Published online Sep 26, 2014. doi: 10.4330/wjc.v6.i9.985
Revised: April 29, 2014
Accepted: July 12, 2014
Published online: September 26, 2014
Processing time: 196 Days and 22.4 Hours
ABO blood type is one of the most readily available laboratory tests, and serves as a vital determinant in blood transfusion and organ transplantation. The ABO antigens are expressed not only on red blood cell membranes, determining the compatibility of transfusion, but also on the surface of other human cells, including epithelium, platelet and vascular endothelium, therefore extending the research into other involvements of cardiovascular disease and postoperative outcomes. ABO blood group has been recognized as a risk factor of venous thrombosis embolism since the 1960’s, effects now understood to be related to ABO dependent variations are procoagulant factor VIII (FVIII) and von Willebrand factor (vWF) levels. Levels of vWF, mostly genetically determined, are strongly associated with venous thromboembolism (VTE). It mediates platelet adhesion aggregation and stabilizes FVIII in plasma. Moreover, many studies have tried to identify the relationship between ABO blood types and ischemic heart disease. Unlike the clear and convincing associations between VTE and ABO blood type, the link between ABO blood type and ischemic heart disease is less consistent and may be confusing. Other than genetic factors, ischemic heart disease is strongly related to diet, race, lipid metabolism and economic status. In this review, we’ll summarize the data relating race and genetics, including ABO blood type, to VTE, ischemic heart disease and postoperative bleeding after cardiac surgery.
Core tip: In this review, we updated the reports regarding the associations between ABO blood groups and venous thrombosis, ischemic heart disease as well as postoperative outcomes after cardiac surgery. ABO blood group is clearly associated with venous thromboembolism whereas critical review of the literature reveals a more controversial relationship with atherosclerosis, arterial thrombosis and postoperative outcomes.
- Citation: Zhou S, Welsby I. Is ABO blood group truly a risk factor for thrombosis and adverse outcomes? World J Cardiol 2014; 6(9): 985-992
- URL: https://www.wjgnet.com/1949-8462/full/v6/i9/985.htm
- DOI: https://dx.doi.org/10.4330/wjc.v6.i9.985
The ABO group of human red cell antigens was discovered by Karl Landsteiner in 1900. ABO antigens are carbohydrate molecules that are the major determinants of the compatibility of red cell transfusions. Naturally occurring, complement fixing IgM antibodies are formed against the A and B antigens in individuals that do not express them on their red cell surfaces and therefore recognize them as foreign antigens. Each individual inherits two ABO alleles. The A and B alleles encode separate glycosyltransferase that add N-acetylgalactosamine and D-galactose of the “H” antigen (group O determinant), converting it into A and B antigens respectively. However, as the O allele does not express either A or B transferase enzymes, continued expression of the unaltered H antigen is the phenotypic marker of the O blood group[1]. The ABO antigens are expressed not only on red blood cell membranes, determining the compatibility of transfusion, but also on the surface of other human cells, including epithelium, platelet and vascular endothelium[2], therefore extending potential pathophysiology into other areas of cardiovascular disease and postoperative outcomes.
Expression of the different ABO phenotypes is partially dependent on racial origin as shown in Table 1, with Group O generally being the most common blood group[3]. Blood groups are basically described by phenotypes, because historically blood groups are determined by commercial antibodies that recognize A and B antigens. By this detection method, both AO and AA genotypes (A1(2)O1, A1A2) will be identified as group A, while BO and BB genotypes as group B. In this review, we updated the reports regarding the associations between ABO blood groups and venous thrombosis, ischemic heart disease as well as postoperative outcomes in terms of both ABO phenotype and genotype.
| Race | Blood group phenotype | Blood group genotypes | ||||
| O1 (O2 rare) | A1 | A2 | B | A1B | A2B | |
| Caucasian | 44% | 33% | 10% | 9% | 3% | 1% |
| Asian | 43% | 27% | Rare | 25% | 5% | Rare |
| African | 49% | 19% | 8% | 20% | 3% | 1% |
Von Willebrand factor (vWF) has two major biological forms and the high molecular weight vWF (HMW vWF) is hemostatically more active than the low molecular weight vWF (LMW vWF)[4]. HMW vWF mediates the interaction between platelets and damaged areas of the blood vessel wall, while LMW vWF acts as a specific carrier molecule for procoagulant factor VIII (FVIII), thereby localizing FVIII to the site of any vascular injury. Both are essential for normal hemostasis[5,6].
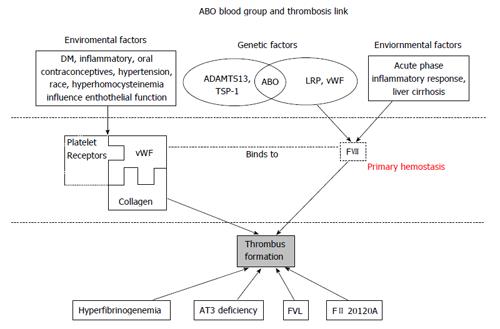
Plasma vWF levels are generally reported to be approximately 25% higher in non-O blood individuals[7]. Synthesized in endothelial cells and megakaryocytes, the HMW vWF, enters the plasma from platelet granules following platelet activation and degranulation at the site of tissue injury, or alternatively being stored in endothelial cell Weibel-Palade bodies, then secreted in response to thrombin, fibrin or histamine stimulation[8]. vWF molecular has three binding sites, platelet glycoprotein 1b binds to A1 domain, while collagen binds to A3 domain, forming the primary hemostatic clot[9,10]. The A2 domain binds to ADAMTS13 and is responsible for vWF cleavage (Figure 1).
Clinical observations that the severity of bleeding in mild von Willebrand’s disease was exaggerated for group O patients led to the recognition of an ABO dependent variation in vWF levels[11-13]. A formal linkage analysis showed the effect of ABO blood type on von Willebrand factor is a direct functional effect of the ABO locus, rather than linkage disequilibrium between the ABO locus and another unidentified VWF regulation locus[14]. vWF levels can also influence procoagulant FVIII levels since vWF is a carrier molecule that protects FVIII from proteolysis in plasma.
Moeller et al[15] compared vWF and FVIII levels in individuals of different ABO phenotype and found ascending order O < A < B < AB for vWF level and O < A < AB < B for FVIII level. This effect becomes more nuanced when considering the specific genotypes that result in ABO phenotypes, as illustrated in Table 2. Within A and B phenotypes, vWF concentrations in AA or BB are slightly higher than AO or BO[16] and A1 and B alleles are found to be associated with higher vWF and FVIII levels, while A2 is comparable to O allele[6,11,13,17].
| Genotype | Median value | ||
| vWF | FVIII | ||
| Low | O1O1 | 69% | 75% |
| Medium | A1O, A2O, BO | 89% | 96% |
| High | AA, BB, A1B | 120% | 117% |
| Highest | A2B | 169% | 112% |
There is no direct evidence demonstrating that the ABO locus is associated with vWF synthesis[8], therefore efforts to elucidate the association between ABO and vWF have focused on vWF metabolism and cleavage. ADAMTS13 cleavages HMW vWF to LMW vWF[8,15,18,19], thereby modulating the tendency of vWf to cause platelet aggregation and thrombus formulation[20]. The biological importance of this is exemplified by thrombotic thrombocytopenic purpura (TTP). In TTP, autoantibodies neutralize ADAMTS13 leading to diffuse microvascular thrombosis from the unregulated action of HMW vWF. This extreme example leads to a proposed mechanism for the ABO group related modulation of vWF levels and therefore tendency to thrombosis. While A, B and H antigens are more commonly known to be expressed on the cell surfaces of erythrocytes and various exocrine cells, they are also expressed on the vWF molecule. The location of the A, B and H antigens on the vWF molecule is thought to be close to the A2 domain binding site for ADAMTS13 and that A and B antigens reduce ADAMTS13 binding and, therefore, cleavage[8,21]. Some studies confirmed this hypothesis by providing evidence that the proteolytic effect of ADAMTS13 on vWF was significantly faster in O group (only H antigen expression) than in non-O groups with A and B antigen expression[22,23] . Factors other than ABO group can also modify vWF metabolism which may limit the direct association of ABO group with vWF levels and thrombosis, explaining some inconsistencies in the various studies we report. For example, thrombospondin-1 (TSP-1) has been reported to control vWF multimer size by both directly cleavage and indirectly, competing with ADAMTS13[24,25]. Thus, any genetic factors influence cleavage (ABO blood type, ADAMTS13 and TSP-1) and environmental risk factors that affect endothelial cell function, such as age, diabetes mellitus, hypertension, inflammatory and oral contraceptive drugs, all contribute to the complex risk factors leading to clinical thrombosis. This concept is illustrated in Figure 1.
The link between ABO blood group, H antigen expression and lower vWF levels has been well established above. How this translates into a clinically relevant risk of thromboembolism manifesting either as venous thromboembolism or coronary artery thrombosis is discussed in detail below.
Venous thromboembolism (VTE) includes deep vein thrombosis and pulmonary embolism and is a serious medical condition with a historical mortality rate of 10% and 15% respectively[26]. ABO blood group has been recognized as a risk factor since the 1960’s, effects now understood to be related to ABO dependent variations are procoagulant FVIII and vWF levels. Levels of vWF, mostly genetically determined, are strongly associated with VTE. It mediates platelet adhesion aggregation and stabilizes FVIII in plasma. In a healthy state, twin studies showed 75% of variance in plasma vWF levels result from genetic determinants[27], 30% of which are associated with ABO blood type[28]. Other non-genetic factors, such as aging, diabetes, free radical formation and inflammation, may have a more important role during acute illnesses or during the perioperative period[29]. As shown in Figure 1, environmental causes of endothelial dysfunction can greatly affect vWF levels.
Numerous studies have reported that individuals with non-O blood types had a higher risk of VTE compared to their O counterparts[30-34]. According to Wiggins, compared to O1O1 group, AB diplotype category has the highest VTE rate, followed by B allele and A1 allele[13]. Other rare genotypes like A3, Ax, Aa, B3, Ba were less amenable to statistically meaningful comparison in this study. These observations were supported by genotype association studies that showed H-antigen rich genotypes (O1O1, O1O2, O1A2) have a lower incidence of VTE than H-antigen poor genotypes (A1B, O1A1, O1B)[17,35,36], establishing ABO blood type as an important risk factor for VTE[37].
In Figure 1, various genetic and environmental factors affecting vWF levels are presented. What’s more, FVIII, circulating bound to vWF, also plays a crucial and independent role in the propagation phase of coagulation activation[6]. Since vWF is the plasma co-carrier of FVIII, ABO blood type, by altering vWF levels, also exerts an effect on FVIII levels. Tirado et al[34] demonstrated that genetic factors explain 40% of the variance of FVIII levels; other studies further identified a quantitative trait locus and the ABO locus as two major genetic factors underlining the variability of FVIII levels[14,38]. Unconnected to ABO group, lipoprotein receptor-related protein has also been identified to be associated with degradation of FVIII, another consideration when evaluating variance in FVIII levels[39]. In summary, while ABO blood type and vWF levels are two important factors commonly known to modulate FVIII plasma level, the biology determining FVIII is a complex interaction of genetic and environmental factors as illustrated in Figure 1.
However, FVIII may have some effect independent of vWF. Some studies demonstrated that a high FVIII level is persistent beyond the acute phase state[40,41], representing a potential risk factor for delayed or recurrent thrombosis. In addition, Morange et al[17] described a residual statistical effect of ABO blood group on FVIII levels after adjustment for vWF levels, postulating that FVIII is an independent VTE risk factor[29,34] Additionally, FVIII was reported to be associated with recurrent disease[34], consistent with reports that non-O carriers had a higher incidence of VTE recurrence than O carriers[42,43].
Unlike the clear and convincing associations between VTE and ABO blood type, the link between ABO blood type and ischemic heart disease is less consistent and may be confusing. In part this can be due to the inclusion of different end-points that may represent different disease processes, such as angina/atherosclerosis (less likely ABO/vWF related) or myocardial infarction (MI)/coronary thrombosis (more likely ABO/vWF related). The pathogenesis of coronary artery disease (CAD) involves the progression of an atherosclerotic disease process, whereas MI (or acute coronary syndrome) results from a platelet rich thrombus forming on abnormal endothelium diseased by the atherosclerotic process. Platelet rich thrombi (MI) are reliant on primary hemostasis, whereas the mechanism linking ABO group to CAD is less obvious. However, it is important to evaluate ABO group as a risk factor for both these devastating conditions: CAD and MI.
Many studies show that non-O group have higher incidence of ischemic heart disease (Table 3). The Framingham Heart study, and others, suggested A blood type has increased risk of CAD[44-46] and MI[47]; more specifically, A blood group seems to be related to early CAD detection[47,48] and predominates in patients with MI[49]. Other studies noted groups B[50,51] or AB[52] have higher incidence of CAD. Conversely, Mitchell[53] reported that towns with a higher prevalence of group O have higher rates of cardiovascular mortality and an Indian study with moderate sample size also showed O blood group is more frequent in CAD and increased the risk of CAD[54]. Further studies do not identify any association between blood type and CAD[55,56]. Based on these inconsistent results and relative small sample sizes. He[57] conducted a meta-analysis of two large, prospective studies consisting of 89501 participants, and found the highest risk of CAD was observed in blood group AB, followed by group B, A and O. This is consistent with what we know about ABO related vWF/FVIII levels with the highest in group AB, followed by group B, A and O According to this meta-analysis, non-O group has an 11% increased risk of CAD, an association not altered by adjusting for other co-morbidities. There was, however, no difference in survival and, paradoxically, a trend towards increased mortality and/or non-fatal myocardial infarction in O blood type patients.
| Ref. | Population | Sample size | Outcome(s) | Findings |
| Garrison et al[44] | United States | “Cardiovascular disease” | O showed the lowest incidence | |
| Whincup et al[45] | United Kingdom (men only) | 7662 | CAD | Individuals with A blood type has higher incidence of CAD (RR = 1.21, CI: 1.01-1.46) |
| Rosenberg et al[46] | United States (young women) | 225 MI vs 802 controls | MI | Blood group A was associated with MI |
| Lee et al[47] | Taiwan (young patients) | 136 CAD vs 129 without CAD | CAD and MI | Group A was associated with increased risk of CAD (OR = 2.61, CI: 1.11-6.14) and MI (OR = 3.53, CI: 1.21-10.29) |
| Sari et al[48] | Turkish | 476 MI vs 203 healthy control | MI | ABO blood type is not associated with development of MI |
| Carpeggiani et al[49] | Italy | 4901 | MI and CAD | Group non-O is associated with increased mortality in patients with CAD, groups A and B prevail in MI |
| Nydegger et al[50] | 177 patients vs 89 control | MI | B allele carriers had higher MI (OR = 2.7, CI: 1.1-6.8) | |
| Stakisaitis et al[51] | Lithuania | 441 | CAD | B blood group can be related with CAD in women |
| Meade et al[52] | United Kingdom | 1393 men with 178 IHDs | CAD and MI | Incidence was significantly higher in blood group AB |
| Mitchell et al[53] | United Kingdom | “Cardiovascular disease” | Towns with higher prevalence of group O have higher rate of cardiovascular mortality | |
| Biswas et al[54] | India | 250 CAD vs 250 controls | CAD | Group O increases the risk of CAD |
| Amirzadegan et al[55] | Iran | 2016 patients | CAD | No correlation |
| Biancari et al[56] | Finland | 1152 CABG patients | MI | No correlation |
| He et al[57] | United States | 89501 | Coronary heart disease | AB group has highest CAD risk, followed by groups B, A and O |
The relationship between ABO genotype and CAD has also been investigated. Wiggins et al[13] reported an 18% increased MI risk associated with A11 allele carriers compared to O1O1 homozygotes, but no other associations were found between B or AB alleles and MI, possibly due to underpowering as B and AB groups are relatively rare. An investigation of postmenopausal women suggested A or B allele carriers almost had two-fold incidence of acute ischemic heart disease compared to OO[58]. Similarly, Nydegger et al[50] showed a three-fold risk of MI with the presence of B allele (genotype AB, BB or BO) compared to non B allele (genotype OO, AO, AA) in a smaller case-control study. Another study[59] with angiography showed O1 allele carriers had a 39% decreased risk of MI compared to non O1. More obviously, von Beckerath et al[59] found a dose-dependent effect with carriage of one or two O1 alleles being associated with decreased risks of acute MI. However, a recently published study by Reilly et al[60] argued that ABO locus did not predict MI in patients with known CAD, but was strongly associated with the presence of CAD in two large genome wide association studies. Whether ABO alleles are associated with the development of MI or only the presence of CAD is not yet clearly defined. It is much easier to investigate the risk factors for CAD prevalence in a cross-sectional study than to evaluate the incidence of MI with a prospective design, as the latter requires a stable cohort with years of detailed follow-up. Currently, the association of MI and ABO blood group has only been well reported in survivors of MI events. This introduces bias, as patients may suffer an asymptomatic MI, not present at hospital, or die before diagnosis.
There are some mechanisms proposed to explain the association between ABO blood type and CAD, but a unifying theory remains elusive. Along with fibrinogen, vWF may play a role in the progression of atherosclerosis by promoting platelet aggregation and adhesion[21]. On the other hand, blood group A has been noted to have higher levels of cholesterol and low density lipoprotein[61], which may partly explain the association with an increased risk of CAD. Additionally, the ABO locus was recently reported to be associated with CAD related inflammatory makers, including intercellular adhesion molecule-1, soluble P-selectin[62], soluble E selectin[63] and tumor necrosis factor-α[53]. Still, the interactions among genetic factors (known genes increasing susceptibly to CAD and the ABO locus) and environmental factors conferring risk for CAD and MI are complicated. It is unclear which ABO phenotypes or genotypes increase CAD and/or MI risk; this risk may differ for the incidence of CAD or MI and survival following MI.
Our group performed a retrospective study to evaluate the relationship between ABO blood types and postoperative bleeding in cardiac surgical patients. This was based on the hypothesis that lower circulating vWF levels seen with group O may reduce primary hemostasis resulting in increased postoperative bleeding. While group O did have impaired baseline measures of primary hemostasis and required less heparin and protamine for perioperative anticoagulation, the result showed no difference of postoperative bleeding between different blood groups[20]. Limitations of such perioperative studies are the lack of intermediate, mechanistic measures of factor levels and the confounding effects of the acute phase response that may drown out an ABO effect. Also, the classification by phenotype is limited. For example, the A2O genotype with low vWF levels and the A1A1 genotype with high vWF levels are both classified as group A. In addition, the statistically convenient categorization into O and non-O phenotype is flawed for the same reason, blurring comparison between H antigen rich and H antigen poor genotypes that have been shown to drive the association between ABO blood type and outcome. As an alternative approach, we have preliminary results suggesting that the AB phenotype (no H antigen) requires less perioperative transfusion than non-AB phenotypes and this is associated with better postoperative survival for the rare AB group. These findings require confirmation with prospective study.
In summary, ABO blood group is an important determinant of vWF and FVIII levels which in turn confer a clear risk of increased VTE with the higher levels seen in the non-O blood types. The associations are far less clear for CAD and MI but a similar pattern emerges with most studies finding group O to be at lower risk. In terms of perioperative bleeding and transfusion, a possible reciprocal for thrombosis, further work needs to be done to determine a consistent ABO effect.
P- Reviewer: Redondo PC, Sabate M S- Editor: Song XX L- Editor: A E- Editor: Liu SQ
| 1. | Lowe JB. The blood group-specific human glycosyltransferases. Baillieres Clin Haematol. 1993;6:465-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 72] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 2. | Franchini M, Rossi C, Mengoli C, Frattini F, Crestani S, Giacomini I, Luppi M, Bonfanti C. ABO blood group and risk of coronary artery disease. J Thromb Thrombolysis. 2013;36:286-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Fang C, Cohen HW, Billett HH. Race, ABO blood group, and venous thromboembolism risk: not black and white. Transfusion. 2013;53:187-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Paulinska P, Spiel A, Jilma B. Role of von Willebrand factor in vascular disease. Hamostaseologie. 2009;29:32-38. [PubMed] |
| 5. | Dentali F, Sironi AP, Ageno W, Crestani S, Franchini M. ABO blood group and vascular disease: an update. Semin Thromb Hemost. 2014;40:49-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Rios DR, Fernandes AP, Figueiredo RC, Guimarães DA, Ferreira CN, Simões E Silva AC, Carvalho MG, Gomes KB, Dusse LM. Relationship between ABO blood groups and von Willebrand factor, ADAMTS13 and factor VIII in patients undergoing hemodialysis. J Thromb Thrombolysis. 2012;33:416-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Gill JC, Endres-Brooks J, Bauer PJ, Marks WJ, Montgomery RR. The effect of ABO blood group on the diagnosis of von Willebrand disease. Blood. 1987;69:1691-1695. [PubMed] |
| 8. | Jenkins PV, O’Donnell JS. ABO blood group determines plasma von Willebrand factor levels: a biologic function after all? Transfusion. 2006;46:1836-1844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 289] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 9. | Mohri H, Yoshioka A, Zimmerman TS, Ruggeri ZM. Isolation of the von Willebrand factor domain interacting with platelet glycoprotein Ib, heparin, and collagen and characterization of its three distinct functional sites. J Biol Chem. 1989;264:17361-17367. [PubMed] |
| 10. | Cruz MA, Yuan H, Lee JR, Wise RJ, Handin RI. Interaction of the von Willebrand factor (vWF) with collagen. Localization of the primary collagen-binding site by analysis of recombinant vWF A domain polypeptides. J Biol Chem. 1995;270:19668. [PubMed] |
| 11. | Dentali F, Sironi AP, Ageno W, Turato S, Bonfanti C, Frattini F, Crestani S, Franchini M. Non-O blood type is the commonest genetic risk factor for VTE: results from a meta-analysis of the literature. Semin Thromb Hemost. 2012;38:535-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 164] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 12. | Cambronero F, Vilchez JA, García-Honrubia A, Ruiz-Espejo F, Moreno V, Hernández-Romero D, Bonacasa B, González-Conejero R, de la Morena G, Martínez P. Plasma levels of von Willebrand factor are increased in patients with hypertrophic cardiomyopathy. Thromb Res. 2010;126:e46-e50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Wiggins KL, Smith NL, Glazer NL, Rosendaal FR, Heckbert SR, Psaty BM, Rice KM, Lumley T. ABO genotype and risk of thrombotic events and hemorrhagic stroke. J Thromb Haemost. 2009;7:263-269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Souto JC, Almasy L, Muñiz-Diaz E, Soria JM, Borrell M, Bayén L, Mateo J, Madoz P, Stone W, Blangero J. Functional effects of the ABO locus polymorphism on plasma levels of von Willebrand factor, factor VIII, and activated partial thromboplastin time. Arterioscler Thromb Vasc Biol. 2000;20:2024-2028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 139] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 15. | Moeller A, Weippert-Kretschmer M, Prinz H, Kretschmer V. Influence of ABO blood groups on primary hemostasis. Transfusion. 2001;41:56-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 88] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Shima M, Fujimura Y, Nishiyama T, Tsujiuchi T, Narita N, Matsui T, Titani K, Katayama M, Yamamoto F, Yoshioka A. ABO blood group genotype and plasma von Willebrand factor in normal individuals. Vox Sang. 1995;68:236-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 76] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Morange PE, Tregouet DA, Frere C, Saut N, Pellegrina L, Alessi MC, Visvikis S, Tiret L, Juhan-Vague I. Biological and genetic factors influencing plasma factor VIII levels in a healthy family population: results from the Stanislas cohort. Br J Haematol. 2005;128:91-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | O’Donnell J, Laffan MA. The relationship between ABO histo-blood group, factor VIII and von Willebrand factor. Transfus Med. 2001;11:343-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 204] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 19. | Sodetz JM, Pizzo SV, McKee PA. Relationship of sialic acid to function and in vivo survival of human factor VIII/von Willebrand factor protein. J Biol Chem. 1977;252:5538-5546. [PubMed] |
| 20. | Welsby IJ, Jones R, Pylman J, Mark JB, Brudney CS, Phillips-Bute B, Mathew JP, Campbell ML, Stafford-Smith M. ABO blood group and bleeding after coronary artery bypass graft surgery. Blood Coagul Fibrinolysis. 2007;18:781-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Blann AD. Plasma von Willebrand factor, thrombosis, and the endothelium: the first 30 years. Thromb Haemost. 2006;95:49-55. [PubMed] |
| 22. | Paiva SG, Sabino AP, Carvalho MG, Ribeiro DD, Gomes KB, Santos MS, Oliveira MS, Lages GG, Dusse LM, Fernandes AP. Polymorphisms in exons 6 and 7 of the ABO locus and their association with venous thrombosis in young Brazilian patients. Blood Coagul Fibrinolysis. 2009;20:122-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Bowen DJ. An influence of ABO blood group on the rate of proteolysis of von Willebrand factor by ADAMTS13. J Thromb Haemost. 2003;1:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 124] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 24. | Bonnefoy A, Daenens K, Feys HB, De Vos R, Vandervoort P, Vermylen J, Lawler J, Hoylaerts MF. Thrombospondin-1 controls vascular platelet recruitment and thrombus adherence in mice by protecting (sub)endothelial VWF from cleavage by ADAMTS13. Blood. 2006;107:955-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 136] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 25. | Bonnefoy A, Hoylaerts MF. Thrombospondin-1 in von Willebrand factor function. Curr Drug Targets. 2008;9:822-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Trégouët DA, Heath S, Saut N, Biron-Andreani C, Schved JF, Pernod G, Galan P, Drouet L, Zelenika D, Juhan-Vague I. Common susceptibility alleles are unlikely to contribute as strongly as the FV and ABO loci to VTE risk: results from a GWAS approach. Blood. 2009;113:5298-5303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 231] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 27. | de Lange M, Snieder H, Ariëns RA, Spector TD, Grant PJ. The genetics of haemostasis: a twin study. Lancet. 2001;357:101-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 206] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 28. | Orstavik KH, Magnus P, Reisner H, Berg K, Graham JB, Nance W. Factor VIII and factor IX in a twin population. Evidence for a major effect of ABO locus on factor VIII level. Am J Hum Genet. 1985;37:89-101. [PubMed] |
| 29. | Ohira T, Cushman M, Tsai MY, Zhang Y, Heckbert SR, Zakai NA, Rosamond WD, Folsom AR. ABO blood group, other risk factors and incidence of venous thromboembolism: the Longitudinal Investigation of Thromboembolism Etiology (LITE). J Thromb Haemost. 2007;5:1455-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 125] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 30. | Jick H, Slone D, Westerholm B, Inman WH, Vessey MP, Shapiro S, Lewis GP, Worcester J. Venous thromboembolic disease and ABO blood type. A cooperative study. Lancet. 1969;1:539-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 210] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 31. | Robinson WM, Roisenberg I. Venous thromboembolism and ABO blood groups in a Brazilian population. Hum Genet. 1980;55:129-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 32. | Wautrecht JC, Galle C, Motte S, Dereume JP, Dramaix M. The role of ABO blood groups in the incidence of deep vein thrombosis. Thromb Haemost. 1998;79:688-689. [PubMed] |
| 33. | Larsen TB, Johnsen SP, Gislum M, Møller CA, Larsen H, Sørensen HT. ABO blood groups and risk of venous thromboembolism during pregnancy and the puerperium. A population-based, nested case-control study. J Thromb Haemost. 2005;3:300-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 34. | Tirado I, Mateo J, Soria JM, Oliver A, Martínez-Sánchez E, Vallvé C, Borrell M, Urrutia T, Fontcuberta J. The ABO blood group genotype and factor VIII levels as independent risk factors for venous thromboembolism. Thromb Haemost. 2005;93:468-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 35. | Schleef M, Strobel E, Dick A, Frank J, Schramm W, Spannagl M. Relationship between ABO and Secretor genotype with plasma levels of factor VIII and von Willebrand factor in thrombosis patients and control individuals. Br J Haematol. 2005;128:100-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 36. | Buil A, Trégouët DA, Souto JC, Saut N, Germain M, Rotival M, Tiret L, Cambien F, Lathrop M, Zeller T. C4BPB/C4BPA is a new susceptibility locus for venous thrombosis with unknown protein S-independent mechanism: results from genome-wide association and gene expression analyses followed by case-control studies. Blood. 2010;115:4644-4650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 37. | Sode BF, Allin KH, Dahl M, Gyntelberg F, Nordestgaard BG. Risk of venous thromboembolism and myocardial infarction associated with factor V Leiden and prothrombin mutations and blood type. CMAJ. 2013;185:E229-E237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 38. | Soria JM, Almasy L, Souto JC, Buil A, Martinez-Sanchez E, Mateo J, Borrell M, Stone WH, Lathrop M, Fontcuberta J. A new locus on chromosome 18 that influences normal variation in activated protein C resistance phenotype and factor VIII activity and its relation to thrombosis susceptibility. Blood. 2003;101:163-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 39. | Schwarz HP, Lenting PJ, Binder B, Mihaly J, Denis C, Dorner F, Turecek PL. Involvement of low-density lipoprotein receptor-related protein (LRP) in the clearance of factor VIII in von Willebrand factor-deficient mice. Blood. 2000;95:1703-1708. [PubMed] |
| 40. | O’Donnell J, Tuddenham EG, Manning R, Kemball-Cook G, Johnson D, Laffan M. High prevalence of elevated factor VIII levels in patients referred for thrombophilia screening: role of increased synthesis and relationship to the acute phase reaction. Thromb Haemost. 1997;77:825-828. [PubMed] |
| 41. | Oger E, Lacut K, Van Dreden P, Bressollette L, Abgrall JF, Blouch MT, Scarabin PY, Mottier D. High plasma concentration of factor VIII coagulant is also a risk factor for venous thromboembolism in the elderly. Haematologica. 2003;88:465-469. [PubMed] |
| 42. | Vormittag R, Bencur P, Ay C, Tengler T, Vukovich T, Quehenberger P, Mannhalter C, Pabinger I. Low-density lipoprotein receptor-related protein 1 polymorphism 663 C & gt; T affects clotting factor VIII activity and increases the risk of venous thromboembolism. J Thromb Haemost. 2007;5:497-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 43. | Gándara E, Kovacs MJ, Kahn SR, Wells PS, Anderson DA, Chagnon I, Le Gal G, Solymoss S, Crowther M, Carrier M. Non-OO blood type influences the risk of recurrent venous thromboembolism. A cohort study. Thromb Haemost. 2013;110:1172-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 44. | Garrison RJ, Havlik RJ, Harris RB, Feinleib M, Kannel WB, Padgett SJ. ABO blood group and cardiovacular disease: the Framingham study. Atherosclerosis. 1976;25:311-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 45. | Whincup PH, Cook DG, Phillips AN, Shaper AG. ABO blood group and ischaemic heart disease in British men. BMJ. 1990;300:1679-1682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 46. | Rosenberg L, Miller DR, Kaufman DW, Helmrich SP, Van de Carr S, Stolley PD, Shapiro S. Myocardial infarction in women under 50 years of age. JAMA. 1983;250:2801-2806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 47] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 47. | Lee HF, Lin YC, Lin CP, Wang CL, Chang CJ, Hsu LA. Association of blood group A with coronary artery disease in young adults in Taiwan. Intern Med. 2012;51:1815-1820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 48. | Sari I, Ozer O, Davutoglu V, Gorgulu S, Eren M, Aksoy M. ABO blood group distribution and major cardiovascular risk factors in patients with acute myocardial infarction. Blood Coagul Fibrinolysis. 2008;19:231-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 49. | Carpeggiani C, Coceani M, Landi P, Michelassi C, L’abbate A. ABO blood group alleles: A risk factor for coronary artery disease. An angiographic study. Atherosclerosis. 2010;211:461-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 50. | Nydegger UE, Wuillemin WA, Julmy F, Meyer BJ, Carrel TP. Association of ABO histo-blood group B allele with myocardial infarction. Eur J Immunogenet. 2003;30:201-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 51. | Stakisaitis D, Maksvytis A, Benetis R, Viikmaa M. [Coronary atherosclerosis and blood groups of ABO system in women (own data and review)]. Medicina (Kaunas). 2002;38 Suppl 2:230-235. [PubMed] |
| 52. | Meade TW, Cooper JA, Stirling Y, Howarth DJ, Ruddock V, Miller GJ. Factor VIII, ABO blood group and the incidence of ischaemic heart disease. Br J Haematol. 1994;88:601-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 176] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 53. | Mitchell JR. An association between abo blood-group distribution and geographical differences in death-rates. Lancet. 1977;1:295-297. [PubMed] |
| 54. | Biswas S, Ghoshal PK, Halder B, Mandal N. Distribution of ABO blood group and major cardiovascular risk factors with coronary heart disease. Biomed Res Int. 2013;2013:782941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 55. | Amirzadegan A, Salarifar M, Sadeghian S, Davoodi G, Darabian C, Goodarzynejad H. Correlation between ABO blood groups, major risk factors, and coronary artery disease. Int J Cardiol. 2006;110:256-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 56. | Biancari F, Satta J, Pokela R, Juvonen T. ABO blood group distribution and severity of coronary artery disease among patients undergoing coronary artery bypass surgery in Northern Finland. Thromb Res. 2002;108:195-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 57. | He M, Wolpin B, Rexrode K, Manson JE, Rimm E, Hu FB, Qi L. ABO blood group and risk of coronary heart disease in two prospective cohort studies. Arterioscler Thromb Vasc Biol. 2012;32:2314-2320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 139] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 58. | Roest M, Voorbij HA, Barendrecht AD, Peeters PH, van der Schouw YT. Risk of acute ischemic heart disease in postmenopausal women depends on von Willebrand factor and fibrinogen concentrations, and blood group genotype. J Thromb Haemost. 2007;5:189-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 59. | von Beckerath N, Koch W, Mehilli J, Gorchakova O, Braun S, Schömig A, Kastrati A. ABO locus O1 allele and risk of myocardial infarction. Blood Coagul Fibrinolysis. 2004;15:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 60. | Reilly MP, Li M, He J, Ferguson JF, Stylianou IM, Mehta NN, Burnett MS, Devaney JM, Knouff CW, Thompson JR. Identification of ADAMTS7 as a novel locus for coronary atherosclerosis and association of ABO with myocardial infarction in the presence of coronary atherosclerosis: two genome-wide association studies. Lancet. 2011;377:383-392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 433] [Cited by in RCA: 400] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 61. | George VT, Elston RC, Amos CI, Ward LJ, Berenson GS. Association between polymorphic blood markers and risk factors for cardiovascular disease in a large pedigree. Genet Epidemiol. 1987;4:267-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 62. | Barbalic M, Dupuis J, Dehghan A, Bis JC, Hoogeveen RC, Schnabel RB, Nambi V, Bretler M, Smith NL, Peters A. Large-scale genomic studies reveal central role of ABO in sP-selectin and sICAM-1 levels. Hum Mol Genet. 2010;19:1863-1872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 203] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 63. | Qi L, Cornelis MC, Kraft P, Jensen M, van Dam RM, Sun Q, Girman CJ, Laurie CC, Mirel DB, Hunter DJ. Genetic variants in ABO blood group region, plasma soluble E-selectin levels and risk of type 2 diabetes. Hum Mol Genet. 2010;19:1856-1862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 133] [Article Influence: 8.9] [Reference Citation Analysis (0)] |