Published online Oct 26, 2015. doi: 10.4330/wjc.v7.i10.621
Peer-review started: May 20, 2015
First decision: June 24, 2015
Revised: July 21, 2015
Accepted: August 13, 2015
Article in press: August 14, 2015
Published online: October 26, 2015
Processing time: 168 Days and 7.5 Hours
In remote ischemic conditioning (RIC), several cycles of ischemia and reperfusion render distant organ and tissues more resistant to the ischemia-reperfusion injury. The intermittent ischemia can be applied before the ischemic insult in the target site (remote ischemic preconditioning), during the ischemic insult (remote ischemic perconditioning) or at the onset of reperfusion (remote ischemic postconditioning). The mechanisms of RIC have not been completely defined yet; however, these mechanisms must be represented by the release of humoral mediators and/or the activation of a neural reflex. RIC has been discovered in the heart, and has been arising great enthusiasm in the cardiovascular field. Its efficacy has been evaluated in many clinical trials, which provided controversial results. Our incomplete comprehension of the mechanisms underlying the RIC could be impairing the design of clinical trials and the interpretation of their results. In the present review we summarize current knowledge about RIC pathophysiology and the data about its cardioprotective efficacy.
Core tip: Remote ischemic conditioning (RIC) is a safe, non-invasive, and inexpensive technique that has the potential to protect the heart against the ischemia-reperfusion injury. Its cardioprotective efficacy is currently being evaluated, and diverging results are emerging. It is thus worth resuming current understanding of RIC pathophysiology and clinical efficacy.
- Citation: Aimo A, Borrelli C, Giannoni A, Pastormerlo LE, Barison A, Mirizzi G, Emdin M, Passino C. Cardioprotection by remote ischemic conditioning: Mechanisms and clinical evidences. World J Cardiol 2015; 7(10): 621-632
- URL: https://www.wjgnet.com/1949-8462/full/v7/i10/621.htm
- DOI: https://dx.doi.org/10.4330/wjc.v7.i10.621
The myocardium can tolerate brief periods (up to 15 min) of severe and even total ischemia. Such ischemic episodes occur in the settings of angina, coronary vasospasm, and balloon angioplasty, and are not associated with concomitant myocyte cell death. With increasing duration and severity of ischemia, greater myocardial damage, and the predisposition to further damage during reperfusion develop. The combined deleterious effects of coronary occlusion and revascularization configure the “ischemia-reperfusion (IR) injury”[1].
The counterintuitive idea to apply several brief episodes of IR cycles to protect the myocardium against IR injury was firstly advanced in 1986, when Murry et al[2] reported that the infarcted area following a 40-min coronary occlusion was reduced if preceded by four 5-min IR cycles. This phenomenon was called “ischemic preconditioning”. Its clinical application is hindered by the unpredictable timing of acute myocardial infarction (AMI), and by the necessity to intervene on coronary vessels[3]. However, several IR cycles were found to confer cardioprotection even when applied at the onset of coronary revascularization (ischemic postconditioning)[4,5], both in animals and in human patients undergoing primary percutaneous coronary intervention (PCI).
In 1993, it was demonstrated in anesthetized dogs that 4 episodes of 5-min ischemia and reperfusion in the left circumflex coronary territory, followed by a 1-h occlusion of the left anterior descending coronary artery, significantly reduced the infarct size[6]. The “remote ischemic conditioning (RIC)” paradigm has been progressively extended[7]. At present, RIC is defined as the phenomenon by which brief episodes of ischemia and reperfusion in one vascular bed, tissue, or organ render distant sites resistant to the ischemia-reperfusion injury[7,8]. The IR cycles are effective when applied before myocardial ischemia (remote ischemic preconditioning), during coronary occlusion (remote ischemic perconditioning), and during cardiac revascularization (remote ischemic postconditioning)[7-12].
The mechanisms conferring protection at distance have not been completely defined[7], yet their characterization would be relevant to achieve a full comprehension of the phenomenon, and to exploit its full potential in clinical practice. In fact, understanding whether humoral mediators, neural mechanisms, or their combination mediate remote ischemic conditioning would be crucial to determine the optimal number of IR cycles, the better site and timing of their application, to select the patients according to age, comorbidities, and medical treatment, and to optimize the overall therapeutic management of the patient.
In the first part of the present review, we analyze current knowledge of the mechanisms underlying RIC, comparing humoral and reflex-mediated mechanisms. In the second half of this work, we attempt a critical analysis of the available literature concerning the cardioprotective potential of RIC in different settings.
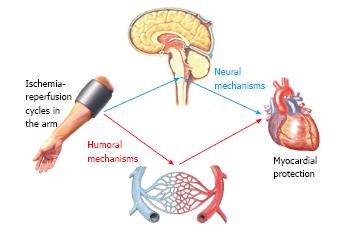
The “humoral hypothesis” has been formulated in the setting of remote ischemic preconditioning (RIPC). It postulates that the IR cycles in a distant site cause the local accumulation of mediators which are then released into the bloodstream and finally reach the heart[7] (Figure 1).
Several data from animal models support this hypothesis. In particular, it has been demonstrated that the effluent from preconditioned hearts could transfer the protection to naïve recipients[13-15]; this protection seems to be mediated by small hydrophobic proteins whose molecular weight ranges between 3.5 and 15-30 kDa[16].
Since these humoral mediators must be effective in remote sites after dilution into the bloodstream, their release from the peripheral tissue has to be massive[16]. The identification of humoral mediators should therefore be relatively easy to perform in animals or humans undergoing a RIPC protocol[16]. Indeed, proteomic approaches have been attempted in both animals and humans, still they have yielded controversial results[17-19].
Among the proteins potentially involved, there are kallistatin, apolipoprotein A-I, and stromal-derived factor 1α (SDF-1α)[16].
Kallistatin is a serine protease which reduces inflammation, apoptosis, and oxidative stress in endothelial cells[20]. It has been recently characterized as a protective factor against renal ischemia-reperfusion injury in mice[21], and has been found to be increased in the plasma of healthy humans undergoing a RIPC protocol[16]. However, its role as a humoral mediator of RIPC has not been properly evaluated yet.
Apolipoprotein A-I has anti-inflammatory properties, which could prove useful in the protection against ischemia-reperfusion injury[18]. In humans, its circulating levels have been found to be either increased[18] or decreased[17,22] after a RIPC protocol; therefore, its exact role is still debated.
Finally, SDF-1α has been proposed to be an important, and possibly the main, mediator of RIPC[23]. In a study on rats, a 50% plasma increment was detected in rats subjected to a RIPC protocol compared to control animals (890 ± 70 pg/mL vs 590 ± 50 pg/mL; n = 8, P < 0.01)[23]. Nevertheless, the administration of a selective inhibitor of SDF-1α did not completely abrogate the reduction in infarct size following RIPC[24], suggesting the existence of other mechanisms of cardioprotection[24].
Several potential other mediators have been identified: among them, there are microRNAs (miRNAs), bradykinin, adenosine, and nitric oxide (NO).
miRNAs have been involved in both muscle ischemia[25] and protection against myocardial IR injury[26]. The circulating levels of miR-144 have been found to increase by 1.6 folds in healthy human subjects undergoing a RIPC protocol, even though the exact mechanism of its action is still unknown[27].
Bradykinin is released by damaged tissues and can activate afferent fibers (see below), possibly contributing to a cardioprotective effect known as “remote preconditioning of trauma”[28]. A release of bradykinin from ischemic tissues into the bloodstream has been reported[29]; the involvement of bradykinin in the RIPC response has been postulated, but the data from an animal study were inconclusive[30].
Finally, both adenosine and NO have been extensively studied in the setting of ischemic preconditioning[31], and have been considered potential mediators of RIPC as well[7,32], although their extremely short half-life makes unlikely that they could exert a significant cardioprotective effect.
To summarize, it is quite established the existence of humoral mechanisms underlying RIPC, but the nature of the mediator(s) is currently unclear. Potential humoral mediators should be assessed with respect to their potential mechanism of action, the increase in their circulating levels following a conditioning protocol, and their half-life; in fact, all these parameters should be compatible with a cardioprotective role. Further studies are required to define the existence and the role of humoral mechanisms underlying RIPC, as well as the other forms of remote ischemic conditioning.
The autonomic nervous system consists of an afferent pathway, integrating centers located into the central nervous system, and two efferent limbs, the sympathetic and the parasympathetic nervous systems[33]. In the heart, sensory innervation is provided by afferent neurons located into the nodose and dorsal root ganglia, and projecting to brainstem areas controlling the activity of both sympathetic and parasympathetic nuclei[33]. Sympathetic efferent fibers innervate the sinoatrial and atrioventricular nodes, the atria, and the ventricles[33]. Parasympathetic efferent fibers are traditionally believed to control exclusively the nodal tissue and the atria[33]. Nevertheless, the presence of cholinergic innervations have been detected in both ventricles, and it has been demonstrated that vagal activation decreases the force of ventricular contraction irrespective of its effect on heart rate, in both animals and humans[33].
The “neural hypothesis” of remote ischemic preconditioning (RIPC) postulates that ischemia-reperfusion (IR) cycles in peripheral sites might activate a neural reflex resulting in myocardial protection against a subsequent myocardial insult[34] (Figure 1).
In animals, cycles of occlusion and reopening of the renal artery, mesenteric artery, or femoral artery resulted in significant cardioprotection; in all these cases, the resection of the afferent fibers projecting to the ischemized territories abolished the cardioprotective effect[35-37]. In rats, IR cycles in the mesenteric artery conferred a cardioprotection similar in entity to that provided by ischemic preconditioning (i.e., IR cycles of the coronary vessel before sustained occlusion); the systemic administration of hexametonium, a blocker of both sympathetic and parasympathetic ganglia, abolished this effect[38].
Conflicting results have been provided by Kingma et al[39], who reported that, in dogs anesthetized with isoflurane, a RIPC protocol conferred robust myocardial protection against a subsequent ischemic injury even during autonomic blockade or surgical denervation of the heart. It should be noted that in this study the animals were anesthetized with isoflurane[39], which is per se a powerful preconditioning agent (see below).
The activation of neural afferents during RIPC has been ascribed to local accumulation of mediators such as calcitonin-gene related peptide (CGRP), adenosine, and bradykinin[40]. Interestingly, the accumulation of adenosine[41,42], bradykinin[43], and other mediators[44,45] in the exercising muscle has been implied as a determinant of the metaboreflex[46], which is a neural mechanism coupling sympathetic tone to exercise requirements[47,48]. It could then be speculated that the IR cycles of a conditioning protocol cause metaboreflex activation. The subsequent increase in sympathetic outflow could confer myocardial protection through the activation of β1 and/or β2 adrenergic receptors; this phenomenon has been discovered in animal hearts perfused with β-agonists, and has been named “β-adrenergic preconditioning”[49-51].
As discussed above, a theoretical framework could be provided for sympathetic outflow as the final mediator of RIPC. Nevertheless, a growing body of evidences points to RIPC as a vagal reflex.
The possibility to precondition the heart by the infusion of acetylcholine (ACh) was demonstrated in 1993 by Yao et al[52] The preconditioning potential of ACh has been confirmed by several other studies[53-55]. It has been demonstrated that cardioprotection by RIPC is suppressed by spinal cord section, bilateral vagotomy or the systemic administration of the muscarinic receptor antagonist atropine, while vagal stimulation closely recapitulates the effects of RIPC[56,57].
Using a viral transfer gene approach in rats, Mastitskaya et al[58] confirmed that an intact parasympathetic outflow is crucial for myocardial protection by RIPC. The neurons in the dorsal motor nucleus of the vagus nerve were selectively silenced, thus abolishing the cardioprotective effect of a RIPC protocol[58]. The selective activation of the same neurons closely recapitulated the cardioprotective effect of RIPC; this response was suppressed by atropine[58]. Again in rats, Basalay et al[59] reported that IR cycles in the limb conferred cardioprotection when applied 25 min prior to myocardial ischemia. The authors then found that the cardioprotective effect was abolished by the denervation of the peripheral ischemic organ or bilateral vagotomy[59].
To our knowledge, only two studies have evaluated the consequences of a RIPC protocol on the autonomic function in humans. In 2005, Loukogeorgakis et al[60] evaluated the possibility to protect against endothelial ischemia-reperfusion injury by RIPC. A cuff inflation to 200 mmHg for 20 min in the non-dominant arm was used as the ischemic insult; the subsequent endothelial damage was denoted by reduced flow-mediated dilation (FMD)[60]. When arm ischemia was preceded by a RIPC protocol in the dominant arm, the ischemic insult in the other arm caused no significant reduction in FMD compared to baseline, suggesting endothelial protection by RIPC[60]. Such response was abolished by the autonomic ganglion blocker trimetaphan[60]. These results suggested an autonomic activation underlying the endothelial protection by RIPC; however, being trimetaphan an aspecific autonomic blocker, it was not possible to ascertain if either the sympathetic or the parasympathetic system accounted for the protection by RIPC[60].
Parasympathetic activation was detected as the underlying mechanism by Enko et al[61] in 2011. After 3 cycles of 5 min ischemia and 5 min reperfusion in the left arm, a significant dilation of the right brachial artery was observed; in the power spectral analysis of heart rate, the high frequency domain displayed a simultaneous increase, denoting increased parasympathetic outflow[61].
The first demonstration of remote ischemic perconditioning was provided in 2007: in pigs, four 5-min cycles of lower limb ischemia during a 40-min left anterior descending coronary artery occlusion caused a significant reduction in infarct size, improved indexes of systolic and diastolic function, and less arrhythmic events during the reperfusion phase[11]. With respect to remote ischemic postconditioning, a cardioprotective effect of IR cycles at the beginning of reperfusion was demonstrated for the first time in 2005[12], and subsequently corroborated by other animal studies[62,63].
To our knowledge, only Basalay et al[59] assessed the pathophysiology of remote ischemic perconditioning and postconditioning. These authors reported that deafferenting the site of IR cycles or cutting both vagus nerves abolished the preconditioning and perconditioning responses in rats, but did not alter the postconditioning effect[59]. These results suggest that remote ischemic perconditioning relies on neural mechanisms, while remote ischemic postconditioning is mediated by humoral mediators[59].
Further studies are required to assess this hypothesis. However, it should be noted that neural mechanisms are more qualified than humoral mechanisms to protect the ischemic myocardium in the setting of remote ischemic perconditioning, at least when the coronary flow is completely blocked. In the same setting, an activation of the parasympathetic system would probably be more effective than a sympathetic response.
Excessive concentrations of catecholamines have been detected in the ischemic area during an acute myocardial infarction[64]. Increased cardiac sympathetic outflow is due to pain, anxiety, and a fall of cardiac output or arterial blood pressure; a further release of catecholamines is promoted by the ischemic damage of nerve endings[64]. As a result, extracellular norepinephrine reaches up to 100-1000 times its normal plasma concentrations within 30 min of coronary occlusion[64]. Far from being protective, local concentrations of this magnitude are capable of producing myocardial necrosis even in nonischemic myocardium, and might promote malignant arrhythmias[64]. This mechanism accounts for the positive effects of early administration of β-blockers during acute myocardial infarction[65,66], and probably excludes increased sympathetic outflow as the final mediator of cardioprotection by remote ischemic perconditioning.
Stable coronary artery disease (SCAD) is associated with impaired quality of life, reduced physical endurance, recurrent hospitalizations and outpatient visits[67]. Revascularization by either elective PCI or CABG can relieve symptoms, reduce the use of anti-ischemic drugs, improve exercise capacity and quality of life, compared to medical therapy alone[67]. The efficacy of elective PCI in addition to medical therapy in patients with SCAD has been demonstrated in a large number of randomized controlled trials, meta-analyses, and large-scale registries[67].
Albeit elective PCI is becoming increasingly safe, balloon inflation during PCI often causes transient ischemia[68]. Myocardial injury with necrosis may derive from recognizable peri-procedural events such as coronary dissection, occlusion of a major coronary artery or a side-branch, disruption of collateral flow, slow flow or no-reflow, distal embolization, and microvascular plugging; alternatively, the ischemic insult can have no detectable cause[68]. Myocardial ischemia is attested by a rise and fall of cardiac biomarkers after the procedure, with values rising five or more folds over the 99th percentile being indicative of peri-procedural myocardial infarction (PMI)[68].
Four recent meta-analyses have demonstrated that RIPC is effective in reducing PMIs in patients undergoing elective PCI[69-72]. For example, in the meta-analysis by Zografos et al[71], PMI occurred in 40.3% of patients in the RIPC group and in 51.3% of patients in the control group (odds ratio 0.57).
Several trials have assessed the long-term outcomes after elective PCI. An improvement in prognosis was not found by Prasad et al[73] over 1 year follow-up. By contrast, in the Cardiac Remote Ischemic Preconditioning in Coronary Stenting study, a significant reduction of major adverse cardiac and cerebral events (MACCE; a composite of all-cause mortality, myocardial infarction, readmission for heart failure, and ischemic stroke or transient ischemic attack) was found at 6 mo[74]. A recent follow-up study evaluating the same cohort demonstrated that the MACCE rate at 6 years remained lower in the RIPC group[75].
The significant heterogeneity of the study protocols could be hindering a careful assessment of RIPC efficacy in the setting of elective PCI. For example, the studies assessed in the meta-analysis by Zografos et al[71] differed with regard to the RIPC procedure (number of IR cycles, duration of the IR periods, site of application of the IR cycles), the percentage of patients with multivessel disease, and the positivity or the negativity of cardiac troponin I (cTnI) before PCI[71]. By contrast, in all the studies evaluated in this meta-analysis the IR cycles were performed immediately before elective PCI[74-80], so that a different time span between the IR cycles and the angioplasty procedure cannot be regarded as a potential confounding factor.
On the whole, a protective role for RIPC in the setting of elective PCI is emerging, even though its efficacy seems to be lower than in primary PCI. Nevertheless, only one long-term follow-up study has been published so far[75]; the prognostic role of RIPC should therefore receive extensive evaluation, as well as the optimal RIPC protocol to achieve effective cardioprotection.
Remote ischemic perconditioning refers to the application of ischemia-reperfusion cycles in a distant site shortly before revascularization. The first evidences of a protective role of remote ischemic perconditioning in human patients was provided in 2010 by Bøtker et al[81], who assessed 333 patients with suspected first ST-elevation myocardial infarction. The primary endpoint was myocardial salvage index (MSI), quantified as the proportion of the area at risk preserved by the treatment, 30 d after primary PCI. MSI was significant higher in the conditioning group than in controls; the protective effect of remote ischemic perconditioning seemed to be strongest in patients with more severe infarctions, i.e., presenting with occluded vessels or infarcts in the left anterior descending artery[81].
The long-term outcome of remote ischemic perconditioning was assessed in the same study population[82]. A significant reduction of MACCE and all cause mortality was observed in the conditioning group over a median follow-up of 3.8 years. There was also a trend toward reduced myocardial reinfarction, readmission for heart failure, and ischemic stroke or transient ischemic attack[82].
In 2013, remote ischemic postconditioning was assessed on 232 patients undergoing elective PCI[83]. In the conditioning group, the patients underwent three 5-min cycles of cuff inflation in the nondominant arm just after the end of the angioplasty. No significant difference was found between the conditioning group and the control group in terms of peak troponin I levels, PMI rate, recurrence of myocardial ischemia[83]. In another study, the incidence of PMIs was similar between all groups, and no difference was remarked with respect to the creatine kinase (CK) levels or the incidence of acute kidney failure[84]. In other studies, significant reductions in the incidence of acute kidney failure were observed; the prevention of acute kidney failure is currently regarded as the most promising perspective for the application of remote ischemic postconditioning during PCI[85,86].
For the details of the studies cited in the present paragraph, see Table 1.
| Ref. | Patients n (CTRLS/RIPC) | ST or LT outcome | Conditioning protocol | Primary endpoint | Results | ||||
| I/R cycles | Cuff pressure | Limb | RIPC | CTRLS | P | ||||
| Remote ischemic preconditioning | |||||||||
| Prasad et al[73], 2013 | 48/47 | ST | 3 × 3’ | 200 mmHg | Upper | Post-PCI myonecrosis (cTnT ≥ 0.03 ng/dL) | 40% | 47% | 0.42 |
| Ahmed et al[76], 2013 | 72/77 | ST | 3 × 5’ | 200 mmHg | Upper | cTnT 16 h post-PCI | 0.02 ng/mL | 0.047 ng/mL | 0.047 |
| Ghaemian et al[77], 2010 | 40/40 | ST | 2 × 5’ | Above systolic | Lower | TnT 24 h post-PCI | 12.50% | 40% | 0.01 |
| Hoole et al[74], 2009 | 117/125 | ST | 3 × 5’ | 200 mmHg | Upper | cTnI 24 h post-PCI | 0.06 ng/mL | 0.16 ng/mL | 0.04 |
| Luo e et al[78], 2013 | 104/101 | ST | 3 × 5’ | 200 mmHg | Upper | hs-cTnI 16 h post-PCI | 0.11 ng/mL | 0.21 ng/mL | < 0.01 |
| Xu et al[79], 2014 | 98/102 | ST | 3 × 5’ | 200 mmHg | Upper | hs-cTnI 16 h post-PCI | 0.29 ng/mL | 0.38 ng/mL | 0.256 |
| Davies et al[75], 2013 | 117/125 | LT | 3 × 5’ | 200 mmHg | Upper | MACCE (6 yr follow up) | 23 | 36 | 0.039 |
| Bøtker et al[81], 2010 | 167/166 | ST | 4 × 5’ | 200 mmHg | Upper | MSI after 30 d | 0.75 | 0.55 | 0.033 |
| Sloth et al[82], 2014 | 167/166 | LT | 4 × 5’ | 200 mmHg | Upper | MACCE rates (5 yr follow-up) | 25.60% | 13.50% | 0.018 |
| Carrasco-Chinchilla et al[83], 2013 | 114/118 | ST | 3 × 5’ | 200 mmHg | Upper | TnI 24 h post-PCI | 0.476 ng/mL | 0.478 ng/mL | 0.378 |
Elective coronary artery bypass surgery (CABG) stands as an alternative to elective PCI for the management of SCAD[87]. The safety and efficacy of both techniques are similar, as well as the incidence of PMIs[87].
In 2007, a randomized controlled study enrolled 57 adult patients undergoing elective CABG surgery[88]. In the RIPC group, three IR cycles were performed after the induction of anesthesia, resulting in a 43% reduction in the 72 h area under the curve (AUC) of cTnT compared with the control group[88]. Other randomized trials confirmed a cardioprotective role of RIPC, in terms of reduced cTnT[89], cTnI[90], and CK isoenzyme MB[91] levels. By contrast, several studies failed to detect significant differences among the RIPC group and the control group[92-95]; the use of volatile anesthetics with preconditioning potential (isoflurane, enflurane, sevoflurane) possibly accounts for discrepant results[96-98].
In a meta-analysis, D’Ascenzo et al[99] reported a significant reduction in cTnI and cTnT levels in the RIPC group after elective CABG surgery. Such difference persisted after excluding the trials with potentially confounding factors (among them, the use of isoflurane)[99]. It has been suggested that the cardioprotective effect of RIPC could be masked by the administration of volatile anesthetics and blunted by the perioperative administration of β-blockers[99,100]. Indeed, previous studies on animals or isolated human atrial trabeculae had demonstrated that β-blockers could attenuate ischemic preconditioning-induced cardioprotection[100,101], perhaps since even the activation of β-adrenergic receptor is protective against ischemia-reperfusion injury (β-adrenergic preconditioning; see paragraph The “neural hypothesis” of RIPC, and potential role of the sympathetic system).
Two studies evaluating the long-term efficacy of RIPC provided diverging results. Lucchinetti et al[94] did not find any difference at 6 mo in terms of deaths or revascularizations, whereas Thielmann et al[102] found significantly lower mortality rates in the RIPC group than in controls over a mean 1.54 year follow-up.
With respect to the settings of elective valve replacement surgery and congenital cardiac surgery, a recent metanalysis detected a significant cardioprotective role for RIPC[103]. Nevertheless, the small number of studies, and the high heterogeneity among them[103] might undermine the reliability of these conclusions. Another recent meta-analysis considered cumulatively CABG, valve replacement surgery, and congenital cardiac surgery, and detected a significant reduction in the post-operative cTnI levels among the patients undergoing RIPC[104]. No subgroup analysis was performed, and the heterogeneity among studies assessing non-CABG surgery was marked[104]. A third meta-analysis took into consideration the studies evaluating RIPC efficacy in adult patients undergoing “major elective or emergency cardiac or vascular surgery”[105]. In such a broad and mixed setting, no significant efficacy of RIPC was detected with regard to several outcomes: perioperative death, myocardial infarction, new-onset cardiac arrhythmias requiring treatment, cerebrovascular accidents, renal failure requiring renal replacement therapy, mesenteric ischemia, length of hospital stay and intensive care unit stay[105].
The few studies evaluating the effects of RIPC in the sole setting of valve replacement yielded conflicting results. For example, Wu et al[106] did not find a significant effect of a standard RIPC protocol on cTnI release after mitral valve replacement surgery, whereas Xie et al[107] reported a significant reduction of the 72 h cTnI-AUC in patients undergoing mitral valve, aortic valve or tricuspid valve surgery.
On the whole, RIPC seems to exert a protective role against PMIs caused by elective CABG surgery, while its long term effects are still uncertain. Furthermore, no definite statement can be made about the RIPC efficacy in other forms of elective cardiac surgery, namely valve replacement surgery and congenital cardiac surgery (Table 2). Finally, volatile anesthetics and β-blockers are emerging as potential confounding factors, although the mechanisms are still unclear.
| Ref. | Patients n (CTRLS/RIPC) | RIPC protocol | Primaryendpoint | Results | Notes | |||||
| I/R cycles | Cuff pressure | Limb | RIPC | CTRLS | P | β-blockers | Isoflurane, desflurane | |||
| Short terms results | ||||||||||
| Hausenloy et al[88], 2007 | 30/27 | 3 × 5 min | 200 mmHg | Upper | cTnT at | + | + | |||
| 6 h | 0.31 mcg/L | 0.59 mcg/L | 0.039 | |||||||
| 12 h | 0.37mcg/L | 0.69 mcg/L | 0.002 | |||||||
| 24 h | 0.30 mcg/L | 0.52 mcg/L | 0.003 | |||||||
| 48 h | 0.30 mcg/L | 0.52 mcg/L | 0.036 | |||||||
| 72 h | 0.25 mcg/L | 0.48 mcg/L | 0.111 | |||||||
| Wagner et al[90], 2010 | 35/33 | 3 × 5 min | 40 mmHg above systolic pressure | Upper | cTnI at 8 h | 2.54 mcg/L | 2.90 mcg/L | 0.043 | - | - |
| Ali et al[91], 2010 | 50/50 | 3 × 5 min | 200 mmHg | Upper | CK-MB at | |||||
| 8 h | 27.22 IU/L | 30.24 IU/L | 0.026 | + | NS | |||||
| 16 h | 33.3 IU/L | 37.2 IU/L | 0.021 | |||||||
| 24 h | 22.74 IU/L | 25.22 IU/L | 0.052 | |||||||
| 48 h | 17.20 IU/L | 19.72 IU/L | 0.003 | |||||||
| Lomivorotov et al[93], 2012 | 40/40 | 3 × 5 min | 200 mmHg | Upper | 48 h cTnI AUC | 54.4 ng/mL | 53.3 ng/mL | > 0.05 | + | + |
| Thielmann et al[102], 2013 | 167/162 | 3 × 5 min | 200 mmHg | Upper | 72 h cTnI AUC | 266 ng/mL | 321 ng/mL | 0.022 | + | + |
| Rahman et al[95], 2010 | 55 | 3 × 5 min | 200 mmHg | Upper | 48 h cTnT AUC | 30 ng/mL | 28 ng/mL | 0.721 | + | + |
| Lucchinetti et al[94], 2012 | 28/27 | 4 × 5 min | 400 mmHg | Lower | 72 h hs-cTnT AUC | 11708 pg/mL | 9574 pg/mL | 0.33 | + | + |
| Long term results | ||||||||||
| Thielmann et al[102], 2013 | 167/162 | 3 × 5 min | 200 mmHg | Upper | ACE at 1.54 yr death MACCE | 3 8 | 11 23 | 0.046 0.005 | + | + |
| Lucchinetti et al[94], 2012 | 28/27 | 4 × 5 min | 400 mmHg | Lower | ACE at 6 mo death rehospitalization | 0 3 | 1 3 | 1.00 1.00 | + | + |
Remote ischemic conditioning (RIC) has been proposed as a “non-invasive, simple, safe, and cheap”[108] strategy to protect the heart against ischemic insults. A great research effort has been performed in order to verify the existence of a myocardial protection by RIC, and to evaluate the extent of such protection. Nevertheless, clinical studies have provided conflicting results. A deeper comprehension of the mechanisms underlying RIC is advisable in order to correctly assess the cardioprotective potential of RIC, and to guide future clinical research.
P- Reviewer: Li YY, Ong HT S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Verma S, Fedak PW, Weisel RD, Butany J, Rao V, Maitland A, Li RK, Dhillon B, Yau TM. Fundamentals of reperfusion injury for the clinical cardiologist. Circulation. 2002;105:2332-2336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 306] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 2. | Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5406] [Cited by in RCA: 5542] [Article Influence: 142.1] [Reference Citation Analysis (0)] |
| 3. | Ovize M, Thibault H, Przyklenk K. Myocardial conditioning: opportunities for clinical translation. Circ Res. 2013;113:439-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 4. | Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285:H579-H588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1396] [Cited by in RCA: 1468] [Article Influence: 66.7] [Reference Citation Analysis (0)] |
| 5. | Touboul C, Angoulvant D, Mewton N, Ivanes F, Muntean D, Prunier F, Ovize M, Bejan-Angoulvant T. Ischaemic postconditioning reduces infarct size: systematic review and meta-analysis of randomized controlled trials. Arch Cardiovasc Dis. 2015;108:39-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Przyklenk K, Bauer B, Ovize M, Kloner RA, Whittaker P. Regional ischemic ‘preconditioning’ protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation. 1993;87:893-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 973] [Cited by in RCA: 1003] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 7. | Heusch G, Bøtker HE, Przyklenk K, Redington A, Yellon D. Remote ischemic conditioning. J Am Coll Cardiol. 2015;65:177-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 507] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 8. | Lim SY, Hausenloy DJ. Remote ischemic conditioning: from bench to bedside. Front Physiol. 2012;3:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 128] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 9. | Kharbanda RK, Mortensen UM, White PA, Kristiansen SB, Schmidt MR, Hoschtitzky JA, Vogel M, Sorensen K, Redington AN, MacAllister R. Transient limb ischemia induces remote ischemic preconditioning in vivo. Circulation. 2002;106:2881-2883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 524] [Cited by in RCA: 543] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 10. | Schmidt MR, Smerup M, Konstantinov IE, Shimizu M, Li J, Cheung M, White PA, Kristiansen SB, Sorensen K, Dzavik V. Intermittent peripheral tissue ischemia during coronary ischemia reduces myocardial infarction through a KATP-dependent mechanism: first demonstration of remote ischemic perconditioning. Am J Physiol Heart Circ Physiol. 2007;292:H1883-H1890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 207] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 11. | Kerendi F, Kin H, Halkos ME, Jiang R, Zatta AJ, Zhao ZQ, Guyton RA, Vinten-Johansen J. Remote postconditioning. Brief renal ischemia and reperfusion applied before coronary artery reperfusion reduces myocardial infarct size via endogenous activation of adenosine receptors. Basic Res Cardiol. 2005;100:404-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 209] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 12. | Vinten-Johansen J, Shi W. Perconditioning and postconditioning: current knowledge, knowledge gaps, barriers to adoption, and future directions. J Cardiovasc Pharmacol Ther. 2011;16:260-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 13. | Breivik L, Helgeland E, Aarnes EK, Mrdalj J, Jonassen AK. Remote postconditioning by humoral factors in effluent from ischemic preconditioned rat hearts is mediated via PI3K/Akt-dependent cell-survival signaling at reperfusion. Basic Res Cardiol. 2011;106:135-145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 14. | Dickson EW, Lorbar M, Porcaro WA, Fenton RA, Reinhardt CP, Gysembergh A, Przyklenk K. Rabbit heart can be “preconditioned” via transfer of coronary effluent. Am J Physiol. 1999;277:H2451-H2457. [PubMed] |
| 15. | Serejo FC, Rodrigues LF, da Silva Tavares KC, de Carvalho AC, Nascimento JH. Cardioprotective properties of humoral factors released from rat hearts subject to ischemic preconditioning. J Cardiovasc Pharmacol. 2007;49:214-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 76] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Helgeland E, Breivik LE, Vaudel M, Svendsen ØS, Garberg H, Nordrehaug JE, Berven FS, Jonassen AK. Exploring the human plasma proteome for humoral mediators of remote ischemic preconditioning--a word of caution. PLoS One. 2014;9:e109279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Hepponstall M, Ignjatovic V, Binos S, Monagle P, Jones B, Cheung MH, d’Udekem Y, Konstantinov IE. Remote ischemic preconditioning (RIPC) modifies plasma proteome in humans. PLoS One. 2012;7:e48284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 18. | Hibert P, Prunier-Mirebeau D, Beseme O, Chwastyniak M, Tamareille S, Lamon D, Furber A, Pinet F, Prunier F. Apolipoprotein a-I is a potential mediator of remote ischemic preconditioning. PLoS One. 2013;8:e77211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Hibert P, Prunier-Mirebeau D, Beseme O, Chwastyniak M, Tamareille S, Pinet F, Prunier F. Modifications in rat plasma proteome after remote ischemic preconditioning (RIPC) stimulus: identification by a SELDI-TOF-MS approach. PLoS One. 2014;9:e85669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Gao L, Li P, Zhang J, Hagiwara M, Shen B, Bledsoe G, Chang E, Chao L, Chao J. Novel role of kallistatin in vascular repair by promoting mobility, viability, and function of endothelial progenitor cells. J Am Heart Assoc. 2014;3:e001194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Zhou S, Sun Y, Zhuang Y, Zhao W, Chen Y, Jiang B, Guo C, Zhang Z, Peng H, Chen Y. Effects of kallistatin on oxidative stress and inflammation on renal ischemia-reperfusion injury in mice. Curr Vasc Pharmacol. 2015;13:265-273. [PubMed] |
| 22. | Pang T, Zhao Y, Zhang NR, Jin SQ, Pan SQ. Transient limb ischemia alters serum protein expression in healthy volunteers: complement C3 and vitronectin may be involved in organ protection induced by remote ischemic preconditioning. Oxid Med Cell Longev. 2013;2013:859056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Davidson SM, Selvaraj P, He D, Boi-Doku C, Yellon RL, Vicencio JM, Yellon DM. Remote ischaemic preconditioning involves signalling through the SDF-1α/CXCR4 signalling axis. Basic Res Cardiol. 2013;108:377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 24. | Przyklenk K. ‘Going out on a limb’: SDF-1α/CXCR4 signaling as a mechanism of remote ischemic preconditioning? Basic Res Cardiol. 2013;108:382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Yang JC, Wu SC, Rau CS, Chen YC, Lu TH, Wu YC, Tzeng SL, Wu CJ, Hsieh CH. TLR4/NF-κB-responsive microRNAs and their potential target genes: a mouse model of skeletal muscle ischemia-reperfusion injury. Biomed Res Int. 2015;2015:410721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Kukreja RC, Yin C, Salloum FN. MicroRNAs: new players in cardiac injury and protection. Mol Pharmacol. 2011;80:558-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 106] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 27. | Li J, Rohailla S, Gelber N, Rutka J, Sabah N, Gladstone RA, Wei C, Hu P, Kharbanda RK, Redington AN. MicroRNA-144 is a circulating effector of remote ischemic preconditioning. Basic Res Cardiol. 2014;109:423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 188] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 28. | Gross GJ, Baker JE, Moore J, Falck JR, Nithipatikom K. Abdominal surgical incision induces remote preconditioning of trauma (RPCT) via activation of bradykinin receptors (BK2R) and the cytochrome P450 epoxygenase pathway in canine hearts. Cardiovasc Drugs Ther. 2011;25:517-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Baumgarten CR, Linz W, Kunkel G, Schölkens BA, Wiemer G. Ramiprilat increases bradykinin outflow from isolated hearts of rat. Br J Pharmacol. 1993;108:293-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 108] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Goto M, Liu Y, Yang XM, Ardell JL, Cohen MV, Downey JM. Role of bradykinin in protection of ischemic preconditioning in rabbit hearts. Circ Res. 1995;77:611-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 332] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 31. | Iliodromitis EK, Andreadou I, Iliodromitis K, Dagres N. Ischemic and postischemic conditioning of the myocardium in clinical practice: challenges, expectations and obstacles. Cardiology. 2014;129:117-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Küntscher MV, Kastell T, Altmann J, Menke H, Gebhard MM, Germann G. Acute remote ischemic preconditioning II: the role of nitric oxide. Microsurgery. 2002;22:227-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Gourine A, Gourine AV. Neural mechanisms of cardioprotection. Physiology (Bethesda). 2014;29:133-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 34. | Gill R, Kuriakose R, Gertz ZM, Salloum FN, Xi L, Kukreja RC. Remote ischemic preconditioning for myocardial protection: update on mechanisms and clinical relevance. Mol Cell Biochem. 2015;402:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 35. | Ding YF, Zhang MM, He RR. Role of renal nerve in cardioprotection provided by renal ischemic preconditioning in anesthetized rabbits. ShenglLixue Bao. 2001;53:7-12. [PubMed] |
| 36. | Liem DA, Verdouw PD, Ploeg H, Kazim S, Duncker DJ. Sites of action of adenosine in interorgan preconditioning of the heart. Am J Physiol Heart Circ Physiol. 2002;283:H29-H37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 136] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 37. | Dong JH, Liu YX, Ji ES, He RR. [Limb ischemic preconditioning reduces infarct size following myocardial ischemia-reperfusion in rats]. Sheng Li Xue Bao. 2004;56:41-46. [PubMed] |
| 38. | Gho BC, Schoemaker RG, van den Doel MA, Duncker DJ, Verdouw PD. Myocardial protection by brief ischemia in noncardiac tissue. Circulation. 1996;94:2193-2200. [PubMed] |
| 39. | Kingma JG, Simard D, Voisine P, Rouleau JR. Role of the autonomic nervous system in cardioprotection by remote preconditioning in isoflurane-anaesthetized dogs. Cardiovasc Res. 2011;89:384-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 40. | Tang ZL, Dai W, Li YJ, Deng HW. Involvement of capsaicin-sensitive sensory nerves in early and delayed cardioprotection induced by a brief ischaemia of the small intestine. Naunyn Schmiedebergs Arch Pharmacol. 1999;359:243-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 41. | Costa F, Biaggioni I. Role of adenosine in the sympathetic activation produced by isometric exercise in humans. J Clin Invest. 1994;93:1654-1660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 42. | Notarius CF, Atchison DJ, Rongen GA, Floras JS. Effect of adenosine receptor blockade with caffeine on sympathetic response to handgrip exercise in heart failure. Am J Physiol Heart Circ Physiol. 2001;281:H1312-H1318. [PubMed] |
| 43. | Scott AC, Wensel R, Davos CH, Kemp M, Kaczmarek A, Hooper J, Coats AJ, Piepoli MF. Chemical mediators of the muscle ergoreflex in chronic heart failure: a putative role for prostaglandins in reflex ventilatory control. Circulation. 2002;106:214-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 44. | Scott AC, Wensel R, Davos CH, Georgiadou P, Kemp M, Hooper J, Coats AJ, Piepoli MF. Skeletal muscle reflex in heart failure patients: role of hydrogen. Circulation. 2003;107:300-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 45. | Rotto DM, Kaufman MP. Effect of metabolic products of muscular contraction on discharge of group III and IV afferents. J Appl Physiol (1985). 1988;64:2306-2313. [PubMed] |
| 46. | Nobrega AC, O’Leary D, Silva BM, Marongiu E, Piepoli MF, Crisafulli A. Neural regulation of cardiovascular response to exercise: role of central command and peripheral afferents. Biomed Res Int. 2014;2014:478965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 134] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 47. | Coats AJ, Clark AL, Piepoli M, Volterrani M, Poole-Wilson PA. Symptoms and quality of life in heart failure: the muscle hypothesis. Br Heart J. 1994;72:S36-S39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 226] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 48. | Piepoli M, Clark AL, Volterrani M, Adamopoulos S, Sleight P, Coats AJ. Contribution of muscle afferents to the hemodynamic, autonomic, and ventilatory responses to exercise in patients with chronic heart failure: effects of physical training. Circulation. 1996;93:940-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 372] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 49. | Salie R, Moolman JA, Lochner A. The mechanism of beta-adrenergic preconditioning: roles for adenosine and ROS during triggering and mediation. Basic Res Cardiol. 2012;107:281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 50. | Robinet A, Hoizey G, Millart H. PI 3-kinase, protein kinase C, and protein kinase A are involved in the trigger phase of beta1-adrenergic preconditioning. Cardiovasc Res. 2005;66:530-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 51. | Bhushan S, Kondo K, Predmore BL, Zlatopolsky M, King AL, Pearce C, Huang H, Tao YX, Condit ME, Lefer DJ. Selective β2-adrenoreceptor stimulation attenuates myocardial cell death and preserves cardiac function after ischemia-reperfusion injury. Arterioscler Thromb Vasc Biol. 2012;32:1865-1874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 52. | Yao Z, Gross GJ. Acetylcholine mimics ischemic preconditioning via a glibenclamide-sensitive mechanism in dogs. Am J Physiol. 1993;264:H2221-H2225. [PubMed] |
| 53. | Richard V, Blanc T, Kaeffer N, Tron C, Thuillez C. Myocardial and coronary endothelial protective effects of acetylcholine after myocardial ischaemia and reperfusion in rats: role of nitric oxide. Br J Pharmacol. 1995;115:1532-1538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 54. | Qian YZ, Levasseur JE, Yoshida K, Kukreja RC. KATP channels in rat heart: blockade of ischemic and acetylcholine-mediated preconditioning by glibenclamide. Am J Physiol. 1996;271:H23-H28. [PubMed] |
| 55. | Yamaguchi F, Nasa Y, Yabe K, Ohba S, Hashizume Y, Ohaku H, Furuhama K, Takeo S. Activation of cardiac muscarinic receptor and ischemic preconditioning effects in in situ rat heart. Heart Vessels. 1997;12:74-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 56. | Katare RG, Ando M, Kakinuma Y, Arikawa M, Handa T, Yamasaki F, Sato T. Vagal nerve stimulation prevents reperfusion injury through inhibition of opening of mitochondrial permeability transition pore independent of the bradycardiac effect. J Thorac Cardiovasc Surg. 2009;137:223-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 57. | Gourine A, Gourine AV, Mastitskaya S, Ackland G. “Remote preconditioning reflex”- a neural pathway of cardioprotection during myocardial ischaemia and reperfusion induced by remote ischaemic preconditioning. Eur Heart J. 2010;31:319. |
| 58. | Mastitskaya S, Marina N, Gourine A, Gilbey MP, Spyer KM, Teschemacher AG, Kasparov S, Trapp S, Ackland GL, Gourine AV. Cardioprotection evoked by remote ischaemic preconditioning is critically dependent on the activity of vagal pre-ganglionic neurones. Cardiovasc Res. 2012;95:487-494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 168] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 59. | Basalay M, Barsukevich V, Mastitskaya S, Mrochek A, Pernow J, Sjöquist PO, Ackland GL, Gourine AV, Gourine A. Remote ischaemic pre- and delayed postconditioning - similar degree of cardioprotection but distinct mechanisms. Exp Physiol. 2012;97:908-917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 60. | Loukogeorgakis SP, Panagiotidou AT, Broadhead MW, Donald A, Deanfield JE, MacAllister RJ. Remote ischemic preconditioning provides early and late protection against endothelial ischemia-reperfusion injury in humans: role of the autonomic nervous system. J Am Coll Cardiol. 2005;46:450-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 322] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 61. | Enko K, Nakamura K, Yunoki K, Miyoshi T, Akagi S, Yoshida M, Toh N, Sangawa M, Nishii N, Nagase S. Intermittent arm ischemia induces vasodilatation of the contralateral upper limb. J Physiol Sci. 2011;61:507-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 72] [Reference Citation Analysis (0)] |
| 62. | Andreka G, Vertesaljai M, Szantho G, Font G, Piroth Z, Fontos G, Juhasz ED, Szekely L, Szelid Z, Turner MS. Remote ischaemic postconditioning protects the heart during acute myocardial infarction in pigs. Heart. 2007;93:749-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 145] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 63. | Gritsopoulos G, Iliodromitis EK, Zoga A, Farmakis D, Demerouti E, Papalois A, Paraskevaidis IA, Kremastinos DT. Remote postconditioning is more potent than classic postconditioning in reducing the infarct size in anesthetized rabbits. Cardiovasc Drugs Ther. 2009;23:193-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 64. | Schömig A. Catecholamines in myocardial ischemia. Systemic and cardiac release. Circulation. 1990;82:II13-II22. [PubMed] |
| 65. | Roberts R, Rogers WJ, Mueller HS, Lambrew CT, Diver DJ, Smith HC, Willerson JT, Knatterud GL, Forman S, Passamani E. Immediate versus deferred beta-blockade following thrombolytic therapy in patients with acute myocardial infarction. Results of the Thrombolysis in Myocardial Infarction (TIMI) II-B Study. Circulation. 1991;83:422-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 242] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 66. | Ibanez B, Macaya C, Sánchez-Brunete V, Pizarro G, Fernández-Friera L, Mateos A, Fernández-Ortiz A, García-Ruiz JM, García-Álvarez A, Iñiguez A. Effect of early metoprolol on infarct size in ST-segment-elevation myocardial infarction patients undergoing primary percutaneous coronary intervention: the Effect of Metoprolol in Cardioprotection During an Acute Myocardial Infarction (METOCARD-CNIC) trial. Circulation. 2013;128:1495-1503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 299] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 67. | Kolh P, Windecker S, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Jüni P. 2014 ESC/EACTS Guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur J Cardiothorac Surg. 2014;46:517-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3295] [Cited by in RCA: 3380] [Article Influence: 307.3] [Reference Citation Analysis (0)] |
| 68. | Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Thygesen K, Alpert JS, White HD, Jaffe AS. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60:1581-1598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2076] [Cited by in RCA: 2312] [Article Influence: 177.8] [Reference Citation Analysis (0)] |
| 69. | Pei H, Wu Y, Wei Y, Yang Y, Teng S, Zhang H. Remote ischemic preconditioning reduces perioperative cardiac and renal events in patients undergoing elective coronary intervention: a meta-analysis of 11 randomized trials. PLoS One. 2014;9:e115500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 70. | Wang X, Yan J, Li L, Su Q. The effect of remote ischemic preconditioning in patients undergoing elective percutaneous coronary intervention: a systematic review and meta-analysis of randomized controlled trials. Exp Clin Cardiol. 2014;20:1411-1435. |
| 71. | Zografos TA, Katritsis GD, Katritsis DG. Remote ischemic preconditioning reduces peri-procedural myocardial injury in elective percutaneous coronary intervention: a meta-analysis. Int J Cardiol. 2014;173:530-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 72. | D’Ascenzo F, Moretti C, Omedè P, Cerrato E, Cavallero E, Er F, Presutti DG, Colombo F, Crimi G, Conrotto F. Cardiac remote ischaemic preconditioning reduces periprocedural myocardial infarction for patients undergoing percutaneous coronary interventions: a meta-analysis of randomised clinical trials. EuroIntervention. 2014;9:1463-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 73. | Prasad A, Gössl M, Hoyt J, Lennon RJ, Polk L, Simari R, Holmes DR, Rihal CS, Lerman A. Remote ischemic preconditioning immediately before percutaneous coronary intervention does not impact myocardial necrosis, inflammatory response, and circulating endothelial progenitor cell counts: a single center randomized sham controlled trial. Catheter Cardiovasc Interv. 2013;81:930-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 74. | Hoole SP, Heck PM, Sharples L, Khan SN, Duehmke R, Densem CG, Clarke SC, Shapiro LM, Schofield PM, O’Sullivan M. Cardiac Remote Ischemic Preconditioning in Coronary Stenting (CRISP Stent) Study: a prospective, randomized control trial. Circulation. 2009;119:820-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 349] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 75. | Davies WR, Brown AJ, Watson W, McCormick LM, West NE, Dutka DP, Hoole SP. Remote ischemic preconditioning improves outcome at 6 years after elective percutaneous coronary intervention: the CRISP stent trial long-term follow-up. Circ Cardiovasc Interv. 2013;6:246-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 136] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 76. | Ahmed RM, Mohamed el-HA, Ashraf M, Maithili S, Nabil F, Rami R, Mohamed TI. Effect of remote ischemic preconditioning on serum troponin T level following elective percutaneous coronary intervention. Catheter Cardiovasc Interv. 2013;82:E647-E653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 77. | Ghaemian A, Nouraei SM, Abdollahian F, Naghshvar F, Giussani DA, Nouraei SA. Remote ischemic preconditioning in percutaneous coronary revascularization: a double-blind randomized controlled clinical trial. Asian Cardiovasc Thorac Ann. 2012;20:548-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 78. | Luo SJ, Zhou YJ, Shi DM, Ge HL, Wang JL, Liu RF. Remote ischemic preconditioning reduces myocardial injury in patients undergoing coronary stent implantation. Can J Cardiol. 2013;29:1084-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 79. | Xu X, Zhou Y, Luo S, Zhang W, Zhao Y, Yu M, Ma Q, Gao F, Shen H, Zhang J. Effect of remote ischemic preconditioning in the elderly patients with coronary artery disease with diabetes mellitus undergoing elective drug-eluting stent implantation. Angiology. 2014;65:660-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 80. | Zografos T, Katritsis G, Korovesis S, Giazitzoglou E, Katrit- sis D. Remote ischemic preconditioning is feasible in ad hoc percutaneous coronary interventions. Eur Heart J. 2013;34 (Suppl 1). |
| 81. | Bøtker HE, Kharbanda R, Schmidt MR, Bøttcher M, Kaltoft AK, Terkelsen CJ, Munk K, Andersen NH, Hansen TM, Trautner S. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet. 2010;375:727-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 738] [Cited by in RCA: 763] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 82. | Sloth AD, Schmidt MR, Munk K, Kharbanda RK, Redington AN, Schmidt M, Pedersen L, Sørensen HT, Bøtker HE. Improved long-term clinical outcomes in patients with ST-elevation myocardial infarction undergoing remote ischaemic conditioning as an adjunct to primary percutaneous coronary intervention. Eur Heart J. 2014;35:168-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 232] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 83. | Carrasco-Chinchilla F, Muñoz-García AJ, Domínguez-Franco A, Millán-Vázquez G, Guerrero-Molina A, Ortiz-García C, Enguix-Armada A, Alonso-Briales JH, Hernández-García JM, de Teresa-Galván E. Remote ischaemic postconditioning: does it protect against ischaemic damage in percutaneous coronary revascularisation? Randomised placebo-controlled clinical trial. Heart. 2013;99:1431-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 84. | Lavi S, D’Alfonso S, Diamantouros P, Camuglia A, Garg P, Teefy P, Jablonsky G, Sridhar K, Lavi R. Remote ischemic postconditioning during percutaneous coronary interventions: remote ischemic postconditioning-percutaneous coronary intervention randomized trial. Circ Cardiovasc Interv. 2014;7:225-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 85. | Crimi G, Ferlini M, Gallo F, Sormani MP, Raineri C, Bramucci E, De Ferrari GM, Pica S, Marinoni B, Repetto A. Remote ischemic postconditioning as a strategy to reduce acute kidney injury during primary PCI: a post-hoc analysis of a randomized trial. Int J Cardiol. 2014;177:500-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 86. | Deftereos S, Giannopoulos G, Tzalamouras V, Raisakis K, Kossyvakis C, Kaoukis A, Panagopoulou V, Karageorgiou S, Avramides D, Toutouzas K. Renoprotective effect of remote ischemic post-conditioning by intermittent balloon inflations in patients undergoing percutaneous coronary intervention. J Am Coll Cardiol. 2013;61:1949-1955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 87. | Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Jüni P. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014;35:2541-2619. [PubMed] |
| 88. | Hausenloy DJ, Mwamure PK, Venugopal V, Harris J, Barnard M, Grundy E, Ashley E, Vichare S, Di Salvo C, Kolvekar S. Effect of remote ischaemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: a randomised controlled trial. Lancet. 2007;370:575-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 523] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 89. | Venugopal V, Hausenloy DJ, Ludman A, Di Salvo C, Kolvekar S, Yap J, Lawrence D, Bognolo J, Yellon DM. Remote ischaemic preconditioning reduces myocardial injury in patients undergoing cardiac surgery with cold-blood cardioplegia: a randomised controlled trial. Heart. 2009;95:1567-1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 183] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 90. | Wagner R, Piler P, Bedanova H, Adamek P, Grodecka L, Freiberger T. Myocardial injury is decreased by late remote ischaemic preconditioning and aggravated by tramadol in patients undergoing cardiac surgery: a randomised controlled trial. Interact Cardiovasc Thorac Surg. 2010;11:758-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 91. | Ali N, Rizwi F, Iqbal A, Rashid A. Induced remote ischemic pre-conditioning on ischemia-reperfusion injury in patients undergoing coronary artery bypass. J Coll Physicians Surg Pak. 2010;20:427-431. [PubMed] |
| 92. | Karuppasamy P, Chaubey S, Dew T, Musto R, Sherwood R, Desai J, John L, Shah AM, Marber MS, Kunst G. Remote intermittent ischemia before coronary artery bypass graft surgery: a strategy to reduce injury and inflammation? Basic Res Cardiol. 2011;106:511-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 93. | Lomivorotov VV, Shmyrev VA, Nepomnyaschih VA, Ponomarev DN, Knyazkova LG, Lomivorotov VN, Karaskov AM. Remote ischaemic preconditioning does not protect the heart in patients undergoing coronary artery bypass grafting. Interact Cardiovasc Thorac Surg. 2012;15:18-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 94. | Lucchinetti E, Bestmann L, Feng J, Freidank H, Clanachan AS, Finegan BA, Zaugg M. Remote ischemic preconditioning applied during isoflurane inhalation provides no benefit to the myocardium of patients undergoing on-pump coronary artery bypass graft surgery: lack of synergy or evidence of antagonism in cardioprotection? Anesthesiology. 2012;116:296-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 95. | Rahman IA, Mascaro JG, Steeds RP, Frenneaux MP, Nightingale P, Gosling P, Townsend P, Townend JN, Green D, Bonser RS. Remote ischemic preconditioning in human coronary artery bypass surgery: from promise to disappointment? Circulation. 2010;122:S53-S59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 192] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 96. | Ang R. Highlights in basic autonomic neurosciences: remote ischaemic preconditioning as an autonomic reflex--a question of timing and circumstances? Auton Neurosci. 2013;173:1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 97. | Zhou C, Liu Y, Yao Y, Zhou S, Fang N, Wang W, Li L. β-blockers and volatile anesthetics may attenuate cardioprotection by remote preconditioning in adult cardiac surgery: a meta-analysis of 15 randomized trials. J Cardiothorac Vasc Anesth. 2013;27:305-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 98. | Yu CH, Beattie WS. The effects of volatile anesthetics on cardiac ischemic complications and mortality in CABG: a meta-analysis. Can J Anaesth. 2006;53:906-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 111] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 99. | D’Ascenzo F, Cavallero E, Moretti C, Omedè P, Sciuto F, Rahman IA, Bonser RS, Yunseok J, Wagner R, Freiberger T. Remote ischaemic preconditioning in coronary artery bypass surgery: a meta-analysis. Heart. 2012;98:1267-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 100. | Spear JF, Prabu SK, Galati D, Raza H, Anandatheerthavarada HK, Avadhani NG. beta1-Adrenoreceptor activation contributes to ischemia-reperfusion damage as well as playing a role in ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2007;292:H2459-H2466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 101. | Lange M, Smul TM, Blomeyer CA, Redel A, Klotz KN, Roewer N, Kehl F. Role of the beta1-adrenergic pathway in anesthetic and ischemic preconditioning against myocardial infarction in the rabbit heart in vivo. Anesthesiology. 2006;105:503-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 102. | Thielmann M, Kottenberg E, Kleinbongard P, Wendt D, Gedik N, Pasa S, Price V, Tsagakis K, Neuhäuser M, Peters J. Cardioprotective and prognostic effects of remote ischaemic preconditioning in patients undergoing coronary artery bypass surgery: a single-centre randomised, double-blind, controlled trial. Lancet. 2013;382:597-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 376] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 103. | Haji Mohd Yasin NA, Herbison P, Saxena P, Praporski S, Konstantinov IE. The role of remote ischemic preconditioning in organ protection after cardiac surgery: a meta-analysis. J Surg Res. 2014;186:207-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 104. | Yang L, Wang G, Du Y, Ji B, Zheng Z. Remote ischemic preconditioning reduces cardiac troponin I release in cardiac surgery: a meta-analysis. J Cardiothorac Vasc Anesth. 2014;28:682-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 105. | Healy DA, Khan WA, Wong CS, Moloney MC, Grace PA, Coffey JC, Dunne C, Walsh SR, Sadat U, Gaunt ME. Remote preconditioning and major clinical complications following adult cardiovascular surgery: systematic review and meta-analysis. Int J Cardiol. 2014;176:20-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 106. | Wu Q, Gui P, Wu J, Ding D, Purusram G, Dong N, Yao S. Effect of limb ischemic preconditioning on myocardial injury in patients undergoing mitral valve replacement surgery. -A randomized controlled trial-. Circ J. 2011;75:1885-1889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 107. | Xie JJ, Liao XL, Chen WG, Huang DD, Chang FJ, Chen W, Luo ZL, Wang ZP, Ou JS. Remote ischaemic preconditioning reduces myocardial injury in patients undergoing heart valve surgery: randomised controlled trial. Heart. 2012;98:384-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 108. | Ovize M, Bonnefoy E. Giving the ischaemic heart a shot in the arm. Lancet. 2010;375:699-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |