Published online Feb 26, 2015. doi: 10.4330/wjc.v7.i2.104
Peer-review started: July 10, 2014
First decision: September 9, 2014
Revised: October 7, 2014
Accepted: November 17, 2014
Article in press: November 19, 2014
Published online: February 26, 2015
Processing time: 217 Days and 6 Hours
A 65-year-old man developed chest pain under cardiogenic shock. Coronary angiography revealed severe stenosis from the ostium of the left main coronary artery (LMCA) to the left anterior descending artery (LAD). Intravascular ultrasound (IVUS) identified a large hematoma that originated from the aorta and extended into the LAD, thereby compressing the true lumen. Type A aortic dissection (TAAD) that involved the LMCA was diagnosed by IVUS. Coronary stenting was performed via the LMCA to the proximal LAD, which resulted in coronary blood flow restoration and no further propagation of dissection. Elective surgical aortic repair was performed 2 wk after the stenting. LMCA stenting under IVUS guidance is effective for prompt diagnosis and precise stent deployment in patients with cardiogenic shock due to TAAD with LMCA dissection.
Core tip: Type A aortic dissection (TAAD) involving the left main coronary artery (LMCA) is a rare but potentially lethal condition. However, the precise diagnosis of TAAD prior to the treatment of acute myocardial infarction is difficult, and percutaneous intervention for LMCA obstruction secondary to TAAD is often complicated. This case report represents successful LMCA stenting under intravascular ultrasound (IVUS) guidance in a patient with cardiogenic shock due to TAAD with LMCA dissection. This procedure, particularly in terms of the use of IVUS, may be effective for rapid hemodynamic stabilization in patients in critical condition.
- Citation: Hanaki Y, Yumoto K, I S, Aoki H, Fukuzawa T, Watanabe T, Kato K. Coronary stenting with cardiogenic shock due to acute ascending aortic dissection. World J Cardiol 2015; 7(2): 104-110
- URL: https://www.wjgnet.com/1949-8462/full/v7/i2/104.htm
- DOI: https://dx.doi.org/10.4330/wjc.v7.i2.104
Acute myocardial infarction (AMI) concomitant with acute type A aortic dissection (TAAD) is associated with a high hospital mortality rate despite improvements in TAAD surgical outcomes[1-3]. In particular, TAAD involving the left main coronary artery (LMCA) is a rare but lethal condition associated with low output syndrome, which results from extensive myocardial necrosis regardless of whether an aortic repair surgery is successful. Early coronary revascularization should be performed to minimize cardiac dysfunction[3]. The treatment of dissected coronary arteries with stent implantation achieves prompt and adequate myocardial blood flow and helps prevent extensive myocardial damage. However, an accurate diagnosis of TAAD prior to treatment for AMI is difficult, particularly in patients with hemodynamic instability[4,5]. Furthermore, percutaneous coronary intervention for LMCA obstruction due to TAAD is a complicated procedure unless the mechanism of the LMCA obstruction has been clarified[6]. Here, we describe a case of successful coronary intervention under intravascular ultrasound (IVUS) guidance in a patient with shock due to an unusually localized TAAD with LMCA obstruction.
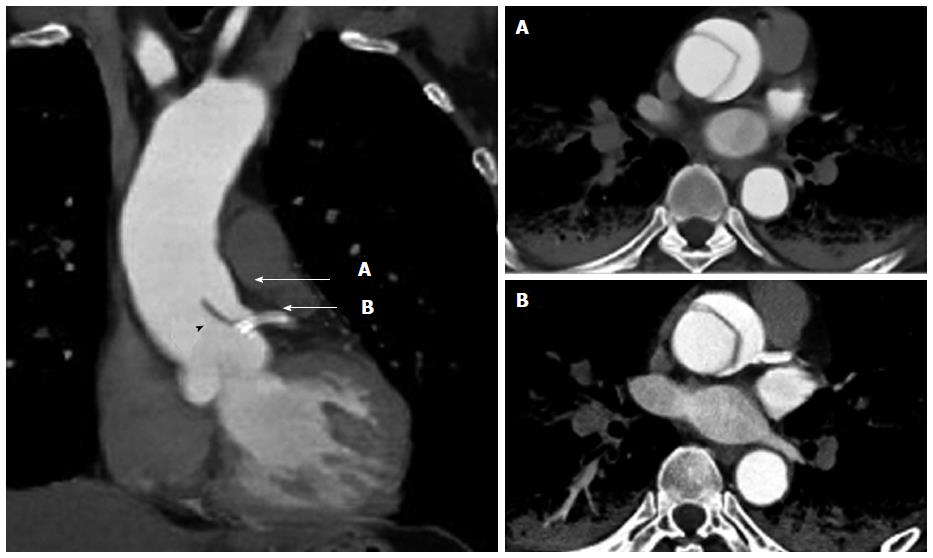
A 65-year-old man was admitted due to sudden-onset chest pain accompanied with cold sweats. The patient had previously undergone a drug-eluting stent implantation in the left anterior descending artery (LAD) 5 years earlier to treat stable angina. His hypertension and hyperlipidemia were well controlled with medication, and he had continued dual antiplatelet therapy (DAPT) since the stent implantation. The patient was transported by ambulance to our hospital within an hour of onset, and his status on arrival included an unmeasurable blood pressure below 60 mmHg and a heart rate of 50 beats/min. The initial electrocardiogram demonstrated bradycardia with an idioventricular rhythm, wide QRS complexes, and ST elevation in the lead aVR (Figure 1). Transthoracic echocardiography (TTE) revealed marked left ventricular dysfunction and a left ventricular ejection fraction of < 30% based on visual estimation. No aortic intimal flap, severe aortic regurgitation, or pericardial effusion was observed. The patient was immediately transferred to the cardiac catheterization laboratory because of ongoing myocardial ischemia and hemodynamic instability. An intra-aortic balloon pump was immediately inserted into the left femoral artery, and coronary angiography was performed from the right femoral artery without difficulty. The right coronary artery did not exhibit stenosis or collateral vessels. The left coronary artery exhibited severe stenosis from the ostium of the LMCA to the proximal LAD, which involved the ostium of the left circumflex artery (LCX) with TIMI grade 1 flow (Figure 2). Coronary intervention was subsequently performed. Run-through NS guidewire (Terumo, Tokyo, Japan) and SION guidewire (Asahi Intecc, Aichi, Japan) were inserted into the LAD and LCX, respectively. To confirm the wire position and evaluate the LMCA obstruction, IVUS (ViewIT, Terumo, Tokyo, Japan) was performed with quick pull-back. The IVUS revealed a large hematoma that originated from the aorta and extended into the LMCA and LAD (Figure 3A-C). The true lumen was compressed by the false lumen throughout the LAD immediately prior to the bifurcation of the diagonal branch (Figure 2D). A large intramural hematoma that involved the ostium of the LCX (Figure 3B) continued into the aortic wall in accordance with the double contour image in the coronary angiogram (Figure 3). We diagnosed an LMCA obstruction due to TAAD; thus, we decided to place a stent for immediate restoration of the coronary blood flow followed by aortic surgery. A 4.0- × 22.0-mm bare metal stent (Integrity; Medtronic, Minnesota, United States) was deployed via the LMCA through the LAD across the LCX ostium with satisfactory restoration of coronary blood flow. IVUS confirmed that the implanted stent appeared well-expanded and completely sealed the false lumen. No propagation of the false lumen into the distal LAD or the ostium of the LCX was observed (Figure 4). The patient’s hemodynamic failure and symptoms improved immediately after stenting. We removed the intra-aortic balloon pump immediately after hemodynamic stabilization because of the potential exacerbation of TAAD. Contrast-enhanced computed tomography (CT) following the coronary intervention revealed a localized retrograde dissection of the ascending aorta that extended to the ostium of the LMCA (Figure 5). The implanted stent protected the LMCA ostium from TAAD. Marked lung congestion was present. The following day, the patient’s maximum creatine kinase and creatine kinase-myocardial band levels were 16190 and 829 IU/L, respectively. The DAPT comprised aspirin (100 mg/d) and clopidogrel (75 mg/d). An elective ascending aortic repair surgery was performed pending improvement of congestive heart failure under continued DAPT 2 wk after coronary stenting. Ascending aortic replacement with an interposition vascular prosthesis graft was performed; coronary artery bypass grafting (CABG), which included the left internal thoracic artery (LITA) to the distal portion of the LAD and a saphenous vein graft (SVG) to the middle portion of the LCX, was performed at the surgeon’s discretion. Intraoperatively, an intimal tear in the ascending aorta with the false lumen that extended to the ostium of the LMCA was identified without an intimal rupture or aortic valvular destruction. Satisfactory restoration of the intracoronary stent for the left coronary artery and wide patency of the ostia of the left and right coronary arteries were visibly confirmed at the time of surgery. The intraoperative bleeding volume was 1230 mL, which necessitated a blood transfusion of 650 mL. The patient was discharged 51 d after admission. The physical status at the one-month follow-up visit was characterized as New York Heart Association class 1 despite a high concentration of brain natriuretic peptide (400 pg/mL: normal range < 20 pg/mL). The TEE demonstrated reduced antero-septal wall motion; however, the overall ejection fraction recovered to 50%. A follow-up coronary angiography 6 mo after discharge revealed that the left coronary artery maintained excellent blood flow without in-stent restenosis. The SVG was patent; however, the LITA revealed shrinkage as a non-functional bypass.
AMI with TAAD is associated with a high risk of extensive and irreversible myocardial damage and hemodynamic instability, which leads to high mortality regardless whether the surgical repair is successful[1,2]. TAAD that involves the LMCA is associated with a particularly high incidence of preoperative cardiopulmonary arrest and high operative mortality[3]. Postoperative low output syndrome due to extensive myocardial damage from AMI involving the LMCA is a major concern in surgical management. Although stent implantation in the treatment of dissected coronary arteries achieves immediate restoration of coronary blood flow and prevents extensive myocardial damage, the confirmation of the correct diagnosis and the performance of the optimal treatment procedure remain challenging[7-9] (Table 1).
| Ref. | Patient(age/sex) | Hemodynamic status | Timing ofdiagnosis | Diagnostic modality | Timing ofoperation | CABG | Outcome |
| Saxena et al[7] | 56 Male | Unstable | During PCI | Aortogram | < 24 h | - | Alive |
| Ohara et al[8] | 67 Male | Unstable | After PCI | CT | Not performed | - | Dead |
| Barabas et al[9] | 74 Male | Stable | During PCI | Aortogram | < 24 h | + | Alive |
| Ravandi et al[17] | 86 Male | Unstable | During PCI | Aortogram | Not performed | - | Uncertain |
| Imoto et al[18] | 71 Male | Unstable | Before PCI | CT | 3 d later | - | Alive |
| Cardozo et al[19] | 68 Male | Stable | During PCI | Aortogram | Not performed | - | Dead |
| Camero et al[20] | 52 Female | Unstable | After PCI | TEE | < 24 h | - | Alive |
| Present case | 65 Male | Unstable | During PCI | IVUS | 14 d later | + | Alive |
AMI with shock might be overlooked as an underlying factor of TAAD[4]. The diagnosis of TAAD with concomitant AMI is difficult because TAAD may be hidden in patients in critical condition. The present patient did not have back pain or a widened mediastinum on the chest X-ray obtained for suspected TAAD. Furthermore, the TTE revealed no evidence of localized TAAD. Although early revascularization should be strongly considered for patients with AMI secondary to cardiogenic shock[10], subsequent thrombolytic therapy and/or coronary intervention can be complicated in patients with underlying TAAD[5]. CT is recommended as the first line of investigation for patients with suspected TAAD. However, CT imaging is more time consuming for critical shock patients with an ST segment elevation myocardial infarction[11]. TTE is also useful; however, it is a limited screening technique for the quick diagnosis of TAAD because of the unavoidable operator dependency, reduced image resolution, and limited field of view[12]. The IVUS findings in the present case enabled the determination of the precise diagnosis of TAAD with typical findings of coronary artery compression[13,14].
Although coronary stenting is helpful in patients with TAAD, it remains challenging because of technical difficulties. The technical issues regarding coronary stenting include the navigation of the guidewire through the true lumen. IVUS imaging can be used to detect the orifice of the dissection, confirm the correct wire placement in the true lumen, and assist in the determination of the precise stent position, size, and length in a short time. Inappropriate ballooning or stenting that fails to completely seal the dissection might propagate the false lumen to the distal or proximal region. Repeated contrast injections should also be avoided because the extension of the dissection may result in the deterioration of the patient’s condition[15].
Imoto et al[3] reported that preoperative cardiopulmonary arrest and myocardial ischemia, particularly of the left coronary artery territory, negatively affected the survival outcomes in patients undergoing surgery for TAAD with coronary artery dissection. Early coronary intervention via stent implantation effectively prevents postoperative low cardiac output syndrome. Several bridge approaches to surgery have consequently been developed to facilitate early coronary intervention and reduce the extent of myocardial cell necrosis.
The timing of surgical repair for TAAD after coronary stenting is important. A delay in surgical repair may lead to the propagation and rupture of the aortic dissection. Prompt intervention can serve as a bridge approach to gain time for critically unstable patients prior to definitive surgery. In contrast, perioperative stent thrombosis is a serious complication that is associated with a significant increase in mortality, particularly in LMCA stenting. This complication is caused by antiplatelet therapy discontinuation and a surgery-induced prothrombotic situation. DAPT is necessary after coronary artery stenting, particularly in the acute phase. Hansson et al[16] studied the association of antiplatelet therapy with bleeding complications and mortality in patients undergoing operations for TAAD. The patients with ongoing platelet inhibition had significantly larger intraoperative and postoperative bleeding volumes; furthermore, the patients on DAPT had high 30-d mortality rates. In the present case, surgical aortic repair was performed after 2 wk while DAPT was continued. The duration of DAPT may be shortened, thus reducing bleeding during aortic surgery, by using a large bare metal stent with IVUS guidance to confirm the proper stent apposition. However, premature cessation of DAPT is likely to induce critical stent thrombosis. The addition of CABG is encouraging even after successful recanalization with stenting during the preparation for stent thrombosis after aortic surgery[3]. The optimal timing of surgical repair, the duration of DAPT and the efficacy of CABG addition after coronary artery stenting have not been established (Table 1).
LMCA stenting prior to the surgical repair of TAAD with LMCA dissection could be effective for an immediate improvement in hemodynamic instability. We emphasize the use of IVUS during the treatment of AMI because LMCA obstruction is necessary to exclude the presence of TAAD.
A 65-year-old male with a history of sudden-onset chest pain with hemodynamic instability.
The shock status, peripheral coldness and electrocardiogram indicated a severe myocardial infarction with hemodynamic instability.
Coronary artery disease with or without coronary atherosclerosis, for example coronary spasm, thrombosis, or Takotsubo cardiomyopathy.
The cardiac enzyme levels were extremely elevated after the catheter procedure, with the following results: WBC 21.6 k/uL; AST 1706 U/L; LDH 2945 U/L; CK 16190 U/L; CK-MB 829 U/L; and D-dimer 10.18 µg/mL.
The intravascular ultrasound (IVUS) findings during coronary intervention revealed a large hematoma that originated from the aorta and extended into the left main coronary artery (LMCA) and left anterior descending artery. An enhanced computed tomography scan revealed a localized retrograde dissection of the ascending aorta that extended to the ostium of the LMCA.
No specimen materials were collected.
The patient was treated with a percutaneous coronary intervention and surgical procedure.
Type A aortic dissection (TAAD) involving the LMCA is rare; however, there are a few case reports of the efficacy of LMCA stenting prior to surgical repair for immediate improvement in hemodynamic instability.
The use of IVUS during acute myocardial infarction secondary to LMCA obstruction is effective for not only achieving appropriate stenting but also excluding the presence of TAAD.
A well-written and interesting case report nicely outlining management strategy of acute myocardial infarction secondary to involvement of left main coronary artery by type A acute aortic dissection.
P- Reviewer: den Uil CA, Eftychiou C, Jankowski P, Raja SG, Rabkin SW S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Neri E, Toscano T, Papalia U, Frati G, Massetti M, Capannini G, Tucci E, Buklas D, Muzzi L, Oricchio L. Proximal aortic dissection with coronary malperfusion: presentation, management, and outcome. J Thorac Cardiovasc Surg. 2001;121:552-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 167] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 2. | Kawahito K, Adachi H, Murata S, Yamaguchi A, Ino T. Coronary malperfusion due to type A aortic dissection: mechanism and surgical management. Ann Thorac Surg. 2003;76:1471-1476; discussion 1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 105] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Imoto K, Uchida K, Karube N, Yasutsune T, Cho T, Kimura K, Masuda M, Morita S. Risk analysis and improvement of strategies in patients who have acute type A aortic dissection with coronary artery dissection. Eur J Cardiothorac Surg. 2013;44:419-424; discussion 424-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 4. | Lentini S, Perrotta S. Aortic dissection with concomitant acute myocardial infarction: From diagnosis to management. J Emerg Trauma Shock. 2011;4:273-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Hansen MS, Nogareda GJ, Hutchison SJ. Frequency of and inappropriate treatment of misdiagnosis of acute aortic dissection. Am J Cardiol. 2007;99:852-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 156] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 6. | Neri R, Migliorini A, Moschi G, Valenti R, Dovellini EV, Antoniucci D. Percutaneous reperfusion of left main coronary disease complicated by acute myocardial infarction. Catheter Cardiovasc Interv. 2002;56:31-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Saxena P, Boyle A, Shetty S, Edwards M. Left main coronary artery stenting prior to surgical repair of a type a aortic dissection. J Card Surg. 2011;26:634-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Ohara Y, Hiasa Y, Hosokawa S. Successful treatment in a case of acute aortic dissection complicated with acute myocardial infarction due to occlusion of the left main coronary artery. J Invasive Cardiol. 2003;15:660-662. [PubMed] |
| 9. | Barabas M, Gosselin G, Crépeau J, Petitclerc R, Cartier R, Théroux P. Left main stenting-as a bridge to surgery-for acute type A aortic dissection and anterior myocardial infarction. Catheter Cardiovasc Interv. 2000;51:74-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Hochman JS, Sleeper LA, Webb JG, Sanborn TA, White HD, Talley JD, Buller CE, Jacobs AK, Slater JN, Col J. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. N Engl J Med. 1999;341:625-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1988] [Cited by in RCA: 2039] [Article Influence: 78.4] [Reference Citation Analysis (0)] |
| 11. | Goran KP. Suggestion to list acute aortic dissection as a possible cause of type 2 myocardial infarction (according to the universal definition). Eur Heart J. 2008;29:2819-2820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Cecconi M, Chirillo F, Costantini C, Iacobone G, Lopez E, Zanoli R, Gili A, Moretti S, Manfrin M, Münch C. The role of transthoracic echocardiography in the diagnosis and management of acute type A aortic syndrome. Am Heart J. 2012;163:112-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Hibi K, Kimura K, Nakatogawa T, Okuda J, Umemura S, Yock PG. Images in cardiovascular medicine. Intracoronary ultrasound diagnosis of an aortic dissection causing anterior acute myocardial infarction. Circulation. 2003;108:e145-e146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Na SH, Youn TJ, Cho YS, Lim C, Chung WY, Chae IH, Choi DJ, Choh JH. Images in cardiovascular medicine. Acute myocardial infarction caused by extension of a proximal aortic dissection flap into the right coronary artery: an intracoronary ultrasound image. Circulation. 2006;113:e669-e671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Sakakura K, Wada H, Taniguchi Y, Mori M, Momomura S, Ako J. Intravascular ultrasound-guided coronary stenting without contrast medium for the treatment of catheter-induced aortocoronary dissection. Cardiovasc Interv Ther. 2013;28:71-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Hansson EC, Dellborg M, Lepore V, Jeppsson A. Prevalence, indications and appropriateness of antiplatelet therapy in patients operated for acute aortic dissection: associations with bleeding complications and mortality. Heart. 2013;99:116-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Ravandi A, Penny WF. Percutaneous intervention of an acute left main coronary occlusion due to dissection of the aortic root. JACC Cardiovasc Interv. 2011;4:713-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Imoto K, Uchida K, Suzuki S, Isoda S, Karube N, Kimura K. Stenting of a left main coronary artery dissection and stent-graft implantation for acute type a aortic dissection. J Endovasc Ther. 2005;12:258-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Cardozo C, Riadh R, Mazen M. Acute myocardial infarction due to left main compression aortic dissection treated by direct stenting. J Invasive Cardiol. 2004;16:89-91. [PubMed] |
| 20. | Camaro C, Wouters NT, Gin MT, Bosker HA. Acute myocardial infarction with cardiogenic shock in a patient with acute aortic dissection. Am J Emerg Med. 2009;27:899.e3-899.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |