WHY SHOULD CARDIOMYOCYTES BE PRODUCED USING NON-CARDIAC CELLS?
Heart contraction is produced by cardiomyocytes, which comprise the cardiac muscle cell population. It was thought that the heart is a refractory organ that is incapable of replacing cardiomyocytes that are lost by normal tissue damage or cardiac disease. However, over the past decade this view has been challenged by numerous studies indicating that the heart can regenerate cardiomyocytes; at least with a capacity to replace those cells lost by regular tissue turnover[1]. Unfortunately, this regenerative capacity is significantly lower compared to skeletal muscle. Thus, major disease insults, such as myocardial infarction (MI), result in an irreversible, catastrophic loss of cardiomyocytes. Typically, MI results in the death of around 20% of the total cardiomyocytes population in the heart. The ventricle is a major site of cardiomyocyte death, with billions of dead cells being eventually replaced by fibrous scar tissue[2-4]. Acute MI produces significant mortality (for example, 36% fatality in the United Kingdom, over the years 2002-2010[5]). For those patients that survive, the poor capacity of heart regeneration means that many are predisposed to eventually develop clinical heart failure[2,6,7].
It can be envisaged that one potential therapy for preventing the progression of MI to heart failure would be the transplantation of functional cardiomyocytes to the infarction site. This cell therapy approach has been demonstrated as an achievable cure for degenerative diseases, such as the transplantation of hematopoietic multipotent stem cells to treat certain types of leukemia[8]. Cardiac cell therapy could prevent progression to heart failure by allowing functional recovery of the heart. Most cell therapy approaches involving cardiomyocyte transplantation aim to treat the consequences of MI, because of the significant impact of this disease on human health.
Cell therapy approaches have been developed to treat cardiac dysfunction, such as MI (reviewed in[9]). Multiple strategies exist for cell delivery and cell source. For example, different types of stem cells have been used for transplantation, including mesenchymal stem cells or cardiac stem cells. More differentiated cells have also been utilized, such as skeletal muscle cells and cardiomyocytes. Unfortunately, the results of clinical trials have only shown a modest improvement after MI. One approach to improve the outcome of cell therapy for MI would be the development of an ideal, optimized cell type for transplantation. This would also require the development of a rigorous, defined experimental methodology to ensure quality control for the cells prior to grafting. However, standardized protocols for culturing transplantable cells are lacking and laboratories tend to develop thief own “in house” techniques and culture media recipes.
The research field of chemical biology is ideally suited to provide reagents that can enhance cell culture and scale-up for transplantation. Chemical biology is a multidisciplinary field that uses chemical “tools” or probes provided by synthetic chemistry to understand and manipulate biological systems[10,11]. These chemical tools are usually small molecules, which are defined as organic compounds with a molecular weight below 800 Daltons. This allows diffusion across the cell membrane and is an upper limit for oral bioavailability[12,13]. A significant example of the contribution of chemical biology to cell research is the generation of induced pluripotent stem cells (iPSCs) from differentiated adult cells[14]. This was originally achieved by overexpressing four “Yamanaka” transcription factors: Oct4, Sox2, c-Myc, and Klf4. Within just a few years, the protocol to produce iPSCs was optimized and simplified by chemical biologists. It was shown that iPSCs could be generated by expressing only the Oct4 transcription factor and a two-step combination of small molecule inhibitors of cell signaling pathways and gene regulatory mechanisms[13]. This is an important example of the ability of small molecules to substitute for transcription factors that have a global influence on genes regulating cell differentiation. Small molecules also possess significant advantages compared to other technologies for controlling cell phenotype, such as genetic methods. In the following section, we briefly discuss these advantages and provide examples of the application of small molecules to the derivation of cardiogenic cells for cell therapy.
Why use small molecules to control cardiogenesis?
Small molecules allow flexibility over the manipulation of the target protein[13,15]. This is not always possible with alternative approaches, such as genetic manipulation. In addition, the effects of small molecule treatment are usually reversible. This means that the target protein can be manipulated with relatively precise timing. An additional level of control can be achieved by fine-tuning the treatment concentration. Small molecules do not always modulate a single protein target in cells; they can produce multiple effects by binding to different protein classes. An example is the molecule BIX-01294, which inhibits different histone-modifying enzymes[16]. Thus, different small molecules can act synergistically to produce multiple effects, with the potential to produce dramatic changes in cell phenotype. The structural diversity of small molecule libraries developed exponentially in the 1990s, due to advances in chemical synthesis, such as combinatorial chemistry and diversity-orientated synthesis[17,18]. This greater diversity increases the potential to control molecular interactions with target proteins. Small molecules also provide logistical advantages for researchers. Compared to protein or nucleic acid reagents, they are cheap to produce, simple to store in the laboratory and more amenable to quality control. Small molecule-based methods do have some disadvantages, such as the potential for off-target effects on other proteins possessing similar structural elements. Notwithstanding, small molecules have become prominently used in stem cell biology and regenerative medicine, including the production of cardiomyocytes or cardiogenic stem cells[3,15,19]. Next, we discuss some prominent examples of small molecule-based strategies for cardiac cell therapy.
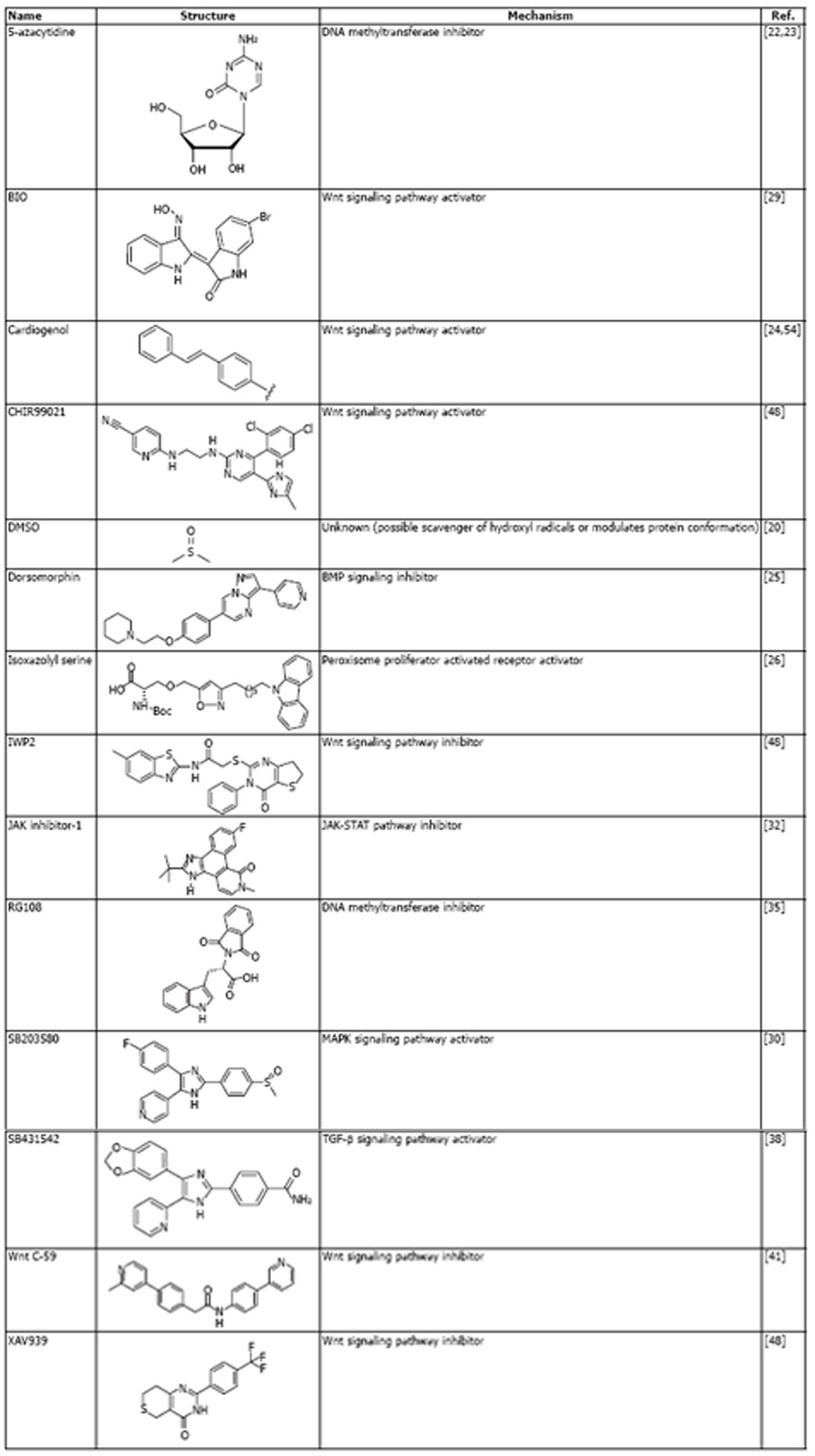
Due to the major health impact of cardiac disease, many small molecules have been developed to simplify the generation of cardiomyocytes or enhance the production of cardiogenic stem cells for potential cell therapy. A selection of these small molecules is shown in Figure 1. The use of small molecules to generate cardiomyocytes can be traced back to 1982, with the discovery that the small organic molecule, DMSO, could induce cardiomyocytes differentiation in murine teratocarcinoma-derived embryonic stem cells[20]. However, it was the development of cell therapy applications for cardiac diseases, such as MI in the 1990s[21] that spurred the discovery of bioactive compounds for enhancing cardiogenesis.
Figure 1 Selected small molecules that are used to regulate cardiogenesis (in alphabetical order).
BMP: Bone morphogenetic; MAPK: Mitogen-activated protein kinase; TGF-β: Transforming growth factor-β.
A significant advance came with the report that a small molecule inhibitor of DNA methylation, 5-azacytidine, could induce cardiomyocyte differentiation in murine bone marrow stromal cells[22]. DNA methylation is an epigenetic modification that regulates global gene expression patterns. Thus, it was demonstrated that a small molecule could modulate the epigenetic status of the cell genome to make it amenable to differentiation when exposed to a cardiogenic environment, such as transplantation into to heart or exposure to cardiomyocyte differentiation factors. A follow-up study showed that 5-azacytidine could also induce cardiomyocyte differentiation in embryonic stem cells (ESCs)[23]. Consequently, numerous studies focused on the application of small molecules to derive cardiomyocytes from ESCs. Prominent examples included the discovery of cardiogenol, which activates the Wnt cell signaling pathway and chromatin remodeling enzymes[24]; dorsomorphin, which inhibits the bone morphogenetic (BMP) signaling pathway[25]; and a series of isoxazoyl serines, which act as peroxisome activated proliferation receptor (PPAR) agonists[26] (Figure 1).
These small molecules are not only useful as tools to induce cardiomyocyte differentiation. Characterizing their biological activity also gives insights into the cellular mechanisms that regulate the differentiation process. For example, the pivotal role of the Wnt signaling pathway was confirmed in a screening study for small molecule inducers of cardiogenesis[27]. Cardiogenesis is initiated in ESCs by the formation of embryonic bodies that induce formation of the mesodermal lineage, from which cardiomyocytes are eventually derived[28]. Small molecule screening at 6 d after embryoid formation revealed that Wnt signaling inhibitors significantly enhance cardiomyocyte differentiation, confirming the important role of this signaling pathway in cardiogenesis. This finding also contrasts with the known importance of Wnt signaling for mesodermal induction at the earlier stage of cardiogenesis[28]. This was confirmed in a study which used the Wnt activating molecule, BIO (Figure 1), at the embryoid body stage to increase the number of beating cells after differentiation[29]. Another interesting finding was that inactivation of the mitogen-activated protein kinase (MAPK) signaling pathway by small molecule SB203580 (Figure 1) enhanced cardiomyocyte differentiation from ESCs[30]. SB203580 was treated to embryoid bodies at 24 h after formation, indicating the important role of the MAPK pathway in maintaining the undifferentiated cell state and inhibiting cardiogenesis.
The development of iPSCs (described above) also provided extra impetus to develop small molecule-based methods to induce cardiogenesis, because iPSCs circumvent the ethical and technical problems associated with using ESCs[31]. In addition, iPSCs can be derived from somatic cells, which offers an opportunity to derive cardiac cells from differentiated cells residing in non-cardiac tissues. An interesting example of a small molecule based approach to induce cardiac differentiation in iPSCs utilizes JAK inhibitor-1 (Figure 1), which blocks signaling by janus protein tyrosine kinase and repression of the JAK-STAT pathway (the major alternative second messenger system in cells)[32]. The usefulness of this approach is that it bypasses the need to induce fully reprogrammed iPSCs from somatic cells. Application of JAK-1 during the iPSC generation step blocked the acquisition of full “stemness” and produced a cell population that could be efficiently induced to form functional cardiomyocytes by culture in chemically defined cardiogenic media. Impressively, 100% of the treated cells underwent spontaneous contractions and the protocol was significantly faster than alternative methods, such as the forced expression of master cardiogenic transcription factors[33].
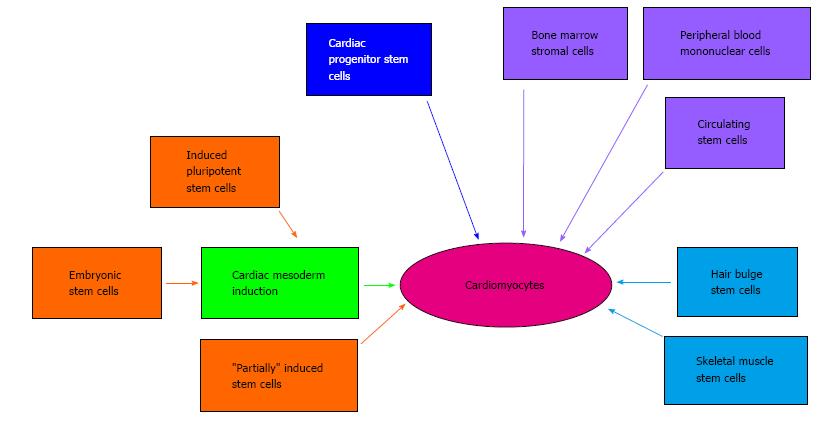
Pluripotent stem cells, such as ESCs and iPSCs, are not the only cell source that has been used for small molecule-induced cardiogenesis. Cells from a diverse range of tissues have been demonstrated to be amenable to cardiomyocyte differentiation (Figure 2). These tissue-specific stem and precursor cells possess different levels of potency, such as skeletal muscle stems (unipotent) or bone marrow stromal cells (multipotent)[34]. These approaches may be useful for patient-specific cell therapy, because tissues such as skeletal muscle and blood are readily assessable. As an example of this small molecule-based approach, the DNA methyltransferase inhibitor RG108 was successfully used to convert skeletal muscle stem cells into a pluripotent state. These cells could then be induced to form embryoid bodies and undergo cardiogenic differentiation[35]. Significantly, transplantation of these cells could improve cardiac performance and reduce scarring in animal models of MI. Interestingly, a stem cell population has also been found to reside at the base of hair follicles (bulge stem cells)[36] and treatment with the small molecule Wnt pathway activator, cardiogenol C, induced expression of cardiomyocyte markers in these cells[37]. Thus, there are multiple “paths” to generate cardiac cells from diverse tissue types, which are facilitated by small molecule treatments (Figure 2). Moreover, small molecule methods can significantly enhance genetic-based approaches for cardiogenesis in somatic cells. For example, compound SB431542 (Figure 1), which inhibits the transforming growth factor-β (TGF-β) can produce a five-fold increase in the direct reprogramming of fibroblasts into cardiomyocytes using master cardiomyocyte transcription factors[38].
Figure 2 Pathways of small molecule-mediated cardiomyocyte production.
It is now established that cardiomyocyte differentiation can be induced in multiple cells types using small molecule-based approaches. Embryonic stem cells and induced pluripotent stem cells are typically induced to undergo cardiac mesoderm differentiation before culture conditions are switched to cardiomyocyte differentiation media. “Partially” induced stem cells are somatic cells that were transfected with induced pluripotent stem cells reprogramming factors and then treated with the small molecule, JAK inhibitor-1 (as described in the text).
It can also be observed that, over time, these small molecule-based approaches are being optimized and simplified by the research community to allow easier derivation of cardiogenic cells. An important development in this regard is the development of chemically defined culture media for cardiogenic differentiation. This is important because serum should be eliminated from cell therapy applications. Serum supplies can suffer from batch variability and contain unspecified growth factors that may interfere with the differentiation process. Serum may also contain xenoantigens or infectious agents, which may induce an immune response in the host after transplantation[39]. To address this problem, a recently published study describes the development of a small molecule-based protocol to induce cardiomyocytes from bone marrow stem cells[40]. The development of these chemically-defined cardiogenic media cocktails are discussed in more detail below.
Latest progress in small molecule mediated cardiogenesis: Development of chemically defined induction media
A recently published study indicates the rapid progress that has been made for developing small molecule-based methods for cardiomyocyte differentiation. The study by Burridge et al[41] represents an impressively detailed investigation to define an optimized, chemically defined protocol for cardiomyocyte differentiation from human iPSCs. Their previous protocol for cardiomyocyte induction required the supplement B27, which is a complex mixture of 21 components. Some of these components are derived from animals, which necessitated the need to develop a chemically defined cardiogenic media. Interestingly, the iPSCs used in this study were also generated using a chemically defined methodology: human primary fibroblasts were transduced/transfected with the Yamanaka factors and cultured sequentially in the defined E8 and E7 culture media containing the small molecule histone deacetylase inhibitor, sodium butyrate. iPSC colonies could be selected after three weeks. The induction of cardiomyocyte differentiation was rigorously investigated using different combinations of small molecules and matrix scaffolds for cell attachment. Before the onset of cardiogenesis, cells were incubated with the small molecule thiazovivin, which is a selective inhibitor of Rho-associated coiled-coil containing protein kinase (ROCK) that has been shown to facilitate iPSC generation[13,42]. As mentioned above, modulation of the Wnt cell signaling pathway is a crucial aspect of the differentiation process, with positive signaling required for mesoderm specification and inhibition required for subsequent cardiomyocyte differentiation. A wide range of small molecule Wnt pathway modulators was tested to find the optimal combination for cardiac induction. Cardiomyocyte differentiation was monitored by measuring expression of the early cardiomyocyte marker gene, troponin T (TNNT)[43]. Interestingly, different small molecule Wnt pathway activators showed markedly diverse effects on cardiogenesis. Of six activators tested, only two [CHIR99021 and BIO (Figure 1)] facilitated the production of TNNT expressing cells without causing cell death. This finding emphasizes the need to compare small molecules that target identical pathways to eliminate the potential for significant off-target effects.
Further optimization of this cardiomyocyte differentiation protocol involved comparison of small molecule inhibitors of various signaling pathways (such as BMP or TGF-β) during the mesoderm induction step and Wnt signaling inhibition during the later cardiomyocyte differentiation step. Remarkably, these optimizations led to the development of a simplified induction protocol that could produce 95% TNNT positive contractile cardiomyocytes, with around 100 cells being derived from a single human iPSC. This protocol is based on the CDM3 media, which contains the chemically defined RPMI-1640 base media and is supplemented with just two reagents: l-ascorbic acid 2-phosphate and human recombinant albumin. iPSCs are cultured with CDM3 plus the Wnt pathway activator, CHIR99021 for two days, followed by two days incubation with CDM3 plus the small molecule Wnt pathway inhibitor, Wnt C-59 (Figure 1). Impressively, cardiomyocyte differentiation was observed just 96 h after this step, using additional incubation with CDM3 media alone. The cardiomyocyte phenotype was assessed by nanopillar-based microscopic observation and it was observed that the majority of cardiomyocytes (> 60%) were ventricular-like compared to atrial-like, with no nodal cardiomyocytes being observed. This observation is important, because new strategies to induce cardiomyocyte differentiation aim to produce specific, adult cardiomyocyte subtypes. The role of small molecules in these differentiation strategies are discussed in the next section.
New small molecule-based methods to induce epicardial cells for facilitating regeneration
As mentioned in the introduction for this editorial, cell therapy approaches for degenerative diseases require a high quality source of purified, functional cells for transplantation. This is also relevant for other applications, such as disease modeling, developmental studies and cell-based drug screening[44]. Early small molecule-based methods for inducing cardiogenesis, such as treatment with 5-azacytidine, were found to produce mixed populations of cells containing approximately 30% cardiomyocytes[22]. As described above, recent developments in producing chemically defined conditions for cardiogenesis from stem cells allows the derivation of cell populations containing up to 95% cardiomyocytes[41]. However, the heart comprises multiple cell types and cardiomyocytes account for only around 30% of total cells[45]. Therefore, for applications such as modeling cardiac development or cell therapy approaches for MI, it would be useful to generate these non-cardiomyocyte cell types. This is especially relevant for the epicardial cell type, because it is known that epicardial tissue plays a pivotal role in both cardiac development and cardiomyocyte regeneration after disease[46,47]. A recently published study demonstrates that it is indeed possible to derive epicardial cells from iPSCs, with small molecules being used as “control switches” to regulate cell differentiation potential[48].
Epicardial cells can be defined by their cobblestone morphology, specific gene marker expression [Wilms tumor protein (WT1) and T-box 18 (TBX18)] and the ability to synthesize retinoic acid[49-51]. In this study, human ESCs were induced to form embryoid bodies and undergo mesoderm formation by treatment with BMP4 alone for one day, followed by treatment with combined BMP, fibroblast growth factors (FGF) and activin A (a TGF pathway stimulator). Crucially, after 96 h the TGF pathway was inhibited with the small molecule SB431542 (Figure 2). This small molecule was removed after 48 h to facilitate the derivation of cardiomyocytes. In an attempt to block this cardiomyocyte differentiation and allow epicardial cells to be generated, the authors of this study focused on manipulating the Wnt signaling pathway after the addition of BMP. Such precise manipulation of Wnt signaling within this time window of differentiation could be achieved using small molecules. The effects of small molecule Wnt inhibitors (XAV939 and IWP2) were compared with a small molecule activator (CHIR99021). The effect of BMP-mediated signaling on differentiation was assessed using the small molecule inhibitor, dorsomorphin. The use of these small molecule combinations showed that BMP signaling has no effect on the canonical Wnt signaling pathway. Most significantly, it was observed that maintaining Wnt signaling using CHIR99021 allowed the derivation of epicardial lineage cells, as assessed by gene marker expression, morphological characteristics, differentiation into vascular or smooth muscle cells and the ability to synthesize retinoic acid[48]. The population of cells expressing the epicardial marker WT1 could be expanded to account for over 95% of the differentiated cell population, with one mesodermal lineage cell at day 4 of differentiation producing 4-5 epicardial cells. This study provides a high profile example of the advantages that small molecule methodologies offer to cell biologists. In this study, small molecules were used with precise timing to derive a valuable cardiac cell population which has been of great interest to investigators of heart development and regeneration after injury.
CONCLUSION
In this editorial, we have described some prominent examples of small molecule-based methods to facilitate the production of cardiac cells from diverse cell sources. We have shown that small molecules have numerous advantages as tools to control cell differentiation. They can be viewed as cheap, simple and reliable “switches” that provide rapid and reversible control of key cell signaling pathways. Our editorial has focused on the generation of cardiomyocytes from non-cardiac cells, but small molecules are also used in many other areas of cardiac regeneration research. Examples include improving the survival of cardiac tissue grafts, inducing cardiomyocyte dedifferentiation/proliferation and activating endogenous cardiac progenitor cells[3,52]. In addition, the impact of small molecule approaches is shown in the recent demonstration that iPSCs can be derived from somatic cells using just these chemicals alone, i.e., without the need for expressing reprogramming transcription factors[53]. This was achieved using a stepwise protocol requiring only seven small molecules: RepSox (a TGF signaling pathway inhibitor), PD0325901 (a MAPK pathway inhibitor), CHIR99021 (a Wnt pathway activator), TTNPB (a retinoic acid analog), 3 deazaneplanocin-A (which reduces histone methylation), valproic acid (which increases histone acetylation) and forskolin (which increases protein kinase A signaling). Remarkably, the efficiency of iPSC generation using this small molecule method was similar to that achieved using the reprogramming factors. It can be envisaged that this iPSC method could be joined with the chemically defined, small molecule method for cardiomyocyte differentiation, described above. Theoretically, this would allow the derivation of cardiac cells from almost any somatic cell source in the body.
Overall, we hope that this editorial has provided convincing evidence of the many advantages of using small molecules in biological research and cardiac regenerative medicine in particular. Chemical biologists continue to develop new bioactive small molecules or optimize the structures of existing compounds, to improve specificity and/or lower effective concentration. In concert with this research effort, cell biologists are also discovering new applications for known bioactive molecule or developing novel small molecule cocktails to manipulate cell behavior. Therefore, it seems likely that even more diverse and exciting progress is the field of cardiac regeneration can be achieved using small molecules.