INTRODUCTION
Cardiovascular disorders (CVDs) are currently the main cause of mortality in developed countries, and atherosclerosis is the major contributor to the CVD risk[1]. The disease affects large arteries causing lipid deposition in the arterial wall, local inflammatory process and the formation of plaques. Atherosclerotic plaques progression leads to luminal narrowing of blood vessels, which by itself can result in the ischemia of corresponding organs. However, most dangerous is the acute thrombus formation on the surface of a ruptured atherosclerotic plaque. Thrombosis can lead to rapid and life-threatening events, such as acute coronary syndrome and stroke[2]. Early stages of atherosclerosis development are mostly clinically silent, and the first manifestations of the disease are often fatal. At the same time, subclinical atherosclerosis is a prevalent condition in the modern society, especially among the ageing population. It has been reported recently that up to 5% of women and 12% of men over the age of 80 suffer from asymptomatic atherosclerotic carotid artery stenosis[3]. Other studies demonstrated the high rate of asymptomatic atherosclerosis incidence in young and middle-aged people, with up to 100% of apparently healthy individuals having atherosclerotic lesions at various stages of progression[4,5]. Along with the well-known benefits of life style correction, the development of more specific therapies could help decreasing the risk of atherosclerosis progression and consequent CVD development. It is therefore important to improve our understanding of the pathological mechanisms that trigger atherosclerotic lesion development in the arterial wall.
The intimal barrier of human arterial wall consists of the endothelium and the subendothelial net-like tissue layer formed by pericyte-like cells that have recently been characterized as true pericytes[6,7]. Whereas the endothelium serves as the first line of defence against the development of atherosclerotic lesion, the pericyte-containing subendothelial layer can be regarded as the second defence line essential for normal functioning and protection of the arterial wall. Apart from pericytes, this layer contains other cell types, including smooth muscle cells, macrophages, lymphocytes, mast cells, dendritic cells, and other cell types. Pericyte-containing subendothelial layer is a characteristic component of both arterial and venous intima[6]. Its role in the development of human pathology remained unknown until recently. However, the accumulating evidence demonstrated its importance in many severe vascular disorders, such as saphenous vein graft disease, thrombosis and atherosclerosis. Macrovascular pericytes are capable of rapid proliferation and participate in immune reactions. Moreover, these cells have been identified as a source of coagulation-inducing tissue factor[7]. In this review we will summarize the current knowledge on the structure and function of the subendothelial leaflet of human macrovascular intima and its role in the pathogenesis of atherosclerosis.
STRUCTURE OF NORMAL AND ATHEROSCLEROTIC ARTERIAL INTIMA
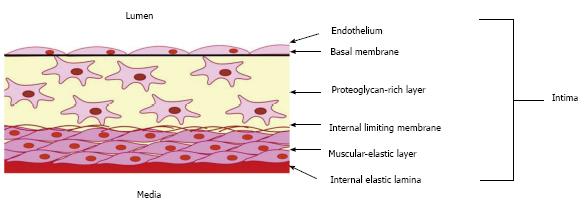
Aortic intima is the part of the blood vessel wall located between the internal elastic lamina and the lumen with endothelial lining, and consists of two distinct layers (Figure 1)[8,9]. Muscular-elastic layer represents the external part of the intima, and is separated from the internal intimal layer by the limiting membrane. This layer is formed by longitudinally oriented elongated cells and elastic fibres. The internal intimal layer, also known as proteoglycan-rich or connective tissue layer[10,11], contains randomly-oriented connective tissue fibres and morphologically heterogeneous cell population[12]. The intimal layers differ from each other by the composition of glycosaminoglycans and fibres. Muscular-elastic layer is rich in elastin fibres, and proteoglycan-rich layer - in collagen and reticulin[13]. Moreover, collagen composition and structure are different between the two layers: longitudinally oriented collagens I and III are common in the muscular-elastic layer, while collagens IV and V are detected primarily in the proteoglycan layer, where they form the endothelial basal membrane.
Figure 1 Schema showing the organization of the intima of the arterial wall.
The proteoglycan-rich layer which contains a heterogeneous population of cells, including macrovascular pericytes, is located just below the endothelial monolayer. Intimal pericytes form a network of cells interconnected through gap junctions. The muscular-elastic layer, formed by elongated contractile smooth muscular cells, lies below the proteoglycan-rich layer.
The development of atherosclerotic lesion has been carefully studied by our group on human aorta samples from healthy subjects and atherosclerotic patients[8-13]. Atherosclerotic process is tightly associated with thickening of the arterial intima. Careful analysis of the intima thickness along the arteries affected by atherosclerosis in comparison to normal tissues revealed that muscular-elastic layer remained unaltered in the fatty streak area and became only slightly thicker in the atherosclerotic plaque area[8-13]. By contrast, the proteoglycan layer was 2-fold thicker in the fatty streak and 4-fold - in the atherosclerotic lesion areas, and was the major contributor of the arterial stenosis. This thickened proteoglycan layer had increased collagen content. Moreover, longitudinal orientation of collagen fibres was altered, and lipid droplets were present between the interstitial collagen fibres[8-13]. Atherosclerotic plaques were characterized by the accumulation of all collagen types, especially in the fibrous cap area. Collagens IV and V formed a thick layer in the endothelial basal membrane area and surrounded the subendothelial cells. At the same time, no prominent alterations of collagen structure and composition were present in the muscular-elastic layer of the intima[8-13].
Changes of lipid content accompany the development of atherosclerotic plaques in the aortic intima. In the normal tissue, extracellular lipid droplets visualised by Oil Red O staining were located along the elastic membrane. In the fatty streaks, both extra- and intracellular droplets were present, mostly in the proximity of the lumen. In atherosclerotic plaques, lipid deposition could be observed in the proteoglycan layer, and total lipid content was 8-fold higher than in the unaffected tissue[6]. Lipid content of muscular-elastic layer was increased to a lesser extent, being 4.4-fold higher than control. Cellular composition of the proteoglycan-rich layer of atherosclerotic intima was also altered in comparison with the healthy tissue[6]. Total cell count assessed by alcohol-alkaline dissociation of the fixed tissue, was 1.5 and 2-fold higher in the fatty streak and atherosclerotic plaque respectively[6].
Taken together, these observations clearly indicate that the subendothelial proteoglycan-rich layer of the arterial intima undergoes the most prominent alterations in course of the atherosclerotic plaque development, including thickening, lipid deposition and the accumulation of collagen fibres with disturbed orientation[6,8-13].
CELLULAR COMPOSITION OF MACROVASCULAR INTIMA
Human macrovascular intima is made of a heterogeneous cell population. Immediately below the endothelial lining and in close contact with it, there is a three-dimensional net formed by pericyte-like cells. Early studies have identified pericytes as mostly microvascular cells that play an important role in angiogenesis and vessel branching and are essential for maintaining the normal structure of the capillary wall and the endothelial barrier function, including the maintenance the blood-brain barrier[14,15]. Pericytes were also demonstrated to contribute to embryonic development of the aorta[16]. It has become evident, however, that pericyte-like cells are also present in the walls of large blood vessels[17]. Apart from vasa vasorum microvessels[18], pericyte-like stellate cells were found in the intima of large arteries[6,7,19-21].
Accurate identification of pericytes, as well as their characterisation in in vitro studies, remains challenging, since these cells have flexible phenotype. The expression of numerous pericyte marker proteins is highly dynamic and depends on the surrounding milieu. Moreover, many marker proteins are shared between pericytes and vascular smooth muscle cells (VSMCs)[14]. One of such markers is the α-smooth muscle actin (αSMA), which is normally expressed in VSMCs, but is also common in pericytes. However, its expression in the latter cell type can vary depending on the surrounding milieu[22]. For instance, pericytes from non-contractile capillaries can be αSMA-negative[23]. The expression of αSMA in pericytes can also be regulated by cell maturity[23] or stimulation by endothelin-1[24]. These considerations have to be taken into account while analyzing the cell population of macrovesicular intima. In the intima of large human arteries, the majority of resident cells were found to be αSMA-positive, especially in the muscular-elastic layer, where this population reached two thirds of the total amount of cells[25]. In the proteoglycan layer, the percentage of αSMA-positive cells was much lower. These cells were different from typical VSMCs, were less densely packed and had a characteristic branched morphology, with long processes forming intracellular contacts. Moreover, some αSMA-positive intimal cells expressed marker proteins that were unusual for muscular cells, such as CD68, which is considered a macrophage-specific antigen[26]. Interestingly, the proportion of αSMA+CD68+ cells increased in atherosclerotic lesions, as well as in primary cell culture exposed to atherogenic modified low-density lipoprotein (LDL). Apparently, the acquisition of the “macrophage”-specific marker reflects the engagement of cells into phagocytic activity[27,28]. The large percentage of αSMA-negative cells in the arterial intima can partly be explained by the loss of contractile apparatus, since these cells acquire other functions than maintaining the vascular tone, such as metabolic homeostasis and nutrition or participation in the immune response in the blood vessel wall[29].
Therefore, accurate identification of pericytes in the intimal layer should not be based on αSMA expression exclusively, and has to include the analysis of other pericyte markers expression and morphological data. Commonly used pericyte markers include platelet-derived growth factor receptor β, CD146, aminopeptidase A and N (CD13), endoglin, neuron-glial 2, non-muscle myosin, desmin, vimentin and nestin[14,30]. However, most of these markers are shared with VSMCs and/or dependent on pericyte maturity or activation state, and therefore should be used in combination to avoid false-positive results. It has been demonstrated that some antigens expressed in resident intimal cells are not present in VSMCs. Antigen 3G5 is an O-sialoganglioside specific for microvascular pericytes[31]. It was found in some intimal resident cells, but not in the tunica media[26,32]. In humans, 3G5-positive pericytes form the net-like subendothelial tissue layer in normal human arterial intima and account for approximately one third of intimal cells (Figure 2)[26]. Another pericyte antigen detected in the macrovascular intima is 2A7, also known as melanoma chondroitin sulphate proteoglycan[26,33]. The expression of this antigen is typical for activated pericytes in the active angiogenesis phase. The expression of these pericyte marker antigens alters in atherogenesis. As demonstrated on primary cultures of subendothelial cells exposed to atherogenic modified LDL, intracellular lipid deposition results in the reduction of 3G5-positive cell fraction, whereas 2A7-expressing fraction increases[33].
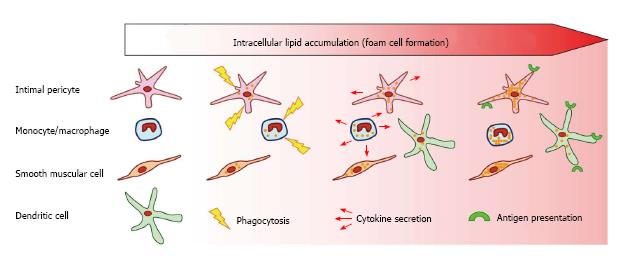
Figure 2 Scheme showing the roles of arterial wall cells in atherogenesis.
Several types of arterial wall cells participate in lipid accumulation and foam cell formation. Macrophages and intimal pericytes accumulate lipids through phagocytosis and participate in the local inflammatory process secreting pro-inflammatory cytokines. Dendritic cells, together with macrophages and intimal pericytes express antigen-presenting complexes, further promoting the inflammatory process.
Studies of the primary cell culture obtained from human macrovascular intima helped revealing the mechanisms of pericyte acquisition of characteristic stellate shape. Pericyte arborisation could be induced by increasing the intracellular cAMP concentration[33]. Interestingly, cells originating from the proteoglycan layer were more susceptible of arborisation, with up to 100% of them being capable of acquiring the stellate shape, whereas in population originating from the muscular-elastic layer it could be induced in only 50% of cells. The arborisation was associated with re-distribution of connexion 43 (Cx43), which is responsible for gap junction formation. Stellate cells formed large Cx43-positive sites located at the ends of cellular processes, where intracellular contacts were formed. In vivo, pericytes form stable contacts with the endothelial cells, which could also be reproduced in co-culture of the two cell types[7].
Apart from pericytes, other cell types populating the arterial intima have been described. These include cells of hematogenous lineage - macrophages[34-36], lymphocytes[37,38], mast cells[39] and dendritic cells. Cells of hematogenous lineage are located exclusively in the subendothelial proteoglycan layer of intima and are enriched in atherosclerotic plaques, where they can account for up to 20% of total cell population.
ROLE OF PERICYTE-LIKE CELLS (INTIMAL PERICYTES) IN THE PATHOGENESIS OF ATHEROSCLEROSIS
According to current consensus, pericytes are pluripotent cells that can serve as progenitors for other cell types of mesenchymal origin, including smooth muscle cells[40], fibroblasts, osteoblasts[41,42], chondrocytes[40] and adipocytes[43]. Pericytes are actively involved in various conditions associated with impaired microcirculation, such as diabetes, inflammation, wound healing and tumor growth. Converging evidence indicates pericyte implication in the pathogenesis of atherosclerosis. In presence of proinflammatory microenvironment and atherogenic modified LDL typical for early stages of atherosclerosis, intimal pericytes can accumulate lipids and become activated. The development of atherosclerotic plaque is associated with the increase of total cell count and the percentage of pericytes indicative of their involvement into the pathological process[21]. In fatty streaks, these cells actively accumulate lipids, which leads to the increase of cell size, acquisition of irregular shape, loss of Cx43-mediated cell contacts[44] and disturbance of the net-like subendothelial tissue[45]. Pericytes may not only proliferate and store lipids contributing to the plaque growth[46,47], but also promote thrombogenesis being a source of tissue factor[17]. Figure 3 depicts the impact of different arterial cells, including intimal pericytes, in the atherosclerotic process.
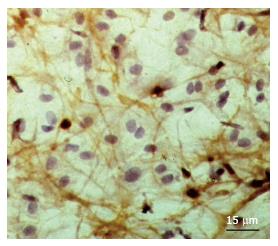
Figure 3 Cellular network formed by 3G5+ cells located under the luminal endothelium in undiseased intima.
This cellular network was identified by means of the application of peroxidase-anti-peroxidase immunohistochemical analysis (brown colour of reaction product) in en face preparation of a tissue specimen of the human aorta. Counterstain with haematoxylin.
Microvascular pericytes are important for atherosclerosis progression, as they participate in neovascularization of the atherosclerotic plaques[48]. The recruitment of pericytes to growing neovessels could potentially be mediated by T-cadherin signalling. T-cadherin is an unusual member of the cadherin family, which is up-regulated in atherosclerosis[49]. It might play a role during LDL-induced pericyte activation through ERK1/2-nuclear factor κB signalling pathway[50]. It has been demonstrated that pericytes could also be recruited to neovessels in atherosclerotic plaques through hepatocyte growth factor signalling triggering c-Met-PI3K/Akt pathway in pericytes[51].
Participation in vascular wall remodelling and calcification is another possible contribution of pericytes to the pathological process in atherosclerosis. Activation of Wnt/β-catenin signalling stimulates chondrogenic differentiation of pericytes[52], which can be enhanced by transforming growth factor-β3[53]. The latter is abundantly produced by macrophages, foam cells and VSMCs in the atherosclerotic plaque[54]. Intimal pericytes were shown to express vascular calcification-associated factor. Moreover, pro-inflammatory environment in the plaque may promote osteogenic differentiation of the resident vascular cells. These observations indicate the potential involvement of pericytes in ectopic vascular calcification associated with atherosclerosis[55,56].
Taken together, the presented observations strongly support the hypothesis that macrovascular pericytes play an important role in all stages of the atherosclerotic lesion development, which makes them an attractive potential target for therapy development[7,57]. Interestingly, pericytes may also take part in the innate immunity reactions, which will be discussed in the next section.
PERICYTE-LIKE CELLS IN INNATE IMMUNITY
The development of atherosclerotic lesion is tightly associated with local inflammatory process. At the initial stages of atherosclerosis, formation of focal intimal thickening is accompanied by the increase of intimal cell count[58-60]. Such increase can be explained by the enhanced proliferation of intimal cells, recruitment of circulating hematologous cells or a combination of these processes. Cell proliferation has been demonstrated to be important for atherosclerotic process[61], although the differences in cell composition depending on different types of large arteries remained unknown. Detailed study of cell count and proliferation was performed on 29 post-mortem specimens of human carotid and coronary arteries with different stages of atherosclerotic plaque development: diffuse intimal thickening (grossly normal tissue), initial lesions, fatty streaks, lipofibrous plaques and fibrous plaques[62]. Proliferating cells in S phase were identified by proliferating cell nuclear antigen (PCNA) staining. The authors report the increased total cell count in atherosclerotic lesions and more frequent macrophages and lymphocytes in comparison with normal tissue. Number of proliferating cells was also increased in atherosclerotic plaques as compared to normal intima. Maximal total cell count and maximal amount of proliferating cells were detected in lipofibrous plaques in both types of arteries. Interestingly, the proliferative index (determined as the percentage of PCNA-positive cells in the total subpopulation) hematogenous cells in atherosclerotic lesions was lower than in the unaffected intima. It is therefore likely that the increase of hematogenous cells in atherosclerotic intima occurs due to immune cell recruitment from the bloodstream and not from the local proliferation[63-65]. By contrast, proliferative index of resident cells was higher in atherosclerotic lesions than in unaffected tissue, reaching its maximum in lipofibrous plaques[62].
It is well documented that modified atherogenic LDL not only causes lipid deposition in the arterial intima, but also initiates pro-inflammatory conditions, stimulating both adaptive and innate immunity[66,67]. During the atherosclerotic plaque formation, inflammatory cells are migrating into the lesion zone, where they release proinflammatory cytokines and chemokines, inducing proliferation of the resident intimal cells[65,68]. This model may explain the observed divergence of inflammatory and resident cells proliferative index dynamics in atherosclerotic lesions. Study of immune-inflammatory processes in diffuse intimal thickening demonstrated the link between lipid deposition in the arterial wall and the immune-inflammatory cell content[69]. Lipid deposition in the proteoglycan-rich layer of intima positively correlated with the expression of major histocompatibility complex class II (MHC II) molecule HLA-DR[70] and the amount of immune-inflammatory cells in that area. These findings demonstrate that lipid accumulation at the early stages of atherosclerotic lesion development is accompanied by local immune activation. HLA-DR is a marker of antigen presenting cells (APCs)[70,71] that include macrophages, B cells and dendritic cells. The latter were described as professional APCs, as they play the most prominent role in antigen presentation and initiation of the immune response. Apart from these cell types of hematogenous origin, some non-hematogenous cells can serve as APCs. For instance, epithelial cells of the thymus are involved in antigen presentation[71]. Human endothelial cells are capable of T cell activation and basally express both MHCI and MHC II, although the expression is less prominent in larger arteries[72]. Moreover, fibroblasts and endothelial cells can become activated to express MHC II under certain conditions both in vitro and in vivo[70,73]. Immunofluorescent analysis of HLA-DR expression in the aortic intima revealed a large population of interconnected HLA-DR-positive cells that formed a net-like tissue (Figure 4)[74]. In some segments of the intima up to 15% of total cell population was expressing HLA-DR[74]. Morphologically, these cells could be identified as pericytes, with their typical net-forming intercellular contacts.
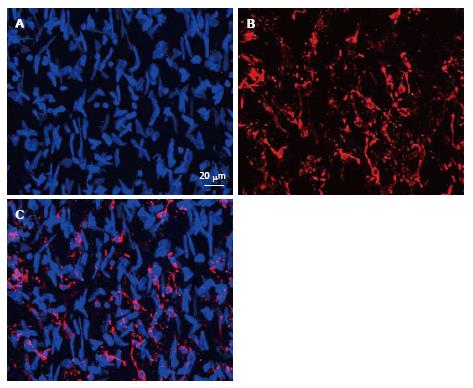
Figure 4 Immunofluorescent images representing the merge of 45 optic “confocal” sections of the intima obtained with interval of 1 μm between consecutive sections (A and B).
A: Nuclei were visualized by staining with ethidium bromide; B: Distribution and networks formed by HLA-DR+ cells, detected with the use of anti-HLA-DR antibody conjugated with Alexa 633; C: A merge of the images shown in (A) and (B). Human aorta specimen. Reproduced from Bobryshev et al[74], with permission from Elsevier. HLA: Human leukocyte antigen.
These results indicate that antigen presentation in the arterial wall can be performed by a variety of intimal cell types, including macrovascular pericytes. Importantly, microscopic study has revealed some HLA-DR-positive cells that also contained apo-B in the perinuclear space, possibly representing the early events of lipid accumulation in the intimal cells[74]. It is therefore possible that pericytes play a double role in atherogenesis, participating both in lipid accumulation and in local immune reaction.
Immunohistochemistry demonstrated the expression of tumor necrosis factor (TNF)-α and CCL18 cytokines in both normal and atherosclerotic human aorta. These two cytokines were expressed by distinct populations of cells, with CCL18 mostly located in the subendothelial layer, and TNF-α - in deeper layers of the unaffected intima. The expression pattern was altered in atherosclerotic plaques, with both TNF-α and CCL18 upregulated compared to grossly normal arterial intima. Apart from macrophages, other cytokine-producing cells types were detected, including stellate pericyte-like cells.
The immune functions of pericytes remain an attractive topic for future investigation[75]. Microvascular placental pericytes were demonstrated to interact with CD4+ T cells in co-culture. Pericytes stimulated the expression of CD25 and CD69 in resting allogenic CD4+ T cells, indicative of MHC recognition. This, however, did not lead to the induction of cytokine production and proliferation of T cells[76]. The possibility that macrovascular pericytes have immune-modulating properties remains to be explored. The three-dimensional network of subendothelial cells participating in immune reactions may play important protective functions in the blood vessel wall. As demonstrated on the biopsy material from arteries, unaffected by atherosclerosis or with different stages of atherosclerotic lesion development, changes in this network occur early in the pathological process.
OTHER CELL TYPES PARTICIPATING IN IMMUNE REACTIONS IN THE SUBENDOTHELIAL INTIMA
Cells of hematogenous origin actively participate in immune reactions in the blood vessel wall. The development of atherosclerotic lesion is accompanied by infiltration and population of the lesion site by lymphocytes and macrophages. In human atherosclerotic lesions, the population of T cells consists mostly of the effector memory T cells. Substantial part of them are CD4+ T helper cells[77]. CD8+ cytotoxic T lymphocytes are also present in atherosclerotic lesions. T cells are especially numerous in so called vulnerable plaques that are susceptible to rupture and thrombosis initiation[78]. B cells have also been demonstrated to participate in atherogenesis[79,80]. However, these cells are mostly found in the adventitia of the affected vessels, and not in the atherosclerotic plaques[77,81].
Pro-inflammatory stimuli in blood vessels affected by atherosclerosis attract circulating monocytes that rapidly migrate to the atherosclerotic lesion site and differentiate where, predominantly to macrophages. This polarization is driven by stimulation of specific receptors, including C-C chemokine receptor type 2 and CX3C chemokine receptor 1[82,83]. Both of these chemokines are implicated in the atherosclerotic plaque progression[84]. Macrophages play a prominent role in the inflammatory process associated with the initiation and progression of atherosclerotic lesions. Macrophages are involved in the uptake of atherogenic modified LDL and form typical foam cells. Toll-like receptor (TLR) signalling in macrophages plays important role in lipid accumulation through regulation of scavenger receptor expression and suppression of cholesterol efflux[85,86]. Macrophages also produce a number of inflammatory cytokines and contribute to vascular remodelling.
In atherosclerotic lesions, two distinct macrophage subpopulations can be found: M1 (classical) and M2 (alternative). Pro-inflammatory M1 phenotype can be induced in response to bacterial infection and pro-inflammatory cytokines, such as interferon (IFN)-γ and TNF-α[87]. They produce a spectrum of cytokines and chemokines stimulating the inflammatory response, as well as nitric oxide and reactive oxygen species. M2 macrophages have anti-inflammatory properties, producing interleukin (IL)-10. In addition, M2 subpopulation of macrophages is heterogeneous: M2a macrophages are induced in response to IL-4 and IL-13 and participate in tissue remodelling, M2b cells are involved in immunoregulation, and M2c, induced by IL-10 and transforming growth factor-β, are involved in clearance of apoptotic cells[88,89]. All three subtypes of M2 macrophages can be found in human atherosclerotic lesions. Human-specific M4 macrophages can be induced by platelet-derived chemokine CXCL4 and are characterized by resistance to excessive lipid uptake. This makes them susceptible to rapid transformation into foam cells and indicates their potential role in atherosclerotic lesion development[90]. Specific populations of macrophages can be found in hemorrhagic atherosclerotic plaques. In humans, they represent M(Hb), HA-mac and Mhem subsets. HA-mac and M(Hb) macrophages are involved in hemoglobine clearance[91]. All three subpopulations possess anti-inflammatory properties and can play a protective role in atherosclerosis.
Dendritic cells are also frequent in the subendothelial layer of intima, just below the liminal endothelium, where they contribute up to 10% of total cell population[74,92,93]. In the subendothelial space, dendritic cells form cellular networks by means of their long interconnected processes[74,92,93]. Dendritic cells play an important role in the immune response, producing a spectrum of cytokines and surface co-stimulatory molecules. They can be differentiated from monocytes at the periphery through stimulation with granulocyte-macrophage colony-stimulating factor (GM-CSF) and TLR4 ligands[94]. These monocyte-derived dendritic cells are involved in antigen presentation and cross-presentation[95]. It has been demonstrated that monocyte-derived DCs from patients with atherosclerosis had increased sensitivity to GM-CSF and IL-4 as compared to healthy subjects[96]. Pro-inflammatory microenvironment of the atherosclerotic plaque accounts for preferential differentiation of monocytes and dendritic cell precursors towards pro-inflammatory dendritic cells that play an important role in the inflammatory process and stimulate differentiation of the naïve T cells to Th1 and Th17 phenotypes[97]. Dendritic cells also produce pro-inflammatory IFN-α and a number of chemokines that induce the migration of inflammatory cells to the lesion site[98]. In summary, pro-inflammatory dendritic cells play a prominent role in the development of atherosclerotic plaque. Populations of atheroprotective tolerogenic and anti-inflammatory dendritic cells could also be found in atherosclerotic plaques. In Apo-E-deficient mice, tolerogenic dendritic cells generated in response to immunogenic bacterial peptides were demonstrated to decrease inflammation and contribute to the plaque decrease through induction of IL-10-producing Tregs[99]. It is possible that employing the pathways of tolerogenic dendritic cell induction can be beneficial for treatment of atherosclerosis.
CONCLUSION
The subendothelial layer of human arterial intima contains a cellular network made of pluripotent pericytes that form multiple intracellular contacts through gap junctions. Together with the professional immune cells, such as macrophages and dendritic cells, pericytes may execute functions, typical for innate immune cell types: participate in LDL trapping by phagocytosis, produce pro- and anti-inflammatory cytokines and other factors and present antigens (Figure 2). Whereas the aortic endothelium plays a role as the first line of defence in atherosclerosis development, the subendothelial pericytes can be regarded as the second defence line and should be considered for the development therapeutic approaches for atherosclerosis treatment.