved (interview or cTTE) in 23 patients of which 6 were surgically closed, 4 died (no device related cause was suspected) and 13 were lost to follow-up.
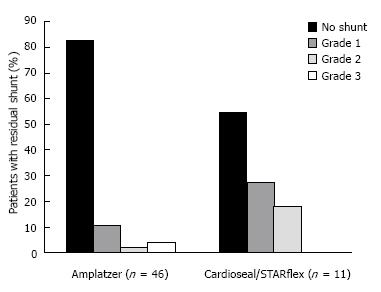
Contrast TTE showed a RLS shunt in eight patients (17.4%) who received an ASO. Of these, five patients (10.9%) had a minimal, one patient (2.2%) a moderate and two patients (4.3%) a severe residual shunt. Five patients who received a CS/SF device (45.5%) had a residual RLS of which three patients (27.3%) had a minimal and two patients (18.2%) a moderate residual RLS. When minimal shunts were excluded, the closure rate was 93.5% for ASO and 81.8% for the CS/SF device, respectively. There was no recurrent LRS at latest follow up. Our analyses showed no significant differences in the diameters of the ASD or the device used between the patients with or without a residual shunt. Secondly, no predictors for a right-to-left shunt at long-term follow-up could be found using univariate analysis.
Recurrent thrombo-embolic events after more than 12 mo of follow-up occurred in two patients (1.9%, 2 ASO). One 40-year-old patient suffered a cerebrovascular accident 2.5 years after ASD closure, while on aspirin because of coronary artery disease. Although there was no history of SVT or device thrombus, oral anticoagulation was initiated after this event. At long-term follow up, a minimal residual RLS was found. The other patient (48-year-old) was known with a history of multiple TIA’s prior to closure and was therefore treated with Aspirin. Despite closure of the ASD and optimal medical treatment, the patient suffered from another TIA more than 5 years after ASD closure. At the long-term follow-up visit no residual shunt or thrombus formation on the device was found. This patient had no history of SVT. In total, 3 patients suffered a recurrent neurological event during a mean follow up of 6.4 years (0.5% per year follow up).
During long-term follow-up, new-onset SVT occurred in 3 patients (3.9%) who received an ASO and in none of the patients who received a CS/SF device.
Long-term residual shunt rate, recurrent thrombo-embolic event rate and new-onset SVT rate are presented in Table 3. Figure 1 shows the percentage of patients with residual right-to-left shunt at more than 5-year follow-up after percutaneous ASD closure.
| Amplatzer | Cardioseal/STARflex | |
| 5 yr follow-up available, n (%) | 58 (76.3) | 23 (82.1) |
| New-onset SVT | ||
| 0-1 yr | 5 (6.6) | 5 (17.9) |
| > 1 yr | 3 (3.9) | 0 |
| Reoccurrence TIA/stroke | ||
| 0-1 yr | 0 | 1 (3.6) |
| > 1 yr | 2 (2.6) | 0 |
| TTE > 5 yr FU available, n | 46 | 11 |
| RLS | ||
| No shunt | 38 (82.6) | 6 (54.5) |
| Minimal | 5 (10.9) | 3 (27.3) |
| Moderate | 1 (2.2) | 2 (18.2) |
| Severe | 2 (4.3) | 0 |
| LRS | 0 | 0 |
| Follow up (yr) | 6.6 (5.0-11.1) | 9.9 (6.3-13.4) |
A flow-chart showing the results of this study during follow-up is presented in Figure 2.
Percutaneous ASD closure has relatively good long-term safety- and efficacy profiles, especially using the ASO device. A high residual RLS was present in CS/SF. Residual LRS was not observed in either the ASO- or the CS/SF group.
Device embolization and dislocation is a well-known complication after percutaneous ASD closure[10,11]. The CS/SF is related to a relatively high embolization rate compared to the ASO[12]. In literature, embolization of the CS/SF has been described between 1.4% and 2.5% and for the ASO between 0.1% and 2.4%[7,10,13,14]. In all studies, embolization occurred during the procedure or the index hospitalization. Kefer et al[15] and Masura et al[16], described 112 and 151 patients with a mean follow-up of 5 and 6.5 years, respectively, and showed no device embolization using the ASO device.
In our study 4 major complications (3.8%) occurred within the first six months after closure. Device migration occurred in 10.7% of the patients with a CS/SF device and in 1.3% using an ASO device. All devices were surgically extracted and the ASD was closed with a patch. Compared to the literature our CS/SF subgroup had a higher complication rate, while the ASO subgroup was similar. Hence, the CS/SF devices are no longer available for ASD closure. However, long-term follow-up of patients who received a CS/SF device is recommended.
Because device embolization occurred more often in patients with a Cardioseal/Startflex device, we analysed potential reasons/risk factors only for this device. Post et al[5] showed that the initial ASD and the device diameter were significantly higher in the patients in whom the device was embolized. However, due to the small sample size of this study it is difficult to make any conclusions.
Kefer et al[15] described a recurrent stroke rate of 0% after percutaneous ASD closure with similar devices and follow-up time as in our study. Masura et al[16] described no thrombo-embolisms during the entire follow-up period. One patient (0.6% per follow-up year) with an ASO device in the study of Spies et al[7] suffered a thrombo-embolic event, which could not be related to a residual shunt or device related thrombus formation.
In our study, the recurrent thrombo-embolic event rate during long-term follow-up was 0.4% per follow-up year for both devices, which is similar when compared to the literature. As described above, all patients were treated with anti-platelet therapy or oral anticoagulation and a minimal residual RLS was found in only one patient.
Arrhythmias
arrhythmias early after ASO or other devices implantation are common and extensively described[10,17,18]. Masura et al[16] also mentioned SVT’s at early follow-up, but none during long-term follow-up. Chessa et al[10] noted that arrhythmias are the second most common complication in their study (2.6%) early after the procedure using both the ASO and the CS/SF. Tomar et al[19] described peri-procedural arrhythmias but none were seen during long-term follow-up (median 56 mo). At 2-year follow-up, Spies et al[18] found an annual incidence of new-onset atrial fibrillation of 4.1%. Butera et al[13] described 274 patients (153 ASO, 121 CS/SF) and showed no arrhythmias at follow-up (respectively 16- and 24-mo).
In our study, 3.9% of the patients with an ASO device had new-onset SVT without any abnormalities during cTTE at long-term follow up.
Residual LRS rates for the ASO has previously been described in several studies and ranged between 0%-12.5% at long-term follow up[13-16,19,20]. Kefer et al[15] described a residual LRS rate of 4%. However, only 2 patients (1.8%) with a residual LRS had received an ASO. At 3-year follow-up, Masura et al[16] showed no residual shunt using color-Doppler. Butera et al[20] described 165 patients with a residual LRS rate of 2% in patients suffering multiple ASD’s. At 24-mo follow-up, a non-significant difference between the ASO and CS/SF was found (respectively 0% vs 4.4%). In a study by Nugent et al[14], 72 patients received a CS/SF device with a total residual LRS of 12.5% between 12- and 24-mo of follow-up.
Our study showed no residual LRS for both devices at more than 5-year of follow-up. However, the prevalence of a RLS is relatively high. Earlier, Luermans et al[12] described a residual RLS rate of 14% 3.4 years after closure in 29 patients who received a CS/SF device.
In our study, the RLS rate for the ASO was 17.4% and 45.5% for the CS/SF device more than five years after closure. When excluding the minimal shunts, the ASO had a RLS rate of 6.5% and the CS/SF of 18.2%. The importance of this relatively high rate of RLS is unclear, as the reason for closure was mainly related to the presence of a LRS. Therefore, the fact that we did not notice residual LRS is an important observation. Moreover, to assess the clinical importance of the presence of a RLS, long-term follow-up might be necessary.
The difference in RLS rate after percutaneous ASD closure might be due to the different closing mechanisms; the ASO has a “stent-like” mechanism and consists of Nitinol metal with rounded disks with a polyester fabric sewn inside the meshed disks. The CS/SF device has a “double patch” mechanism and the fabric is directly exposed to blood[13,21]. The latter might delay the endothelialisation of the devices, which is important for complete closure of the ASD. Why endothelialisation happens in some patients better than others is unclear.
Firstly, it is a single-centre design with a small sample-size. Secondly, we used cTTE at follow-up for residual shunt classification while the gold standard is contrast TEE. Though, we did not use contrast TEE, literature describes mostly studies where only color Doppler is used for the assessment of residual shunts. Thirdly, an independent core lab did not review the TTE’s. Fourthly, the long-term follow up data was available in about 80% of patients; this might lead to an under- or overestimation.
Percutaneous closure of a secundum-type atrial septal defect seems to be safe using the ASO. Though, the RLS rate is relatively high, a residual LRS is absent more than 5-year after closure. The CS/SF appears to be associated with a relatively high complication- and residual RLS rate. Because of the unclear importance of a RLS after percutaneous ASD closure, long-term follow-up might be necessary.
An atrial septal defect (ASD) is a common cardiac defect and accounts for one third of all congenital heart diseases detected in adults. The diagnosis in adults is often made when complications of the shunt occur, such as pulmonary hypertension, heart failure, arrhythmias, or paradoxical embolism. Most of these complications might be prevented by closure.
Since the first description of transcatheter device closure of an ASD in 1976, percutaneous closure of an ASD has been practiced and described extensively. Other studies showed the efficacy and safety of percutaneous closure of ASD’s with different devices, mainly during mid-term follow up. Little is known about follow-up more than 5 years after percutaneous ASD closure in adults. We report the efficacy of ASD device closure at more than 5 years follow-up (long-term follow-up).
Previous studies showed that a percutaneous closed ASD using a Cardioseal/Startflex (CS/SF) is associated with a high residual right-to-left shunt (RLS) at mid-term follow-up. This study confirmed that a high residual RLS is still present during long-term follow-up. However, no left-to-right shunt (LRS) was present. Percutaneous closure using a Amplatzer device (ASO) has proven to be efficient at mid-term follow-up. During long-term follow-up no LRS was found. However, a relatively high RLS was present. The importance of RLS at follow-up is unclear. The safety for both devices is similar when compared to literature.
During long-term follow-up, percutaneous closure of ASD’s seems to be safe using different devices, especially using the ASO device. A high residual RLS is present in CS/SF, however there was no residual LRS observed using both the ASO- and the CS/SF device.
An ASD is an opening in the septum between the right- and left atrium. It is a congenital heart disease and therefore present at birth. An ASD can cause symptoms due to heart failure, arrhythmia’s, paradoxical embolism and pulmonary hypertension. Percutaneous closure of an ASD is a relatively simple procedure where a Nitinol device is placed in the opening between the right- and left atrium. Tansthoracic ultrasound of the heart is used to check whether there is a residual opening in the atrial septum.
The paper by Dr. Snijder et al reports the experience in percutaneous closure of atrial septal defects in 104 patients using two devices. Interestingly, in a long-term follow-up a residual left-to-right shunt is absent, although the rate of a residual right-to-left shunt is relatively high.
P- Reviewer: De Ponti R, Ferrer-Hita JJ, Patanè S S- Editor: Gong XM L- Editor: A E- Editor: Lu YJ
| 1. | Campbell M. Natural history of atrial septal defect. Br Heart J. 1970;32:820-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 247] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 2. | Hagen PT, Scholz DG, Edwards WD. Incidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal hearts. Mayo Clin Proc. 1984;59:17-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1669] [Cited by in RCA: 1577] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 3. | King TD, Mills NL. Nonoperative closure of atrial septal defects. Surgery. 1974;75:383-388. [PubMed] |
| 4. | Van Den Branden BJ, Post MC, Plokker HW, Ten Berg JM, Suttorp MJ. Percutaneous atrial shunt closure using the novel Occlutech Figulla device: 6-month efficacy and safety. J Interv Cardiol. 2011;24:264-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Post MC, Suttorp MJ, Jaarsma W, Plokker HW. Comparison of outcome and complications using different types of devices for percutaneous closure of a secundum atrial septal defect in adults: a single-center experience. Catheter Cardiovasc Interv. 2006;67:438-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Pac A, Polat TB, Cetin I, Oflaz MB, Balli S. Figulla ASD occluder versus Amplatzer Septal Occluder: a comparative study on validation of a novel device for percutaneous closure of atrial septal defects. J Interv Cardiol. 2009;22:489-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Spies C, Timmermanns I, Schräder R. Transcatheter closure of secundum atrial septal defects in adults with the Amplatzer septal occluder: intermediate and long-term results. Clin Res Cardiol. 2007;96:340-346. [PubMed] |
| 8. | Khairy P, O’Donnell CP, Landzberg MJ. Transcatheter closure versus medical therapy of patent foramen ovale and presumed paradoxical thromboemboli: a systematic review. Ann Intern Med. 2003;139:753-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 265] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 9. | van Gent MW, Post MC, Luermans JG, Snijder RJ, Westermann CJ, Plokker HW, Overtoom TT, Mager JJ. Screening for pulmonary arteriovenous malformations using transthoracic contrast echocardiography: a prospective study. Eur Respir J. 2009;33:85-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Chessa M, Carminati M, Butera G, Bini RM, Drago M, Rosti L, Giamberti A, Pomè G, Bossone E, Frigiola A. Early and late complications associated with transcatheter occlusion of secundum atrial septal defect. J Am Coll Cardiol. 2002;39:1061-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 404] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 11. | Levi DS, Moore JW. Embolization and retrieval of the Amplatzer septal occluder. Catheter Cardiovasc Interv. 2004;61:543-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 120] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 12. | Luermans JG, Post MC, ten Berg JM, Plokker HW, Suttorp MJ. Long-term outcome of percutaneous closure of secundum-type atrial septal defects in adults. EuroIntervention. 2010;6:604-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Butera G, Carminati M, Chessa M, Delogu A, Drago M, Piazza L, Giamberti A, Frigiola A. CardioSEAL/STARflex versus Amplatzer devices for percutaneous closure of small to moderate (up to 18 mm) atrial septal defects. Am Heart J. 2004;148:507-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Nugent AW, Britt A, Gauvreau K, Piercey GE, Lock JE, Jenkins KJ. Device closure rates of simple atrial septal defects optimized by the STARFlex device. J Am Coll Cardiol. 2006;48:538-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Kefer J, Sluysmans T, Hermans C, El Khoury R, Lambert C, Van de Wyngaert F, Ovaert C, Pasquet A. Percutaneous transcatheter closure of interatrial septal defect in adults: procedural outcome and long-term results. Catheter Cardiovasc Interv. 2012;79:322-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Masura J, Gavora P, Podnar T. Long-term outcome of transcatheter secundum-type atrial septal defect closure using Amplatzer septal occluders. J Am Coll Cardiol. 2005;45:505-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 171] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 17. | Hill SL, Berul CI, Patel HT, Rhodes J, Supran SE, Cao QL, Hijazi ZM. Early ECG abnormalities associated with transcatheter closure of atrial septal defects using the Amplatzer septal occluder. J Interv Card Electrophysiol. 2000;4:469-474. [PubMed] |
| 18. | Spies C, Khandelwal A, Timmermanns I, Schräder R. Incidence of atrial fibrillation following transcatheter closure of atrial septal defects in adults. Am J Cardiol. 2008;102:902-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 19. | Tomar M, Khatri S, Radhakrishnan S, Shrivastava S. Intermediate and long-term followup of percutaneous device closure of fossa ovalis atrial septal defect by the Amplatzer septal occluder in a cohort of 529 patients. Ann Pediatr Cardiol. 2011;4:22-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Butera G, Romagnoli E, Saliba Z, Chessa M, Sangiorgi G, Giamberti A, Cappato R, Bussadori C, Abella R, Pelissero G. Percutaneous closure of multiple defects of the atrial septum: procedural results and long-term follow-up. Catheter Cardiovasc Interv. 2010;76:121-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Anzai H, Child J, Natterson B, Krivokapich J, Fishbein MC, Chan VK, Tobis JM. Incidence of thrombus formation on the CardioSEAL and the Amplatzer interatrial closure devices. Am J Cardiol. 2004;93:426-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 101] [Article Influence: 4.8] [Reference Citation Analysis (0)] |