Published online Sep 26, 2014. doi: 10.4330/wjc.v6.i9.968
Revised: June 17, 2014
Accepted: July 14, 2014
Published online: September 26, 2014
Processing time: 264 Days and 1.2 Hours
Systemic vascular disease, especially hypertension, has been suspected as a risk factor for some eye diseases including, diabetic retinopathy and age-related macular degeneration. Hypertension can contribute to chronic diseases by hemodynamic injury and/or cellular actions induced by hypertension-related hormones or growth factors. Among the most important is Angiotensin II (Ang II), which controls blood pressure and induces different cellular functions that may be dependent or independent of its effect on blood pressure. Importantly, as is true for heart, kidney and other organs, the renin-angiotensin system (RAS) is present in the eye. So, even in the absence of hypertension, local production of Ang II could be involved in eye diseases. The goal of this manuscript is to review the most relevant scientific evidence supporting the role of the RAS activation, in the development of age-related macular degeneration and diabetic retinopathy, and highlight the importance of Ang II in the etiology of these diseases.
Core tip: Association between eye diseases and systemic hypertension has been revealed. The developments of some ocular diseases, as well as, alterations in the severity of these diseases have been associated with disregulation of the ocular renin-angiotensin system and activation of the angiotensin type 1 receptor. In this paper we reviewed the importance of angiotensin II in the etiology of age-related macular degeneration and diabetic retinopathy, two ocular diseases that can rob people of their vision.
- Citation: Marin Garcia PJ, Marin-Castaño ME. Angiotensin II-related hypertension and eye diseases. World J Cardiol 2014; 6(9): 968-984
- URL: https://www.wjgnet.com/1949-8462/full/v6/i9/968.htm
- DOI: https://dx.doi.org/10.4330/wjc.v6.i9.968
Knowledge of the renin-angiotensin system (RAS) has advanced remarkably over recent years from that of a classical endocrine system that explained homeostasis for maintenance of circulating intravascular volume and thereby restoration of arterial pressure to a newer concept including a number of local RASs that operate independently within several organs[1-5], including the eye[6,7].
Angiotensin II (Ang II), a hormone that raises blood pressure, is derived either from the circulation or from local production. Ang II causes vasoconstriction, sympathetic nervous stimulation, release of aldosterone, and renal actions which contribute to control the blood pressure[8]. The effects of Ang II provoke different responses in tissue, which are mostly mediated via the Ang II type 1 receptor (AT1R). According to previous studies, the systemic RAS is not supposed to be directly accountable for the increase in blood pressure, it appears to be that the blood pressure and local blood flow (BF) adjustment are due to the local RASs[9]. Ang II directly or indirectly also promotes apoptosis, hypertrophy, neovascularization, inflammation and fibrosis via AT1R activation[10-13].
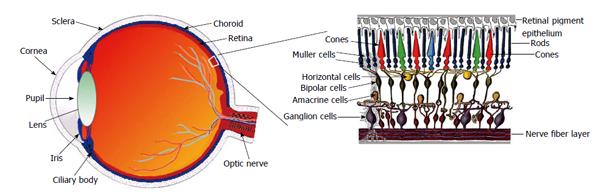
Ophthalmic literature concerning the RAS started in 1977 with a study by Igić et al[14] on the detection of angiotensin-converting-enzyme (ACE) activity in homogenates of the retina. Since then, and as shown in Table 1, the presence of all constituent of the RAS has been confirmed in different parts of the eye (Figure 1), where the mediators of the RAS are locally released, conferring the molecular basis for a biological function of these mediators in the eye[15-18] . However, the origin of intraocular mediators such as Ang II and renin has been debated. Local synthesis of both renin and ACE has been suggested in the retina of rats[19]. In this way, the secretion of renin by retinal pigment epithelium (RPE) to the retinal side was demonstrated by Milenkovic et al[20] (2010). It has been also suggested that Ang I, Ang II, and angiotensinogen are not able to cross the barriers between eye and circulating blood[21,22]. On the other hand, the presence of a ocular local production of Ang II has been indicated[22,23]. As a result, increased local or tissue Ang II formation in the retina in the absence of elevated circulating Ang II may indeed be deleterious.
| RAS molecule | Eye part | Species | Ref. |
| Prorenin | Retina | Human | Sramek et al[207], 1988 |
| Ciliary body | Human | Danser et al[33], 1989 | |
| Vitreous body | Human | Danser et al[33], 1989 | |
| Retina | Retina | Human, rabbit | Danser et al[33], 1989 |
| Ciliary body | Rabbit | Wagner et al[19], 1996 | |
| Choroid | Human, Rabbit | Ramirez et al[208], 1996 | |
| Iris | Rabbit | Ramirez et al[208], 1996 | |
| Vitreous | Human, rabbit | Ramirez et al[208], 1996 | |
| Aqueous humor | Rabbit | Ramirez et al[208], 1996 | |
| Angiotensinogen | Retina | Human, rabbit | Sramek et al[209], 1992 |
| Ciliary body | Human, rabbit | Ramirez et al[208], 1996 | |
| Choroid | Human, rabbit | Wagner et al[19], 1996 | |
| Iris | Human, rabbit | Wagner et al[19], 1996 | |
| Vitreous | Human, rabbit | Wagner et al[19], 1996 | |
| Aqueous humor | Rabbit | ||
| ACE1 | Retina | Dog, monkey, human | Vita et al[210], 1981 |
| Rabbit, porcine | Weinreb et al[211], 1985 | ||
| Ciliary body | Human, rabbit, porcine | Immonen et al[212], 1987 | |
| Choroid | Dog, monkey, human | Ramirez et al[208], 1996 | |
| Rabbit, porcine | Wagner et al[19], 1996 | ||
| Sclera | Dog, monkey | Shiota et al[213], 1997 | |
| Iris | Rabbit, porcine | Geng et al[214], 2003 | |
| Cornea | Human | Savaskan et al[16], 2004 | |
| Vitreous | Dog, monkey, rabbit | Savaskan et al[16], 2004 | |
| Aqueous humor | Human, dog, monkey, rabbit | Savaskan et al[16], 2004 | |
| Tear fluid | Human, rabbit | Savaskan et al[16], 2004 | |
| ACE2 | Retina | Rodent | Tikellis et al[215], 2004 |
| Human | Senanayake et al[17], 2007 | ||
| Chymase | Choroid | Dog | Shiota et al[213], 1997 |
| Sclera | Dog | Maruichi et al[216], 2004 | |
| Vitreous body | Human | ||
| AT1R | Retina | Human | Savaskan et al[16], 2004 |
| Cornea | Human | Senanayake et al[17], 2007 | |
| RPE | Human | Striker et al[18], 2008 | |
| Rodent | Praddaude et al[104], 2009 | ||
| AT2R | Retina | Human | Senanayake et al[17], 2007 |
| RPE | Human | Striker et al[18], 2008 | |
| Rodent | Praddaude et al[104], 2009 | ||
| Ang I | Retina | Porcine | Danser et al[22], 1994 |
| Choroid | Porcine | ||
| Vitreous body | Porcine, human | ||
| Aqueous humor | Human | ||
| Ang II | Retina | Human, porcine, rabbit | Danser et al[22], 1994 |
| Ciliary body | Human, rabbit | Ramirez et al[208], 1996 | |
| Choroid | Porcine, human, rabbit | Savaskan et al[16], 2004 | |
| Iris | Rabbit | Senanayake et al[17], 2007 | |
| Cornea | Human | ||
| Vitreous body | Porcine, human, rabbit | ||
| Aqueous humor | Human, rabbit | ||
| RPE | Rodent | Praddaude et al[104], 2009 | |
| Ang 1-7 | Retina | Human | Senanayake et al[17], 2007 |
The RPE, a cell layer between the neurosensory retina and choroid, nourishes retinal visual cells and forms part of the blood-retinal barrier, therefore, playing a central role in maintaining retinal function. For example, the presence of the AT1R in the RPE basolateral membrane[20], indicates that the systemic RAS is a part of that retinal function signaling. Interestingly, by using electroretinography, it was previously demonstrated that regulation of the systemic RAS changes the neuro-sensory retina activity[24-26]. Furthermore, plasma Ang II cannot pass into the eye[7], and modifications of the renin expression in the RPE by regulators of the systemic RAS alter, have been observed[24]. Overall, these data lead to think the systemic RAS credits the presence of an intraocular RAS through the RPE.
The presence of the most important RAS components in the retina and the Ang II actions observed in the eye (Surveying PubMed for eye, ocular, or retina, and Ang yields 734 citations dating back to 1963), imply an important role of RAS in the eye. However, its exact role, remains inadequately recognized. Of special focus are the components of the RAS and its receptors in the retina, as the RAS is increasingly recognized as a mediator of the pathogenesis of ocular diseases such as age-related macular degeneration (AMD) and diabetic retinopathy (DR)[27-36], which are two major causes of severe vision loss and blindness. Therefore, in this manuscript we review the most relevant scientific evidence supporting the function of the RAS activation, in the development of AMD and DR, and highlight the importance of Ang II in the etiology of these two ocular diseases.
Given that vascular pathology in the retina is an important contributor of vision loss, the greatest research examining retinopathy and the possible role played by the RAS has been focused on the microvasculature. The circulatory system of the retina supplies oxygen and nutrients to retinal tissue, which is essential for a correct function.
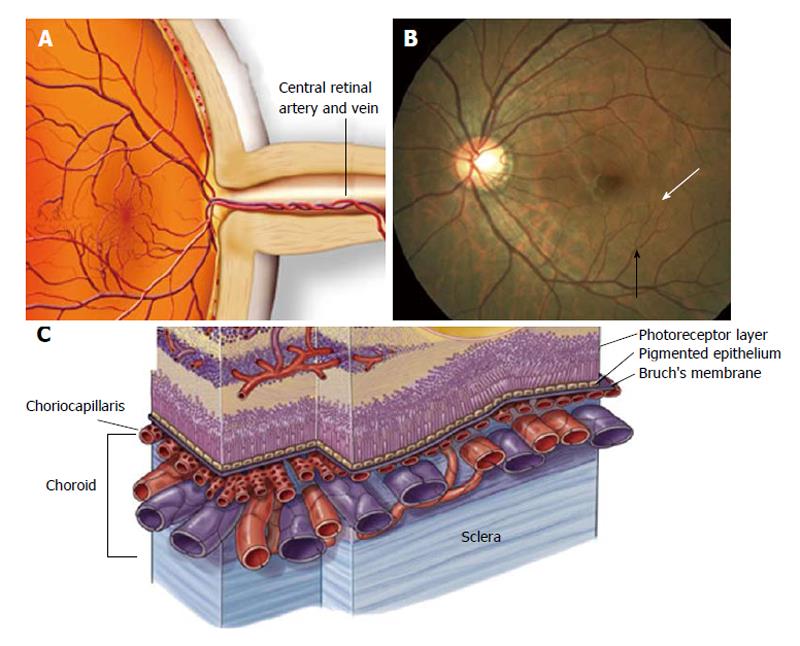
The retina circulation essentially comprises two parts: (1) a retinal circulation without autonomic innervation; and (2) a choroidal vasculature with autonomic innervations[37]. Evidence is accumulating that the retinal microvasculature is an interactive complex that includes a network of capillaries and a tertiary arteriole that links the capillaries with a secondary arteriole (Figure 2). The capillary is formed by an uninterrupted endothelium and inner pericytes[38]. Both endothelial cells and pericytes are directly communicated and share a common basement membrane[39]. It was previously demonstrated that contraction and relaxation of pericytes leads to alterations in the capillary lumen, which could regulates local perfusion[40-45]. Moreover, evidence suggests that a capillary network including pre-capillary at the tertiary arteriole form a working unit which is able to control local perfusion within the retinal vessels[39,46,47].
The retina tends to keep its BF constant through an autoregulatory response that is intrinsic[48,49]. The utoregulation of the retinal microcirculation is evaluated by some methods, including changes in systemic blood pressure[50]. The main regulators of BF are the vascular perycites[51,52], endothelium cells and the neural and glial cells[53]. One of the most important peptides playing a crucial role in the regulation of vasculature tone is Ang II[54-58]. For instance, it has been demonstrated that Ang II induces retinal endothelial cells apoptosis[59] and constriction of pericytes[60-63], therefore, decreasing the mean retinal arterioles and capillaries diameter, which leads to BF reduction[51,52].
Modifications in the retinal BF has been observed in some eye disorders. For example disturbances in the ocular circulation have been reported in AMD[31-33], supporting the presence of hemodynamic abnormalities in this disease. AMD is the main cause of severe visual loss and legal blindness in elderly. There are three stages of AMD: (1) early AMD, which is diagnosed by the presence of medium-sized drusen: (2) intermediate AMD, characterized by the presence of large drusen and/or pigment changes in the retina: and (3) late AMD, in which in addition to drusen, there is damage of the macula with severe vision loss[64]. Both local ocular and systemic vascular risk factors, such as systemic hypertension seem to be connected with the etiology of AMD. A relationship between AMD and modifications in the eye circulation was previously reported[27,29,65-72] and numerous studies have proposed a decrease in the vascularity of the choroid[73-75], reinforcing the existence of hemodynamic abnormalities in this disease. The relationship between impaired choroidal perfusion, reduced choroidal BF and clinical manifestations of AMD has been recently reported by previous studies[70,71,75-79].
Association between AMD and systemic hypertension has been studied by many epidemiological studies[80-84]. The Macular Photocoagulation Study has demonstrated that patients with both, AMD and hypertension responded less to laser photocoagulation treatment than patients with only AMD[85]. These observations, suggested that hypertension could have a harmful effect on the stages of AMD. A decrease in the choroidal BF in individuals with hypertension versus those without was previously reported[31,32]. These authors, also showed that this reduction becomes more marked with increasing AMD severity[31,32]. Therefore, the observed decrease in choroidal BF in AMD patients with hypertension suggests the implication of an ischemic mechanism in the etiology of AMD.
AMD is a slow progressing disease that can rob people of their vision. This ocular disease is a public health problem that will remain a major threat to vision.
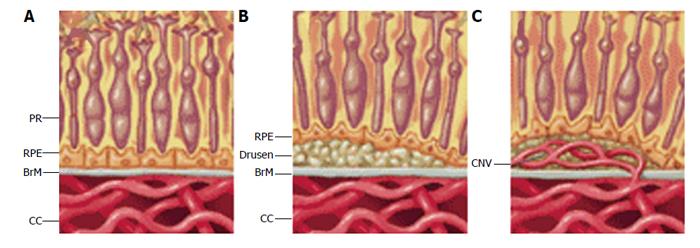
There are two forms of AMD; early (dry) AMD and late (wet) form. Wet AMD is always preceded by early disease, and in about one-third of cases dry AMD can lead to wet macular degeneration which progresses much more rapidly and leads to greater loss of central vision. Death of photoreceptors is the ultimate cause of vision loss. However, the initial cellular target of this deseade is the RPE, its extracellular matrix, and the subjacent vascular bed (called choriocapillaris; Figure 2C), the blood supply for the outer retina.
Dry AMD is characterized by the accumulation of debris and other lipid rich extracellular deposits in form of drusen under the RPE and within Bruch’s membrane (BrM) (Figure 3B)[86,87]. During aging, deposits initially accumulate between the RPE and its basement membrane (called BLD), but progression into AMD requires additional deposit formation within BrM, (called BLiD and “nodular” drusen). These are yellowish lesions that can be seen in the macula at the earliest stages of dry AMD. A finding in dry AMD that represents disease progression and can be used as a surrogate endpoint is the presence, size, and appearance of drusen. Over time, these drusen enlarge, coalesce, become pigmented, and eventually can disappear when they progress to the late form of AMD. We observed that when drusen go away, there are three possible outcomes; formation of geographic atrophy, formation of abnormal blood vessels known as wet AMD or choroidal neovascularization (CNV) (Figure 3C), or disappearance of drusen without any significant anatomic abnormality. The endpoint that represents the progression of the disease is the growth and enlargement rate of drusen[88-90]. Wet AMD is always preceded by early disease.
Our understanding of this disease has increased; however, no one knows exactly what causes AMD. Age is the major factor determinant for developing AMD. However, it has been suggested that the disease results from some interactions between different issues: genetic susceptibility, environmental factors and systemic health co-factors[91-95]. Because the increasing frequency of hypertension, the RAS is of special interest among these systemic health co-factors. In this context, epidemiological demonstrated an association between hypertension and incidence of drusen[28] and with wet AMD development[29,96-98]. Exciting findings which showed a strong link between hypertension and progression of early AMD to the wet form were recently published[99]. However, the mechanism(s) by which hypertension contribute to the progression from early form to CNV was not elucidated. In recent years, evidence has revealed that Ang II, AT1R signaling, and prorenin, may play a significant role in the mentioned pathologic processes[100-104]. Moreover, recent studies revealed the participation of AT2R, Ang I and Ang 1-7[24]. Consequently, investigation of the local RAS in the retina will allow find out new approach for the development of new treatments.
As mentioned previously, RPE-derived debris and other debris accumulated between the RPE and within BrM is a very well-known histopathologic sign of the dry AMD[105-108]. Studies in eyes from AMD patient found out deposits of RPE-derived debris within BrM[109]. Nevertheless, the mechanism(s) by which the debris accumulate were not studied. Based in the idea that a relationship between matrix metalloproteinases (MMPs) and inhibitors of matrix metalloproteinases and development of dry AMD exits. We proposed that the evolution of the sub-RPE deposits into BrM necessitates breakdown of the RPE basement membrane’s components by digestion or degradation of these compounds (i.e., type IV and I collagens and laminin)[110,111], and that ECM turnover up-regulation through activation of MMP-2 and MMP-14 is required for the interruption of these physical barriers. We evaluated the regulatory effects of Ang II and prorenin-activated prorenin receptor (PRR) on the MMP-2 and basement membrane component proteins, in the RPE. The objective of our work was to describe the expression and function of Ang II receptors in the RPE and at explore the contribution of this hormone and PRR in the etiology of dry AMD. Mice were rendered hypertensive either by exogenous administration of Ang II or by using a model of experimental renovascular hypertension (1K1C). Measurements of systolic blood pressure (BP) revealed a progressive increase during Ang II infusion period reaching a peak value on day 14 and remaining at plateau through day 30. However, after 24 h of exposure to Ang II, BP was not modified. Similarly, BP was significantly higher in 1K1C mice compared with the corresponding sham-operated group. No significant differences in BP were observed between control and sham-operated groups. Treatment using Ang II in combination with angiotensin receptors blockers showed that the AT1R blocker eliminated the modifications in the BP due to Ang II. However, the AT2R blocker did not alter the effect of Ang II on systolic BP, demonstrating, that the effect on BP caused by Ang II was AT1R mediated[104,112].
Our study in human and mouse also confirmed that both ATRs were expressed and upregulated by Ang II in the RPE and showed that the activation of the AT1R by Ang II increased the intracellular calcium levels[18,105]. These results clearly evidenced the functionality of the RPE’s AT1R, which could be coupled to the phospholypase C-pathway. In contrast, activation of the AT2R by Ang II did not mobilize intracellular calcium. AT2R could be coupled to the cytosolic phospholipase A2 and not to the PLC pathway as shown for other tissues[113]. Consequently, regulation of the AT2R transduction pathway is a possibility to be explored.
Ang II also up-regulated the activity of MMP-2, MMP-14, and basigin (also known as extracellular matrix metalloproteinase inducer or cluster of differentiation 147) as well as digestion of type IV collagen[18,104,112]. The Ang II observed effects were blocked by the AT1R antagonist candesartan. In vivo, the Ang II-derived decrease in collagen IV was AT1R/AT2R mediated, implying a synergistic effect. Therefore, Ang II through MMP-2, MMP-14, and basigin regulation could stimulate RPE basement membrane breakdown allowing the migration of BLD and buildup of BLiD deposits or drusen.
It is important to note that the majority of intracellular effects of Ang II in most tissues are MAPKs mediated. MAPKs are a group of serine/threonine kinases[114-116] which can be divided into three major groups: ERK, p38, and Jun N-terminus kinase (JNK) and participate in a wide array of cellular responses including proliferation, differentiation, migration, and stress responses among others[117-120]. We explored the involvement of MAPK as intracellular modulator of Ang II-induced up-regulation of MMPs in the RPE. Our study showed that Ang II-induced increase in MMP-2 activity is mediated by ErK(1/2) and p38 MAPK in the human RPE cell line ARPE-19. We also demonstrate that Ang II increased the expression of MMP-14, MMP-2 activity major regulator, in an ErK(1/2) and p38 MAPK-dependent way while basigin does not appear to be involved in RPE cells. In addition, we reported that ErK/p38 MAP kinase signaling pathway is AT1R mediated, which could be an important mechanism by which Ang II up-regulates MMPs in RPE cells. Moreover, we show that RPE from mice exposed to Ang II for 4 wk showed increased MMP-14 and basigin protein expression as well as increased phosphorylated ErK(1/2), p38, and JNK MAPK. The increase in MMP-14 protein expression and activation of ErK(1/2), p38, and JNK MAPK were AT1 receptor-mediated, whereas the increase in basigin expression increase was mediated by AT2 receptor[112]. Blockade of extracellular signal-regulated kinases or p38 MAPK abolished the up-regulation of MMPs in RPE cells[112]. Given that MMP-14 and basigin are major inducers of MMP-2, our results lead us to speculate that MMP-14 and basigin might regulate Ang II-induced MMP-2 activity through MAPKS- and AT1 receptor-dependent signaling pathways in the RPE. These original observations highlight the potential importance of this signaling pathway as a potential mediator of RPE response to Ang II-induced ECM dysregulation and disruption of the RPE basement membrane believed to be involved in sub-RPE deposits progression in the pathogenesis of AMD. Based on our observations, MAPKs inhibitors and AT1R blockers may prevent these changes in the ECM, which are essential in the development of early AMD.
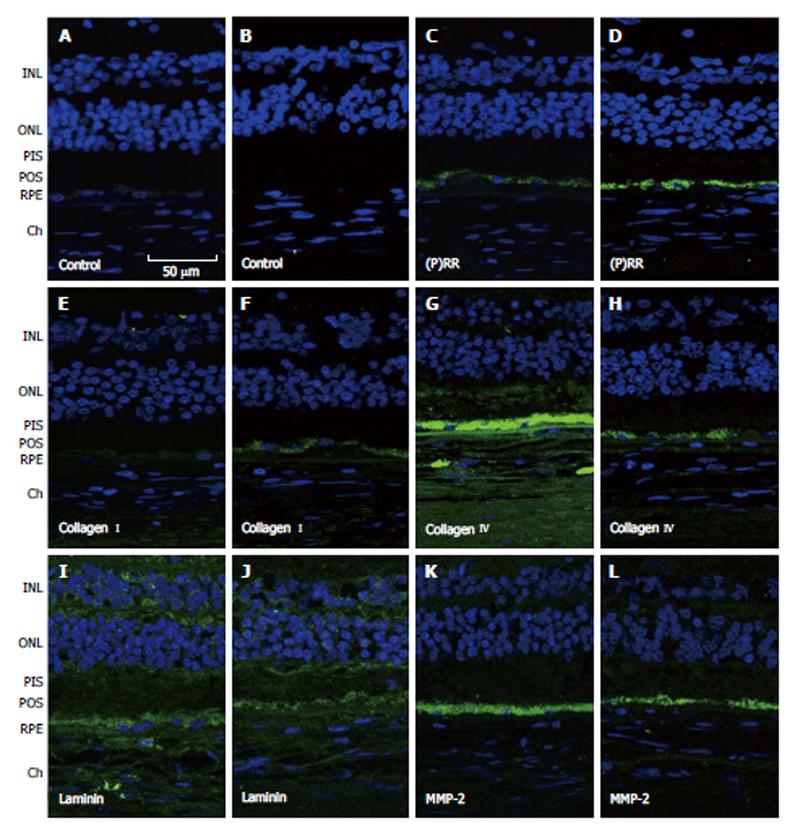
We also provided evidence that activation of the PRR may be involved in ECM-remodeling through increase of collagen I[121]. Interestingly, we confirmed that PRR and type I collagen were present in human retinas and that the expression of both proteins was higher in the RPE from dry AMD hypertensive donors (Figure 4), supporting our in vitro findings. Overall, our studies suggest a molecular mechanism by which hypertension may aggravate the pathology of dry AMD.
Even though dry AMD is not a retinal vascular pathology, we reviewed this form of the disease here because hypertension-related Ang II has been implicated in dry AMD pathogenesis[28], and wet AMD is always preceded by the early form of the disease.
As mentioned previously, about one-third of cases dry AMD can lead to wet macular degeneration which progresses much more rapidly and leads to loss of central vision. CNV is a retinal vasculature related pathology[120] associated with several common retinal degenerative or inflammatory diseases[87,120,122,123]. Inflammation and hypoxia are key cellular processes involved in the development of CNV[17-25], in that choroidal monocytes processes, for example, have been noted to insert into BrM deposits suggesting that these sub-RPE deposits may generate inflammatory stimulus at the BrM and sub-RPE space. Macrophage infiltration to the damaged sites by chemotactic factors may be responsible for the production of inflammatory cytokines and angiogenic factors such as intercellular adhesion molecule 1 (ICAM-1) and monocyte chemoattractant protein-1 (MCP-1)[124] and vascular endothelial growth factor (VEGF)[125] which will ultimately contribute to induction and/or progression of CNV[26-28]. Blockade of AT1R by systemic administration of telmisartan reduced CNV formation, macrophage infiltration and expression of VEGF, VEGF receptor-2 (VEGFR-1), ICAM-1 MCP-1 and interleukin 6 in eyes from a laser induced CNV mouse model of AMD[125]. This suggests that AT1R mediated up-regulation of these molecules and mediators participate in the development of CNV.
Ang II has been shown to act as an indirect mitogenic agent for retinal vascular endothelial cells by increasing VEGFR-2 expression[23] which could lead to formation of CNV. Blockade of AT1R signaling suppresses pathologic but not normal retinal neovascularization by inhibiting inflammatory processes[34,116]. Additionally, it has been shown that excised choroidal neovascular membranes from patients with AMD express AT1R, AT2R and Ang II on the vascular endothelium[126]. Similar findings were seen in the laser-induced mouse model of CNV[126]. As noted above, formation of CNV was suppressed with the AT1R blocker telmisartan but not with an AT2R antagonist[127]. In a laser induced model of CNV using AT1R knockout mice, the ACE inhibitor, imidapril, significantly reduced choroidal and retinal neovascularization in wild type mice to levels detected in laser treated AT1R KO mice[128]. Additionally, in a rat model of laser-induced CNV, losartan was shown to inhibit the incidence of new vessel formation from 99.5% to 72.5%[129].
Increasing evidence support the notion that increase in the production of of chemokines happens in diseases related to an inflammatory component. Several of these chemokines are expressed in the RPE cells, including MCP-1[29,30], which has been proposed to be implicated in the development of dry and wet AMD[31-33]. During inflammatory responses, RPE cells have been shown to secrete MCP-1 toward the choroid, consequently, implying that RPE cells might induce recruitment of macrophage to the choroid[34]. There is clear evidence for the role of MCP-1 in angiogenesis in several angiogenic-related disorders[35-37]. Interestingly, expression of the recently discovered novel zinc finger protein MCP-1 induced protein (MCPIP) has been shown to induce tube formation in human umbilical vein endothelial cells[38].
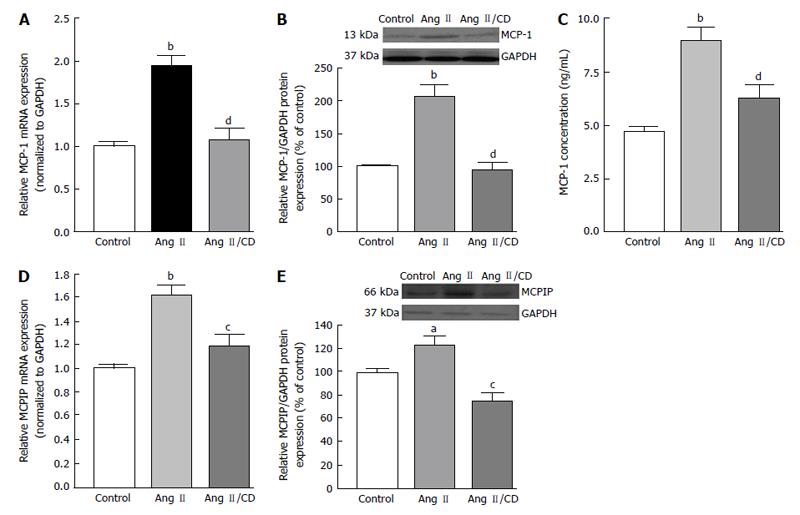
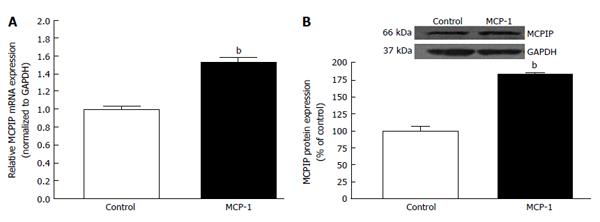
As mentioned previously, hypoxia, which was proposed to be one of the most significant driving forces for CNV formation[130], is another key cellular process which stimulates the expression of VEGF in AMD. Angiogenic factor expression occurring secondary to hypoxia is mediated by the family of transcription regulators know as hypoxia inducible factors (HIF). HIF-1 and -2 have been found to be expressed in human choroidal neovascular membranes[131], and HIF-1 has been shown to upregulate expression of VEGF in RPE[132,133]. Hypertension-associated Ang II is known to induce inflammation, macrophage infiltration, and angiogenesis by stimulating expression of MCP-1, HIF-1 and VEGF through the AT1R[126,134-137]. Up-regulation of MCP-1 has been demonstrated in hypoxic animals[138] and recently, it has been demonstrated that MCP-1 promotes angiogenesis via MCPIP, HIF-1 and VEGF induction[139]. Interestingly, previous works also suggest that the BF in the choroidal and retinal is down-regulated in AMD hypertensive patients[31,32], which leads to think about the possibility that an ischaemic/hypoxia mechanism plays a role in the CNV development. Given that a positive correlation between elevated levels of circulating MCP-1 and hypertension has been previously shown, we studied whether hypertension-induced Ang II influences the development of CNV and characterized the role played by MCP-1/MCPIP in this event. We addressed this by setting goals of understanding the mechanisms underlying the interactions between the RPE, choroidal microvascular endothelial cells (cEC) and Ang II which may contribute to CNV development in hypertensive dry AMD patients.
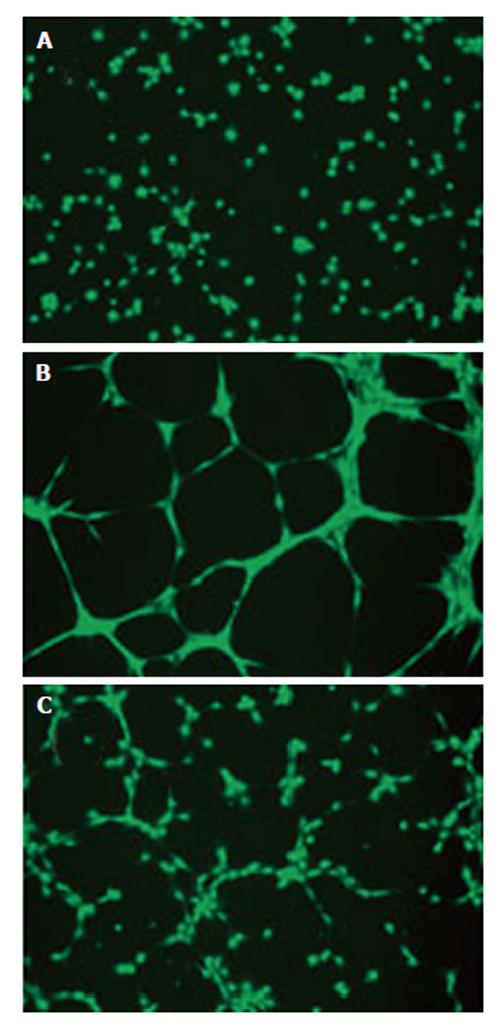
Our results indicated that hypertension-induced Ang II increases MCP-1 and MCPIP expression in mouse RPE-choroid through AT1 receptor. In vitro, MCP-1 and MCPIP expression was up-regulated by Ang II in RPE cells. Moreover, MCP-1 induced expression of MCPIP in RPE cells, which led to cEC tube formation (Figures 5-7) (Marin-Castano et al[140] IOVS 2013; ARVO E-Abstract 6089). Therefore, our data support the hypothesis that Ang II, through MCP-1/MCPIP may contribute to CNV, proposing a possible mechanism linking hypertension and CNV, which can provide new targets for more effective early preventive and novel therapeutic interventions.
The incidence of DR is alarming. A recent study emphasizes that 93 million people have DR, and that about 17 million have the blinding form of the disease[141]. Patients with type 1 or type 2 diabetes are at risk for the development of DR. The longer a person has diabetes, the more likely they are to develop DR[142]. DR is classified into two types: (1) non-proliferative DR (NPDR), the early state of the disease. In NPDR, the blood vessels in the retina are weakened causing tiny bulges called microanuerysms. The microanuerysms may leak fluid into the retina, which may lead to swelling of the macula: and (2) proliferative DR (PDR), which is the more advanced form of the disease. At this stage, the retina becomes oxygen deprived. New blood vessels can start to grow in the retina and into the vitreous causing clouding vision. If left untreated, PDR can cause severe vision loss and even blindness[143]. The progression to PDR looks like to be a result of tissue ischemia and the consequent increase in the production of angiogenic growth factors such as VEGF.
The report that some components of the RAS are augmented in blood and eyes from DR patients[46,144,145], suggests the RAS may be implicated in the pathogenesis of DR[28]. An increase of angiotensinogen, ACE, ACE2, and AT1R in retinas from diabetic animals was described previously[35,146,147]. Up to now, research addressed to find a link between the RAS and retinopathy has been based on the retinal microvasculature. Strong evidence supporting a role of Ang II in pericytes and endothelial cells in the retinal microvasculature has been shown. Ang II has a mitogenic effect on retinal endothelial cells[23,59,148]. This peptide also decreases the expression of pigment epithelium derived growth factor[148] and enhances proliferation of endothelial cells in retina through VEGF up-regulation[23,149]. Moreover, glucose ingestion by the retinal tissue might be instantly regulated by Ang II[150,151]. This increase in glucose in turn could induce VEGF expression and potentates the effect of Ang II on VEGF expression as demonstrated previously in vascular smooth muscle cells[152]. Since it is clear that reactive oxygen species (ROS) contribute to cellular damage in DR by inducing VEGF[153,154], and that both Ang II and high glucose can lead to ROS formation,[155,156] ROS may be a common pathway linking a synergistic effect between Ang II and high glucose on the activation of VEGF.
The actions of Ang II on the retinal vasculature have been well described in pericytes. These microvascular cells are incriminated in the regulation of capillary tone[157], and it has been suggested they have other extra roles such as preservation of microvascular homeostasis[149]. For instance, death of pericytes has been linked to the initial sign of DR. It has been reported that Ang II uncouples pericytes from the vasculature[48,158]. Studies in vitro have shown activation of pericyte migration by Ang II through the AT1R[159,160]. Moreover, Ang II also has an effect on pericyte viability, by increasing apoptosis[33,59]. Therefore, it is evident that Ang II impacts the retinal microvasculature. Research in diabetic animals showed a reduction in the retinal microvascular injury by exposure to ACE inhibitors and AT1R blockers. These data revealed a decrease in the vascular leakage, acellular capillaries formation, VEGF production[161-164], leukostasis and adhesion molecules[164-167]. Comparable advantages were observed in different animal models of diabetes, which were treated with renin inhibitors[167], PRR inhibitor[35], and gene delivery of ACE2[160] respectively. Diabetes may also affect neuronal retina in DR. For example, diabetic retina may reveal releasing of pro-inflammatory factors by microglia[168] , death of retinal neurons[169], apoptosis of ganglion cells[170], glial dysfunction[170] and photoreceptors loss[171]. These pathological neuronal effects may be translated to electrophysiological abnormalities[172-174]. Color vision, contrast sensitivity and dark adaption[24,175] can be altered by diabetes before the presence of any apparent pathological sign in the vessels[175]. Given that treatment with ACE inhibitors and AT1R blockers decreases these deficits in retinal function[176-179], the advantages of RAS blockade could extend to non-vascular cells.
It is also interesting to note that discovery of other important players on the RAS such as ACE2 and Ang (1-7) has resulted in the emerging new role ascribed to these RAS components beyond the classic ACE/Ang II/AT1R axis of the RAS[179-180]. Nevertheless, the force of this novel axis stays inadequately elucidated[180,182-184]. This new protective axis antagonizes the classic role of the vasoconstrictor axis. Thus, it was assumed that a disproportion in the vasoprotective/vasodeleterious axis of the RAS, could result in the development and progression of DR. Many studies in non-ocular tissues have emphasized the beneficial effect of the balance displacement of the RAS towards the ACE2/Ang (1-7)[180,185-189]. Therefore, activation of the vasoprotective axis is currently considered to be part of the beneficial actions of ACEi and ATRs blocker drugs[180,182], which neutralize the actions of Ang II, in spite of its origins of generation[146].
High blood pressure is a great risk factor for DR. Several studies have been addressed to elucidate if the contribution of the Ang II to the development of DR is via blood pressure dependent or independent. This is an intricate search, given that blockers of some compound of the RAS decrease both blood pressure and the actions of the Ang II at cellular levels. Studies in Ren-2 rat with hypertension showed that both AT1R and β-adrenergic blockade regularize blood pressure[158]. Nevertheless, the retinal vascular pathology only becomes better using AT1R blockers. Additional determination of the blood pressure-independent effects of the RAS blockade in DR is crucial for diabetic patients without hypertension.
The mechanism(s) by which the RAS exerts its effects in the retina are being investigated. There is proof that hypertension and mechanical stretch up-regulate the RAS and VEGF expression[190]. It has been previously demonstrated an increase of VEGF in the RPE[191] and in retinal endothelial cells[192] due to mechanical stretch. Moreover, rats with hypertension showed increased expression of the VEGFR-2 in the retina[191]. Therefore, it could be probable that the decrease in VEGF reported in DR[193] following RAS blockade could be due to the antihypertensive properties of this treatment, rather than, suppression of the growth factor effects of Ang II. Moreover, given that a relationship between ROS and cellular damage in DR has been demonstrated and the fact that ROS production is induced by Ang II[153,154,194,195], it is likely that ROS are essential in the pathogenesis of DR. The main origin of the ROS is nicotinamide adenine dinucleotide phosphate (NADPH, or NOX) and ROS originated from NOX have been associated with the development of DR[196,197]. Ang II modulates NOX to generate ROS[194,198]. However, the connection between the RAS and NOX in retinopathy is not completely clarified yet[199,200]. Obviously, the link involving RAS and NOX in DR guarantees further study.
Clinical trials evaluated the influence of Ang II in the development and progression of DR. To elucidate this, three major studies addressed to evaluate the blockade of the RAS were done: (1) the DIabetic REtinopathy Candesartan trial[201-204]; (2) the Appropriate Blood Pressure Control in Diabetes trial[205]; and (3) the Action in Diabetes and Vascular Disease Controlled Evaluation (ADVANCE) trial[206]. The first study showed that candesartan, an AT1R blocker, modestly avoid the evolution of retinopathy in type 1 diabetic patients without hypertension. From another point of view, this AT1R blocker caused reversion of retinopathy in type 2 diabetic patients in a 34% regression of retinopathy and decreased the risk of microaneurysm evolution in both types of diabetes[202]. The second trial study, showed notably benefit for RAS blockade[203], whereas the ADVANCE study reported that treatment with a combination of an ACE inhibitor and a diuretic, did not affect the retinopathy risk[205]. I summary, these data document the influence of Ang II in the development of DR. Further evaluation of the RAS blockade in DR is still to be determined.
Hypertension is a potential link between cardiovascular pathologies and eye diseases. A large amount of information has demonstrated the presence of a RAS in the retina which is greatly spread in the vasculature. To date, findings from epidemiological studies indicate an association between AMD and hypertension. Moreover, studies in vitro and in vivo show that Ang II contributes to sub-RPE deposit formation and CNV development and that these events can be improved by Ang II receptor blockers (ARBs). However, the utility of ARBs for the treatment of eye AMD is still to be determined. In terms of DR, there is documented evidence showing a clear contribution of Ang II to the development of this disease. Therefore, the use of ARBs can confer retinoprotection and arrest the progression of DR.
We thank Khasimuddin Syed for editorial review of the manuscript.
P- Reviewer: Nacak M, Shimada Y, Zhao Di S- Editor: Wen LL L- Editor: A E- Editor: Liu SQ
| 1. | Metsärinne KP, Helin KH, Saijonmaa O, Stewen P, Sirviö ML, Fyhrquist FY. Tissue-specific regulation of angiotensin-converting enzyme by angiotensin II and losartan in the rat. Blood Press. 1996;5:363-370. [PubMed] |
| 2. | Bader M, Peters J, Baltatu O, Müller DN, Luft FC, Ganten D. Tissue renin-angiotensin systems: new insights from experimental animal models in hypertension research. J Mol Med (Berl). 2001;79:76-102. [PubMed] |
| 3. | Kramkowski K, Mogielnicki A, Buczko W. The physiological significance of the alternative pathways of angiotensin II production. J Physiol Pharmacol. 2006;57:529-539. [PubMed] |
| 4. | Rong P, Wilkinson-Berka JL, Skinner SL. Control of renin secretion from adrenal gland in transgenic Ren-2 and normal rats. Mol Cell Endocrinol. 2001;173:203-212. [PubMed] |
| 5. | Wilkinson-Berka JL, Kelly DJ, Rong P, Campbell DJ, Skinner SL. Characterisation of a thymic renin-angiotensin system in the transgenic m(Ren-2)27 rat. Mol Cell Endocrinol. 2002;194:201-209. [PubMed] |
| 6. | Berka JL, Stubbs AJ, Wang DZ, DiNicolantonio R, Alcorn D, Campbell DJ, Skinner SL. Renin-containing Müller cells of the retina display endocrine features. Invest Ophthalmol Vis Sci. 1995;36:1450-1458. [PubMed] |
| 7. | Sarlos S, Rizkalla B, Moravski CJ, Cao Z, Cooper ME, Wilkinson-Berka JL. Retinal angiogenesis is mediated by an interaction between the angiotensin type 2 receptor, VEGF, and angiopoietin. Am J Pathol. 2003;163:879-887. [PubMed] |
| 8. | Fyhrquist F, Metsärinne K, Tikkanen I. Role of angiotensin II in blood pressure regulation and in the pathophysiology of cardiovascular disorders. J Hum Hypertens. 1995;9 Suppl 5:S19-S24. [PubMed] |
| 9. | Beevers G, Lip GY, O’Brien E. ABC of hypertension: The pathophysiology of hypertension. BMJ. 2001;322:912-916. [PubMed] |
| 10. | Benigni A, Cassis P, Remuzzi G. Angiotensin II revisited: new roles in inflammation, immunology and aging. EMBO Mol Med. 2010;2:247-257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 440] [Cited by in RCA: 568] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 11. | Moravski CJ, Kelly DJ, Cooper ME, Gilbert RE, Bertram JF, Shahinfar S, Skinner SL, Wilkinson-Berka JL. Retinal neovascularization is prevented by blockade of the renin-angiotensin system. Hypertension. 2000;36:1099-1104. [PubMed] |
| 12. | Stegbauer J, Coffman TM. New insights into angiotensin receptor actions: from blood pressure to aging. Curr Opin Nephrol Hypertens. 2011;20:84-88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 13. | Willis LM, El-Remessy AB, Somanath PR, Deremer DL, Fagan SC. Angiotensin receptor blockers and angiogenesis: clinical and experimental evidence. Clin Sci (Lond). 2011;120:307-319. [PubMed] |
| 14. | Igić R, Robinson CJ, Milosević Z, Wilson CM, Erdös EG. [Activity of renin and angiotensin I converting enzyme in retina and ciliary body (author’s transl)]. Lijec Vjesn. 1977;99:482-484. [PubMed] |
| 15. | Wheeler-Schilling TH, Kohler K, Sautter M, Guenther E. Angiotensin II receptor subtype gene expression and cellular localization in the retina and non-neuronal ocular tissues of the rat. Eur J Neurosci. 1999;11:3387-3394. [PubMed] |
| 16. | Savaskan E, Löffler KU, Meier F, Müller-Spahn F, Flammer J, Meyer P. Immunohistochemical localization of angiotensin-converting enzyme, angiotensin II and AT1 receptor in human ocular tissues. Ophthalmic Res. 2004;36:312-320. [PubMed] |
| 17. | Senanayake Pd, Drazba J, Shadrach K, Milsted A, Rungger-Brandle E, Nishiyama K, Miura S, Karnik S, Sears JE, Hollyfield JG. Angiotensin II and its receptor subtypes in the human retina. Invest Ophthalmol Vis Sci. 2007;48:3301-3311. [PubMed] |
| 18. | Striker GE, Praddaude F, Alcazar O, Cousins SW, Marin-Castaño ME. Regulation of angiotensin II receptors and extracellular matrix turnover in human retinal pigment epithelium: role of angiotensin II. Am J Physiol Cell Physiol. 2008;295:C1633-C1646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Wagner J, Jan Danser AH, Derkx FH, de Jong TV, Paul M, Mullins JJ, Schalekamp MA, Ganten D. Demonstration of renin mRNA, angiotensinogen mRNA, and angiotensin converting enzyme mRNA expression in the human eye: evidence for an intraocular renin-angiotensin system. Br J Ophthalmol. 1996;80:159-163. [PubMed] |
| 20. | Milenkovic VM, Brockmann M, Meyer C, Desch M, Schweda F, Kurtz A, Todorov V, Strauss O. Regulation of the renin expression in the retinal pigment epithelium by systemic stimuli. Am J Physiol Renal Physiol. 2010;299:F396-F403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Cunha-Vaz J. The blood-ocular barriers. Surv Ophthalmol. 1979;23:279-296. [PubMed] |
| 22. | Danser AH, Derkx FH, Admiraal PJ, Deinum J, de Jong PT, Schalekamp MA. Angiotensin levels in the eye. Invest Ophthalmol Vis Sci. 1994;35:1008-1018. [PubMed] |
| 23. | Otani A, Takagi H, Suzuma K, Honda Y. Angiotensin II potentiates vascular endothelial growth factor-induced angiogenic activity in retinal microcapillary endothelial cells. Circ Res. 1998;82:619-628. [PubMed] |
| 24. | Fletcher EL, Phipps JA, Ward MM, Vessey KA, Wilkinson-Berka JL. The renin-angiotensin system in retinal health and disease: Its influence on neurons, glia and the vasculature. Prog Retin Eye Res. 2010;29:284-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 25. | Jacobi PC, Osswald H, Jurklies B, Zrenner E. Neuromodulatory effects of the renin-angiotensin system on the cat electroretinogram. Invest Ophthalmol Vis Sci. 1994;35:973-980. [PubMed] |
| 26. | Jurklies B, Eckstein A, Jacobi P, Kohler K, Risler T, Zrenner E. The renin-angiotensin system--a possible neuromodulator in the human retina? Ger J Ophthalmol. 1995;4:144-150. [PubMed] |
| 27. | Hyman L, Schachat AP, He Q, Leske MC. Hypertension, cardiovascular disease, and age-related macular degeneration. Age-Related Macular Degeneration Risk Factors Study Group. Arch Ophthalmol. 2000;118:351-358. [PubMed] |
| 28. | Jonas JB, Hayreh SS, Martus P. Influence of arterial hypertension and diet-induced atherosclerosis on macular drusen. Graefes Arch Clin Exp Ophthalmol. 2003;241:125-134. [PubMed] |
| 29. | Klein R, Klein BE, Tomany SC, Cruickshanks KJ. The association of cardiovascular disease with the long-term incidence of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 2003;110:1273-1280. [PubMed] |
| 30. | Skov Jensen P, Jeppesen P, Bek T. Differential diameter responses in macular and peripheral retinal arterioles may contribute to the regional distribution of diabetic retinopathy lesions. Graefes Arch Clin Exp Ophthalmol. 2011;249:407-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Metelitsina TI, Grunwald JE, DuPont JC, Ying GS. Effect of systemic hypertension on foveolar choroidal blood flow in age related macular degeneration. Br J Ophthalmol. 2006;90:342-346. [PubMed] |
| 32. | Metelitsina TI, Grunwald JE, DuPont JC, Ying GS, Brucker AJ, Dunaief JL. Foveolar choroidal circulation and choroidal neovascularization in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2008;49:358-363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 114] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 33. | Danser AH, van den Dorpel MA, Deinum J, Derkx FH, Franken AA, Peperkamp E, de Jong PT, Schalekamp MA. Renin, prorenin, and immunoreactive renin in vitreous fluid from eyes with and without diabetic retinopathy. J Clin Endocrinol Metab. 1989;68:160-167. [PubMed] |
| 34. | Nagai N, Noda K, Urano T, Kubota Y, Shinoda H, Koto T, Shinoda K, Inoue M, Shiomi T, Ikeda E. Selective suppression of pathologic, but not physiologic, retinal neovascularization by blocking the angiotensin II type 1 receptor. Invest Ophthalmol Vis Sci. 2005;46:1078-1084. [PubMed] |
| 35. | Downie LE, Pianta MJ, Vingrys AJ, Wilkinson-Berka JL, Fletcher EL. AT1 receptor inhibition prevents astrocyte degeneration and restores vascular growth in oxygen-induced retinopathy. Glia. 2008;56:1076-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 36. | Lonchampt M, Pennel L, Duhault J. Hyperoxia/normoxia-driven retinal angiogenesis in mice: a role for angiotensin II. Invest Ophthalmol Vis Sci. 2001;42:429-432. [PubMed] |
| 37. | Flammer J, Konieczka K, Bruno RM, Virdis A, Flammer AJ, Taddei S. The eye and the heart. Eur Heart J. 2013;34:1270-1278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 207] [Cited by in RCA: 267] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 38. | Hughes S, Chan-Ling T. Characterization of smooth muscle cell and pericyte differentiation in the rat retina in vivo. Invest Ophthalmol Vis Sci. 2004;45:2795-2806. [PubMed] |
| 39. | Oku H, Kodama T, Sakagami K, Puro DG. Diabetes-induced disruption of gap junction pathways within the retinal microvasculature. Invest Ophthalmol Vis Sci. 2001;42:1915-1920. [PubMed] |
| 41. | Bonkowski D, Katyshev V, Balabanov RD, Borisov A, Dore-Duffy P. The CNS microvascular pericyte: pericyte-astrocyte crosstalk in the regulation of tissue survival. Fluids Barriers CNS. 2011;8:8. [PubMed] |
| 42. | Funk RH. Blood supply of the retina. Ophthalmic Res. 1997;29:320-325. [PubMed] |
| 43. | Iuchtman M, Auslander L. Ectopic pancreas cyst in the mesocolon. J Clin Gastroenterol. 1991;13:716-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 783] [Cited by in RCA: 858] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 44. | Puro DG. Physiology and pathobiology of the pericyte-containing retinal microvasculature: new developments. Microcirculation. 2007;14:1-10. [PubMed] |
| 45. | Hamilton NB, Attwell D, Hall CN. Pericyte-mediated regulation of capillary diameter: a component of neurovascular coupling in health and disease. Front Neuroenergetics. 2010;2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 297] [Cited by in RCA: 370] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 46. | Matsushita K, Fukumoto M, Kobayashi T, Kobayashi M, Ishizaki E, Minami M, Katsumura K, Liao SD, Wu DM, Zhang T. Diabetes-induced inhibition of voltage-dependent calcium channels in the retinal microvasculature: role of spermine. Invest Ophthalmol Vis Sci. 2010;51:5979-5990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 47. | Zhang T, Wu DM, Xu GZ, Puro DG. The electrotonic architecture of the retinal microvasculature: modulation by angiotensin II. J Physiol. 2011;589:2383-2399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 48. | Flammer J, Mozaffarieh M. Autoregulation, a balancing act between supply and demand. Can J Ophthalmol. 2008;43:317-321. [PubMed] |
| 49. | Delaey C, Van De Voorde J. Regulatory mechanisms in the retinal and choroidal circulation. Ophthalmic Res. 2000;32:249-256. [PubMed] |
| 50. | Blum M, Bachmann K, Wintzer D, Riemer T, Vilser W, Strobel J. Noninvasive measurement of the Bayliss effect in retinal autoregulation. Graefes Arch Clin Exp Ophthalmol. 1999;237:296-300. [PubMed] |
| 51. | Schönfelder U, Hofer A, Paul M, Funk RH. In situ observation of living pericytes in rat retinal capillaries. Microvasc Res. 1998;56:22-29. [PubMed] |
| 52. | Kulkarni PS, Hamid H, Barati M, Butulija D. Angiotensin II-induced constrictions are masked by bovine retinal vessels. Invest Ophthalmol Vis Sci. 1999;40:721-728. [PubMed] |
| 53. | Kur J, Newman EA, Chan-Ling T. Cellular and physiological mechanisms underlying blood flow regulation in the retina and choroid in health and disease. Prog Retin Eye Res. 2012;31:377-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 495] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 54. | Brown SM, Jampol LM. New concepts of regulation of retinal vessel tone. Arch Ophthalmol. 1996;114:199-204. [PubMed] |
| 55. | Ito S, Arima S, Ren YL, Juncos LA, Carretero OA. Endothelium-derived relaxing factor/nitric oxide modulates angiotensin II action in the isolated microperfused rabbit afferent but not efferent arteriole. J Clin Invest. 1993;91:2012-2019. [PubMed] |
| 56. | Dollery CT, Hill DW, Hodge JV. The response of normal retinal blood vessels to angiotensin and noradrenaline. J Physiol. 1963;165:500-507. [PubMed] |
| 57. | Vacek L, Bravený P. Effect of angiotensin II on blood pressure and on microvascular beds in mesentery, skin, and skeletal muscle of the rat. Microvasc Res. 1978;16:43-50. [PubMed] |
| 58. | Desjardins-Giasson S, Gutkowska J, Garcia R, Genest J. Effect of angiotensin ii and norepinephrine on release of prostaglandins E2 and I2 by the perfused rat mesenteric artery. Prostaglandins. 1982;24:105-114. [PubMed] |
| 59. | Miller AG, Tan G, Binger KJ, Pickering RJ, Thomas MC, Nagaraj RH, Cooper ME, Wilkinson-Berka JL. Candesartan attenuates diabetic retinal vascular pathology by restoring glyoxalase-I function. Diabetes. 2010;59:3208-3215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 60. | Anderson DR. Glaucoma, capillaries and pericytes. 1. Blood flow regulation. Ophthalmologica. 1996;210:257-262. [PubMed] |
| 61. | Anderson DR, Davis EB. Glaucoma, capillaries and pericytes. 5. Preliminary evidence that carbon dioxide relaxes pericyte contractile tone. Ophthalmologica. 1996;210:280-284. [PubMed] |
| 62. | Haefliger IO, Zschauer A, Anderson DR. Relaxation of retinal pericyte contractile tone through the nitric oxide-cyclic guanosine monophosphate pathway. Invest Ophthalmol Vis Sci. 1994;35:991-997. [PubMed] |
| 63. | Matsugi T, Chen Q, Anderson DR. Suppression of CO2-induced relaxation of bovine retinal pericytes by angiotensin II. Invest Ophthalmol Vis Sci. 1997;38:652-657. [PubMed] |
| 64. | Bird AC, Bressler NM, Bressler SB, Chisholm IH, Coscas G, Davis MD, de Jong PT, Klaver CC, Klein BE, Klein R. An international classification and grading system for age-related maculopathy and age-related macular degeneration. The International ARM Epidemiological Study Group. Surv Ophthalmol. 1995;39:367-374. [PubMed] |
| 65. | Grunwald JE, Hariprasad SM, DuPont J, Maguire MG, Fine SL, Brucker AJ, Maguire AM, Ho AC. Foveolar choroidal blood flow in age-related macular degeneration. Invest Ophthalmol Vis Sci. 1998;39:385-390. [PubMed] |
| 66. | Grunwald JE, Metelitsina TI, Dupont JC, Ying GS, Maguire MG. Reduced foveolar choroidal blood flow in eyes with increasing AMD severity. Invest Ophthalmol Vis Sci. 2005;46:1033-1038. [PubMed] |
| 67. | Böker T, Fang T, Steinmetz R. Refractive error and choroidal perfusion characteristics in patients with choroidal neovascularization and age-related macular degeneration. Ger J Ophthalmol. 1993;2:10-13. [PubMed] |
| 68. | Chen JC, Fitzke FW, Pauleikhoff D, Bird AC. Functional loss in age-related Bruch’s membrane change with choroidal perfusion defect. Invest Ophthalmol Vis Sci. 1992;33:334-340. [PubMed] |
| 69. | Pauleikhoff D, Spital G, Radermacher M, Brumm GA, Lommatzsch A, Bird AC. A fluorescein and indocyanine green angiographic study of choriocapillaris in age-related macular disease. Arch Ophthalmol. 1999;117:1353-1358. [PubMed] |
| 70. | Ciulla TA, Harris A, Kagemann L, Danis RP, Pratt LM, Chung HS, Weinberger D, Garzozi HJ. Choroidal perfusion perturbations in non-neovascular age related macular degeneration. Br J Ophthalmol. 2002;86:209-213. [PubMed] |
| 71. | Ciulla TA, Harris A, Chung HS, Danis RP, Kagemann L, McNulty L, Pratt LM, Martin BJ. Color Doppler imaging discloses reduced ocular blood flow velocities in nonexudative age-related macular degeneration. Am J Ophthalmol. 1999;128:75-80. [PubMed] |
| 72. | Sarks SH. Changes in the region of the choriocapillaris in aging and degeneration. XXIII Concilium Ophthalmologicum, Kyoto, 1978. Amsterdam: Excerpta Medica 1978; 228-238. |
| 73. | Sarks JP, Sarks SH, Killingsworth MC. Evolution of geographic atrophy of the retinal pigment epithelium. Eye (Lond). 1988;2:552-577. [PubMed] |
| 74. | Ramrattan RS, van der Schaft TL, Mooy CM, de Bruijn WC, Mulder PG, de Jong PT. Morphometric analysis of Bruch’s membrane, the choriocapillaris, and the choroid in aging. Invest Ophthalmol Vis Sci. 1994;35:2857-2864. [PubMed] |
| 75. | Harris A, Chung HS, Ciulla TA, Kagemann L. Progress in measurement of ocular blood flow and relevance to our understanding of glaucoma and age-related macular degeneration. Prog Retin Eye Res. 1999;18:669-687. [PubMed] |
| 76. | Lutty G, Grunwald J, Majji AB, Uyama M, Yoneya S. Changes in choriocapillaris and retinal pigment epithelium in age-related macular degeneration. Mol Vis. 1999;5:35. [PubMed] |
| 77. | Pemp B, Schmetterer L. Ocular blood flow in diabetes and age-related macular degeneration. Can J Ophthalmol. 2008;43:295-301. [PubMed] |
| 78. | Uretmen O, Akkin C, Erakgün T, Killi R. Color Doppler imaging of choroidal circulation in patients with asymmetric age-related macular degeneration. Ophthalmologica. 2003;217:137-142. [PubMed] |
| 79. | Prünte C, Niesel P. Quantification of choroidal blood-flow parameters using indocyanine green video-fluorescence angiography and statistical picture analysis. Graefes Arch Clin Exp Ophthalmol. 1988;226:55-58. [PubMed] |
| 80. | Kornzweig AL. Changes in the choriocapillaris associated with senile macular degeneration. Ann Ophthalmol. 1977;9:753-756, 759-762. [PubMed] |
| 81. | Sperduto RD, Hiller R. Systemic hypertension and age-related maculopathy in the Framingham Study. Arch Ophthalmol. 1986;104:216-219. [PubMed] |
| 82. | Goldberg J, Flowerdew G, Smith E, Brody JA, Tso MO. Factors associated with age-related macular degeneration. An analysis of data from the first National Health and Nutrition Examination Survey. Am J Epidemiol. 1988;128:700-710. [PubMed] |
| 83. | Age-Related Eye Disease Study Research Group. Risk factors associated with age-related macular degeneration. A case-control study in the age-related eye disease study: Age-Related Eye Disease Study Report Number 3. Ophthalmology. 2000;107:2224-2232. [PubMed] |
| 84. | van Leeuwen R, Ikram MK, Vingerling JR, Witteman JC, Hofman A, de Jong PT. Blood pressure, atherosclerosis, and the incidence of age-related maculopathy: the Rotterdam Study. Invest Ophthalmol Vis Sci. 2003;44:3771-3777. [PubMed] |
| 85. | Laser photocoagulation for juxtafoveal choroidal neovascularization. Five-year results from randomized clinical trials. Macular Photocoagulation Study Group. Arch Ophthalmol. 1994;112:500-509. [PubMed] |
| 86. | Klein R, Peto T, Bird A, Vannewkirk MR. The epidemiology of age-related macular degeneration. Am J Ophthalmol. 2004;137:486-495. [PubMed] |
| 87. | Young RW. Pathophysiology of age-related macular degeneration. Surv Ophthalmol. 1987;31:291-306. [PubMed] |
| 88. | Bressler SB, Maguire MG, Bressler NM, Fine SL. Relationship of drusen and abnormalities of the retinal pigment epithelium to the prognosis of neovascular macular degeneration. The Macular Photocoagulation Study Group. Arch Ophthalmol. 1990;108:1442-1447. [PubMed] |
| 89. | Sarks SH, Van Driel D, Maxwell L, Killingsworth M. Softening of drusen and subretinal neovascularization. Trans Ophthalmol Soc UK. 1980;100:414-422. [PubMed] |
| 90. | Vinding T. Occurrence of drusen, pigmentary changes and exudative changes in the macula with reference to age-related macular degeneration. An epidemiological study of 1000 aged individuals. Acta Ophthalmol (Copenh). 1990;68:410-414. [PubMed] |
| 91. | Clemons TE, Milton RC, Klein R, Seddon JM, Ferris FL. Risk factors for the incidence of Advanced Age-Related Macular Degeneration in the Age-Related Eye Disease Study (AREDS) AREDS report no. 19. Ophthalmology. 2005;112:533-539. [PubMed] |
| 92. | Evans JR. Risk factors for age-related macular degeneration. Prog Retin Eye Res. 2001;20:227-253. [PubMed] |
| 93. | Khan JC, Thurlby DA, Shahid H, Clayton DG, Yates JR, Bradley M, Moore AT, Bird AC. Smoking and age related macular degeneration: the number of pack years of cigarette smoking is a major determinant of risk for both geographic atrophy and choroidal neovascularisation. Br J Ophthalmol. 2006;90:75-80. [PubMed] |
| 94. | Sepp T, Khan JC, Thurlby DA, Shahid H, Clayton DG, Moore AT, Bird AC, Yates JR. Complement factor H variant Y402H is a major risk determinant for geographic atrophy and choroidal neovascularization in smokers and nonsmokers. Invest Ophthalmol Vis Sci. 2006;47:536-540. [PubMed] |
| 95. | Smith W, Assink J, Klein R, Mitchell P, Klaver CC, Klein BE, Hofman A, Jensen S, Wang JJ, de Jong PT. Risk factors for age-related macular degeneration: Pooled findings from three continents. Ophthalmology. 2001;108:697-704. [PubMed] |
| 96. | Olea JL, Tuñón J. Patients with neovascular age-related macular degeneration in Spain display a high cardiovascular risk. Eur J Ophthalmol. 2012;22:404-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 97. | Hogg RE, Woodside JV, Gilchrist SE, Graydon R, Fletcher AE, Chan W, Knox A, Cartmill B, Chakravarthy U. Cardiovascular disease and hypertension are strong risk factors for choroidal neovascularization. Ophthalmology. 2008;115:1046-1052.e2. [PubMed] |
| 98. | Thapa R, Paudyal G, Shrestha MK, Gurung R, Ruit S. Age-related macular degeneration in Nepal. Kathmandu Univ Med J (KUMJ). 2011;9:165-169. [PubMed] |
| 99. | Hogg RE, McKay GJ, Hughes AE, Muldrew KA, Chakravarthy U. Genotype-phenotype associations in neovascular age-related macular degeneration. Retina. 2012;32:1950-1958. [PubMed] |
| 100. | Burcklé CA, Jan Danser AH, Müller DN, Garrelds IM, Gasc JM, Popova E, Plehm R, Peters J, Bader M, Nguyen G. Elevated blood pressure and heart rate in human renin receptor transgenic rats. Hypertension. 2006;47:552-556. [PubMed] |
| 101. | Ichihara A, Suzuki F, Nakagawa T, Kaneshiro Y, Takemitsu T, Sakoda M, Nabi AH, Nishiyama A, Sugaya T, Hayashi M. Prorenin receptor blockade inhibits development of glomerulosclerosis in diabetic angiotensin II type 1a receptor-deficient mice. J Am Soc Nephrol. 2006;17:1950-1961. [PubMed] |
| 102. | Ichihara A, Kaneshiro Y, Takemitsu T, Sakoda M, Suzuki F, Nakagawa T, Nishiyama A, Inagami T, Hayashi M. Nonproteolytic activation of prorenin contributes to development of cardiac fibrosis in genetic hypertension. Hypertension. 2006;47:894-900. [PubMed] |
| 103. | Kaneshiro Y, Ichihara A, Sakoda M, Takemitsu T, Nabi AH, Uddin MN, Nakagawa T, Nishiyama A, Suzuki F, Inagami T. Slowly progressive, angiotensin II-independent glomerulosclerosis in human (pro)renin receptor-transgenic rats. J Am Soc Nephrol. 2007;18:1789-1795. [PubMed] |
| 104. | Praddaude F, Cousins SW, Pêcher C, Marin-Castaño ME. Angiotensin II-induced hypertension regulates AT1 receptor subtypes and extracellular matrix turnover in mouse retinal pigment epithelium. Exp Eye Res. 2009;89:109-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 106. | Ishibashi T, Patterson R, Ohnishi Y, Inomata H, Ryan SJ. Formation of drusen in the human eye. Am J Ophthalmol. 1986;101:342-353. [PubMed] |
| 107. | Burns RP, Feeney-Burns L. Clinico-morphologic correlations of drusen of Bruch’s membrane. Trans Am Ophthalmol Soc. 1980;78:206-225. [PubMed] |
| 108. | Zhu ZR, Goodnight R, Nishimura T, Sorgente N, Ogden TE, Ryan SJ. Experimental changes resembling the pathology of drusen in Bruch‘s membrane in the rabbit. Curr Eye Res. 1988;7:581-592. [PubMed] |
| 109. | Sarks S, Cherepanoff S, Killingsworth M, Sarks J. Relationship of Basal laminar deposit and membranous debris to the clinical presentation of early age-related macular degeneration. Invest Ophthalmol Vis Sci. 2007;48:968-977. [PubMed] |
| 110. | Deryugina EI, Bourdon MA, Reisfeld RA, Strongin A. Remodeling of collagen matrix by human tumor cells requires activation and cell surface association of matrix metalloproteinase-2. Cancer Res. 1998;58:3743-3750. [PubMed] |
| 111. | Atkinson SJ, Patterson ML, Butler MJ, Murphy G. Membrane type 1 matrix metalloproteinase and gelatinase A synergistically degrade type 1 collagen in a cell model. FEBS Lett. 2001;491:222-226. [PubMed] |
| 112. | Pons M, Cousins SW, Alcazar O, Striker GE, Marin-Castaño ME. Angiotensin II-induced MMP-2 activity and MMP-14 and basigin protein expression are mediated via the angiotensin II receptor type 1-mitogen-activated protein kinase 1 pathway in retinal pigment epithelium: implications for age-related macular degeneration. Am J Pathol. 2011;178:2665-2681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 113. | Cui XL, Ding Y, Alexander LD, Bao C, Al-Khalili OK, Simonson M, Eaton DC, Douglas JG. Oxidative signaling in renal epithelium: Critical role of cytosolic phospholipase A2 and p38(SAPK). Free Radic Biol Med. 2006;41:213-221. [PubMed] |
| 114. | Ichijo H, Nishida E, Irie K, ten Dijke P, Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K, Gotoh Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275:90-94. [PubMed] |
| 115. | Sugden PH, Clerk A. Regulation of the ERK subgroup of MAP kinase cascades through G protein-coupled receptors. Cell Signal. 1997;9:337-351. [PubMed] |
| 116. | Taniyama Y, Ushio-Fukai M, Hitomi H, Rocic P, Kingsley MJ, Pfahnl C, Weber DS, Alexander RW, Griendling KK. Role of p38 MAPK and MAPKAPK-2 in angiotensin II-induced Akt activation in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2004;287:C494-C499. [PubMed] |
| 117. | Chen Z, Gibson TB, Robinson F, Silvestro L, Pearson G, Xu B, Wright A, Vanderbilt C, Cobb MH. MAP kinases. Chem Rev. 2001;101:2449-2476. [PubMed] |
| 118. | Lewis TS, Shapiro PS, Ahn NG. Signal transduction through MAP kinase cascades. Adv Cancer Res. 1998;74:49-139. [PubMed] |
| 119. | Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153-183. [PubMed] |
| 120. | Snow KK, Seddon JM. Do age-related macular degeneration and cardiovascular disease share common antecedents? Ophthalmic Epidemiol. 1999;6:125-143. [PubMed] |
| 121. | Alcazar O, Cousins SW, Striker GE, Marin-Castano ME. (Pro)renin receptor is expressed in human retinal pigment epithelium and participates in extracellular matrix remodeling. Exp Eye Res. 2009;89:638-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 122. | Ferris FL. Senile macular degeneration: review of epidemiologic features. Am J Epidemiol. 1983;118:132-151. [PubMed] |
| 123. | Starr CE, Guyer DR, Yannuzzi LA. Age-related macular degeneration. Can we stem this worldwide public health crisis? Postgrad Med. 1998;103:153-156, 161-164. [PubMed] |
| 124. | Sakurai E, Taguchi H, Anand A, Ambati BK, Gragoudas ES, Miller JW, Adamis AP, Ambati J. Targeted disruption of the CD18 or ICAM-1 gene inhibits choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44:2743-2749. [PubMed] |
| 125. | Ishibashi T, Hata Y, Yoshikawa H, Nakagawa K, Sueishi K, Inomata H. Expression of vascular endothelial growth factor in experimental choroidal neovascularization. Graefes Arch Clin Exp Ophthalmol. 1997;235:159-167. [PubMed] |
| 126. | Nagai N, Oike Y, Izumi-Nagai K, Urano T, Kubota Y, Noda K, Ozawa Y, Inoue M, Tsubota K, Suda T. Angiotensin II type 1 receptor-mediated inflammation is required for choroidal neovascularization. Arterioscler Thromb Vasc Biol. 2006;26:2252-2259. [PubMed] |
| 127. | Kurihara T, Ozawa Y, Ishida S, Okano H, Tsubota K. Renin-Angiotensin system hyperactivation can induce inflammation and retinal neural dysfunction. Int J Inflam. 2012;2012:581695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 128. | Nagai N, Oike Y, Izumi-Nagai K, Koto T, Satofuka S, Shinoda H, Noda K, Ozawa Y, Inoue M, Tsubota K. Suppression of choroidal neovascularization by inhibiting angiotensin-converting enzyme: minimal role of bradykinin. Invest Ophthalmol Vis Sci. 2007;48:2321-2326. [PubMed] |
| 129. | Hikichi T, Mori F, Takamiya A, Sasaki M, Horikawa Y, Takeda M, Yoshida A. Inhibitory effect of losartan on laser-induced choroidal neovascularization in rats. Am J Ophthalmol. 2001;132:587-589. [PubMed] |
| 130. | Schlingemann RO. Role of growth factors and the wound healing response in age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2004;242:91-101. [PubMed] |
| 131. | Sheridan CM, Pate S, Hiscott P, Wong D, Pattwell DM, Kent D. Expression of hypoxia-inducible factor-1alpha and -2alpha in human choroidal neovascular membranes. Graefes Arch Clin Exp Ophthalmol. 2009;247:1361-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 132. | Martin G, Schlunck G, Hansen LL, Agostini HT. Differential expression of angioregulatory factors in normal and CNV-derived human retinal pigment epithelium. Graefes Arch Clin Exp Ophthalmol. 2004;242:321-326. [PubMed] |
| 133. | Arjamaa O, Nikinmaa M. Oxygen-dependent diseases in the retina: role of hypoxia-inducible factors. Exp Eye Res. 2006;83:473-483. [PubMed] |
| 134. | Shirotake S, Miyajima A, Kosaka T, Tanaka N, Kikuchi E, Mikami S, Okada Y, Oya M. Regulation of monocyte chemoattractant protein-1 through angiotensin II type 1 receptor in prostate cancer. Am J Pathol. 2012;180:1008-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 135. | Skultetyova D, Filipova S, Riecansky I, Skultety J. The role of angiotensin type 1 receptor in inflammation and endothelial dysfunction. Recent Pat Cardiovasc Drug Discov. 2007;2:23-27. [PubMed] |
| 136. | Richard DE, Berra E, Pouyssegur J. Nonhypoxic pathway mediates the induction of hypoxia-inducible factor 1alpha in vascular smooth muscle cells. J Biol Chem. 2000;275:26765-26771. [PubMed] |
| 137. | Satofuka S, Kanda A, Ishida S. Receptor-associated prorenin system in the pathogenesis of retinal diseases. Front Biosci (Schol Ed). 2012;4:1449-1460. [PubMed] |
| 138. | Niu J, Azfer A, Zhelyabovska O, Fatma S, Kolattukudy PE. Monocyte chemotactic protein (MCP)-1 promotes angiogenesis via a novel transcription factor, MCP-1-induced protein (MCPIP). J Biol Chem. 2008;283:14542-14551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 181] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 139. | Lam SY, Liu Y, Ng KM, Lau CF, Liong EC, Tipoe GL, Fung ML. Chronic intermittent hypoxia induces local inflammation of the rat carotid body via functional upregulation of proinflammatory cytokine pathways. Histochem Cell Biol. 2012;137:303-317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 140. | Marin-Castano ME, Lee WS, Hernandez E, Praddaude F, Pecher C, Cousins SW. Hypertension-induced Angiotensin II increases production of MCP-1 and MCPIP in the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2013;54: E-Abstract 6089. |
| 141. | Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, Chen SJ, Dekker JM, Fletcher A, Grauslund J. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556-564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3524] [Cited by in RCA: 3108] [Article Influence: 239.1] [Reference Citation Analysis (3)] |
| 142. | Fong DS, Aiello L, Gardner TW, King GL, Blankenship G, Cavallerano JD, Ferris FL, Klein R. Retinopathy in diabetes. Diabetes Care. 2004;27 Suppl 1:S84-S87. [PubMed] |
| 143. | Wilkinson CP, Ferris FL, Klein RE, Lee PP, Agardh CD, Davis M, Dills D, Kampik A, Pararajasegaram R, Verdaguer JT. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110:1677-1682. [PubMed] |
| 144. | Downie LE, Vessey K, Miller A, Ward MM, Pianta MJ, Vingrys AJ, Wilkinson-Berka JL, Fletcher EL. Neuronal and glial cell expression of angiotensin II type 1 (AT1) and type 2 (AT2) receptors in the rat retina. Neuroscience. 2009;161:195-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 145. | Ishizaki E, Takai S, Ueki M, Maeno T, Maruichi M, Sugiyama T, Oku H, Ikeda T, Miyazaki M. Correlation between angiotensin-converting enzyme, vascular endothelial growth factor, and matrix metalloproteinase-9 in the vitreous of eyes with diabetic retinopathy. Am J Ophthalmol. 2006;141:129-134. [PubMed] |
| 146. | Verma A, Shan Z, Lei B, Yuan L, Liu X, Nakagawa T, Grant MB, Lewin AS, Hauswirth WW, Raizada MK. ACE2 and Ang-(1-7) confer protection against development of diabetic retinopathy. Mol Ther. 2012;20:28-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 131] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 147. | Wakisaka M, Yoshinari M, Nakamura S, Asano T, Sonoki K, Shi Ah, Iwase M, Takata Y, Fujishima M. Suppression of sodium-dependent glucose uptake by captopril improves high-glucose-induced morphological and functional changes of cultured bovine retinal pericytes. Microvasc Res. 1999;58:215-223. [PubMed] |
| 148. | Otani A, Takagi H, Oh H, Koyama S, Honda Y. Angiotensin II induces expression of the Tie2 receptor ligand, angiopoietin-2, in bovine retinal endothelial cells. Diabetes. 2001;50:867-875. [PubMed] |
| 149. | Yamagishi S, Imaizumi T. Pericyte biology and diseases. Int J Tissue React. 2005;27:125-135. [PubMed] |
| 150. | Zhang JZ, Gao L, Widness M, Xi X, Kern TS. Captopril inhibits glucose accumulation in retinal cells in diabetes. Invest Ophthalmol Vis Sci. 2003;44:4001-4005. [PubMed] |
| 151. | Gilbert RE, Kelly DJ, Cox AJ, Wilkinson-Berka JL, Rumble JR, Osicka T, Panagiotopoulos S, Lee V, Hendrich EC, Jerums G. Angiotensin converting enzyme inhibition reduces retinal overexpression of vascular endothelial growth factor and hyperpermeability in experimental diabetes. Diabetologia. 2000;43:1360-1367. [PubMed] |
| 152. | Natarajan R, Bai W, Lanting L, Gonzales N, Nadler J. Effects of high glucose on vascular endothelial growth factor expression in vascular smooth muscle cells. Am J Physiol. 1997;273:H2224-H2231. [PubMed] |
| 153. | Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058-1070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3956] [Cited by in RCA: 3623] [Article Influence: 241.5] [Reference Citation Analysis (0)] |
| 154. | Mataftsi A, Dimitrakos SA, Adams GG. Mediators involved in retinopathy of prematurity and emerging therapeutic targets. Early Hum Dev. 2011;87:683-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 155. | Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141-1148. [PubMed] |
| 156. | Wolff SP, Dean RT. Glucose autoxidation and protein modification. The potential role of ‘autoxidative glycosylation’ in diabetes. Biochem J. 1987;245:243-250. [PubMed] |
| 157. | Kawamura H, Kobayashi M, Li Q, Yamanishi S, Katsumura K, Minami M, Wu DM, Puro DG. Effects of angiotensin II on the pericyte-containing microvasculature of the rat retina. J Physiol. 2004;561:671-683. [PubMed] |
| 158. | Nadal JA, Scicli GM, Carbini LA, Nussbaum JJ, Scicli AG. Angiotensin II and retinal pericytes migration. Biochem Biophys Res Commun. 1999;266:382-385. [PubMed] |
| 159. | Nadal JA, Scicli GM, Carbini LA, Scicli AG. Angiotensin II stimulates migration of retinal microvascular pericytes: involvement of TGF-beta and PDGF-BB. Am J Physiol Heart Circ Physiol. 2002;282:H739-H748. [PubMed] |
| 160. | Yamagishi S, Takeuchi M, Matsui T, Nakamura K, Imaizumi T, Inoue H. Angiotensin II augments advanced glycation end product-induced pericyte apoptosis through RAGE overexpression. FEBS Lett. 2005;579:4265-4270. [PubMed] |
| 161. | Wilkinson-Berka JL, Tan G, Jaworski K, Ninkovic S. Valsartan but not atenolol improves vascular pathology in diabetic Ren-2 rat retina. Am J Hypertens. 2007;20:423-430. [PubMed] |
| 162. | Zhang JZ, Xi X, Gao L, Kern TS. Captopril inhibits capillary degeneration in the early stages of diabetic retinopathy. Curr Eye Res. 2007;32:883-889. [PubMed] |
| 163. | Chen P, Scicli GM, Guo M, Fenstermacher JD, Dahl D, Edwards PA, Scicli AG. Role of angiotensin II in retinal leukostasis in the diabetic rat. Exp Eye Res. 2006;83:1041-1051. [PubMed] |
| 164. | Mori F, Hikichi T, Nagaoka T, Takahashi J, Kitaya N, Yoshida A. Inhibitory effect of losartan, an AT1 angiotensin II receptor antagonist, on increased leucocyte entrapment in retinal microcirculation of diabetic rats. Br J Ophthalmol. 2002;86:1172-1174. [PubMed] |
| 165. | Silva KC, Pinto CC, Biswas SK, Souza DS, de Faria JB, de Faria JM. Prevention of hypertension abrogates early inflammatory events in the retina of diabetic hypertensive rats. Exp Eye Res. 2007;85:123-129. [PubMed] |
| 166. | Wilkinson-Berka JL, Tan G, Binger KJ, Sutton L, McMaster K, Deliyanti D, Perera G, Campbell DJ, Miller AG. Aliskiren reduces vascular pathology in diabetic retinopathy and oxygen-induced retinopathy in the transgenic (mRen-2)27 rat. Diabetologia. 2011;54:2724-2735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 167. | Rungger-Brändle E, Dosso AA, Leuenberger PM. Glial reactivity, an early feature of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2000;41:1971-1980. [PubMed] |
| 168. | Krady JK, Basu A, Allen CM, Xu Y, LaNoue KF, Gardner TW, Levison SW. Minocycline reduces proinflammatory cytokine expression, microglial activation, and caspase-3 activation in a rodent model of diabetic retinopathy. Diabetes. 2005;54:1559-1565. [PubMed] |
| 169. | Gastinger MJ, Singh RS, Barber AJ. Loss of cholinergic and dopaminergic amacrine cells in streptozotocin-diabetic rat and Ins2Akita-diabetic mouse retinas. Invest Ophthalmol Vis Sci. 2006;47:3143-3150. [PubMed] |
| 170. | Lieth E, Barber AJ, Xu B, Dice C, Ratz MJ, Tanase D, Strother JM. Glial reactivity and impaired glutamate metabolism in short-term experimental diabetic retinopathy. Penn State Retina Research Group. Diabetes. 1998;47:815-820. [PubMed] |
| 171. | Park SH, Park JW, Park SJ, Kim KY, Chung JW, Chun MH, Oh SJ. Apoptotic death of photoreceptors in the streptozotocin-induced diabetic rat retina. Diabetologia. 2003;46:1260-1268. [PubMed] |
| 172. | Bresnick GH, Korth K, Groo A, Palta M. Electroretinographic oscillatory potentials predict progression of diabetic retinopathy. Preliminary report. Arch Ophthalmol. 1984;102:1307-1311. [PubMed] |
| 173. | Bresnick GH, Palta M. Predicting progression to severe proliferative diabetic retinopathy. Arch Ophthalmol. 1987;105:810-814. [PubMed] |
| 174. | Phipps JA, Fletcher EL, Vingrys AJ. Paired-flash identification of rod and cone dysfunction in the diabetic rat. Invest Ophthalmol Vis Sci. 2004;45:4592-4600. [PubMed] |
| 175. | Bui BV, Armitage JA, Tolcos M, Cooper ME, Vingrys AJ. ACE inhibition salvages the visual loss caused by diabetes. Diabetologia. 2003;46:401-408. [PubMed] |
| 176. | Kurihara T, Ozawa Y, Nagai N, Shinoda K, Noda K, Imamura Y, Tsubota K, Okano H, Oike Y, Ishida S. Angiotensin II type 1 receptor signaling contributes to synaptophysin degradation and neuronal dysfunction in the diabetic retina. Diabetes. 2008;57:2191-2198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 177. | Nakamura H, Inoue T, Arakawa N, Shimizu Y, Yoshigae Y, Fujimori I, Shimakawa E, Toyoshi T, Yokoyama T. Pharmacological and pharmacokinetic study of olmesartan medoxomil in animal diabetic retinopathy models. Eur J Pharmacol. 2005;512:239-246. [PubMed] |
| 178. | Phipps JA, Wilkinson-Berka JL, Fletcher EL. Retinal dysfunction in diabetic ren-2 rats is ameliorated by treatment with valsartan but not atenolol. Invest Ophthalmol Vis Sci. 2007;48:927-934. [PubMed] |
| 179. | Sugiyama T, Okuno T, Fukuhara M, Oku H, Ikeda T, Obayashi H, Ohta M, Fukui M, Hasegawa G, Nakamura N. Angiotensin II receptor blocker inhibits abnormal accumulation of advanced glycation end products and retinal damage in a rat model of type 2 diabetes. Exp Eye Res. 2007;85:406-412. [PubMed] |
| 180. | Ferreira AJ, Santos RA, Bradford CN, Mecca AP, Sumners C, Katovich MJ, Raizada MK. Therapeutic implications of the vasoprotective axis of the renin-angiotensin system in cardiovascular diseases. Hypertension. 2010;55:207-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 140] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 181. | Ferrario CM, Trask AJ, Jessup JA. Advances in biochemical and functional roles of angiotensin-converting enzyme 2 and angiotensin-(1-7) in regulation of cardiovascular function. Am J Physiol Heart Circ Physiol. 2005;289:H2281-H2290. [PubMed] |
| 182. | Keidar S, Kaplan M, Gamliel-Lazarovich A. ACE2 of the heart: From angiotensin I to angiotensin (1-7). Cardiovasc Res. 2007;73:463-469. [PubMed] |
| 183. | Iwai M, Horiuchi M. Devil and angel in the renin-angiotensin system: ACE-angiotensin II-AT1 receptor axis vs. ACE2-angiotensin-(1-7)-Mas receptor axis. Hypertens Res. 2009;32:533-536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 227] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 184. | Der Sarkissian S, Huentelman MJ, Stewart J, Katovich MJ, Raizada MK. ACE2: A novel therapeutic target for cardiovascular diseases. Prog Biophys Mol Biol. 2006;91:163-198. [PubMed] |
| 185. | Huentelman MJ, Grobe JL, Vazquez J, Stewart JM, Mecca AP, Katovich MJ, Ferrario CM, Raizada MK. Protection from angiotensin II-induced cardiac hypertrophy and fibrosis by systemic lentiviral delivery of ACE2 in rats. Exp Physiol. 2005;90:783-790. [PubMed] |
| 186. | Hernández Prada JA, Ferreira AJ, Katovich MJ, Shenoy V, Qi Y, Santos RA, Castellano RK, Lampkins AJ, Gubala V, Ostrov DA. Structure-based identification of small-molecule angiotensin-converting enzyme 2 activators as novel antihypertensive agents. Hypertension. 2008;51:1312-1317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 213] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 187. | Ferreira AJ, Shenoy V, Yamazato Y, Sriramula S, Francis J, Yuan L, Castellano RK, Ostrov DA, Oh SP, Katovich MJ. Evidence for angiotensin-converting enzyme 2 as a therapeutic target for the prevention of pulmonary hypertension. Am J Respir Crit Care Med. 2009;179:1048-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 205] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 188. | Fraga-Silva RA, Sorg BS, Wankhede M, Dedeugd C, Jun JY, Baker MB, Li Y, Castellano RK, Katovich MJ, Raizada MK. ACE2 activation promotes antithrombotic activity. Mol Med. 2010;16:210-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 112] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 189. | Der Sarkissian S, Grobe JL, Yuan L, Narielwala DR, Walter GA, Katovich MJ, Raizada MK. Cardiac overexpression of angiotensin converting enzyme 2 protects the heart from ischemia-induced pathophysiology. Hypertension. 2008;51:712-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 190. | Malhotra R, Sadoshima J, Brosius FC, Izumo S. Mechanical stretch and angiotensin II differentially upregulate the renin-angiotensin system in cardiac myocytes In vitro. Circ Res. 1999;85:137-146. [PubMed] |
| 191. | Suzuma I, Suzuma K, Ueki K, Hata Y, Feener EP, King GL, Aiello LP. Stretch-induced retinal vascular endothelial growth factor expression is mediated by phosphatidylinositol 3-kinase and protein kinase C (PKC)-zeta but not by stretch-induced ERK1/2, Akt, Ras, or classical/novel PKC pathways. J Biol Chem. 2002;277:1047-1057. [PubMed] |
| 192. | Seko Y, Seko Y, Fujikura H, Pang J, Tokoro T, Shimokawa H. Induction of vascular endothelial growth factor after application of mechanical stress to retinal pigment epithelium of the rat in vitro. Invest Ophthalmol Vis Sci. 1999;40:3287-3291. [PubMed] |
| 193. | Moravski CJ, Skinner SL, Stubbs AJ, Sarlos S, Kelly DJ, Cooper ME, Gilbert RE, Wilkinson-Berka JL. The renin-angiotensin system influences ocular endothelial cell proliferation in diabetes: transgenic and interventional studies. Am J Pathol. 2003;162:151-160. [PubMed] |
| 194. | Garrido AM, Griendling KK. NADPH oxidases and angiotensin II receptor signaling. Mol Cell Endocrinol. 2009;302:148-158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 299] [Cited by in RCA: 294] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 195. | Schramm A, Matusik P, Osmenda G, Guzik TJ. Targeting NADPH oxidases in vascular pharmacology. Vascul Pharmacol. 2012;56:216-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 173] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 196. | Al-Shabrawey M, Rojas M, Sanders T, Behzadian A, El-Remessy A, Bartoli M, Parpia AK, Liou G, Caldwell RB. Role of NADPH oxidase in retinal vascular inflammation. Invest Ophthalmol Vis Sci. 2008;49:3239-3244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 166] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 197. | Li J, Wang JJ, Yu Q, Chen K, Mahadev K, Zhang SX. Inhibition of reactive oxygen species by Lovastatin downregulates vascular endothelial growth factor expression and ameliorates blood-retinal barrier breakdown in db/db mice: role of NADPH oxidase 4. Diabetes. 2010;59:1528-1538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 163] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 198. | The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med. 2000;342:381-389. |
| 199. | Chen P, Guo AM, Edwards PA, Trick G, Scicli AG. Role of NADPH oxidase and ANG II in diabetes-induced retinal leukostasis. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1619-R1629. [PubMed] |
| 200. | Fukumoto M, Takai S, Ishizaki E, Sugiyama T, Oku H, Jin D, Sakaguchi M, Sakonjo H, Ikeda T, Miyazaki M. Involvement of angiotensin II-dependent vascular endothelial growth factor gene expression via NADPH oxidase in the retina in a type 2 diabetic rat model. Curr Eye Res. 2008;33:885-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 201. | Chaturvedi N, Porta M, Klein R, Orchard T, Fuller J, Parving HH, Bilous R, Sjølie AK. Effect of candesartan on prevention (DIRECT-Prevent 1) and progression (DIRECT-Protect 1) of retinopathy in type 1 diabetes: randomised, placebo-controlled trials. Lancet. 2008;372:1394-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 318] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 202. | Sola A, Saldeño YP, Favareto V. Clinical practices in neonatal oxygenation: where have we failed? What can we do? J Perinatol. 2008;28 Suppl 1:S28-S34. [PubMed] |
| 203. | Sjølie AK, Klein R, Porta M, Orchard T, Fuller J, Parving HH, Bilous R, Aldington S, Chaturvedi N. Retinal microaneurysm count predicts progression and regression of diabetic retinopathy. Post-hoc results from the DIRECT Programme. Diabet Med. 2011;28:345-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 204. | Mauer M, Zinman B, Gardiner R, Suissa S, Sinaiko A, Strand T, Drummond K, Donnelly S, Goodyer P, Gubler MC. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med. 2009;361:40-51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 626] [Cited by in RCA: 523] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 205. | Estacio RO, Jeffers BW, Gifford N, Schrier RW. Effect of blood pressure control on diabetic microvascular complications in patients with hypertension and type 2 diabetes. Diabetes Care. 2000;23 Suppl 2:B54-B64. [PubMed] |
| 206. | Patel A, MacMahon S, Chalmers J, Neal B, Woodward M, Billot L, Harrap S, Poulter N, Marre M, Cooper M. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet. 2007;370:829-840. [PubMed] |
| 207. | Sramek SJ, Wallow IH, Day RP, Ehrlich EN. Ocular renin-angiotensin: immunohistochemical evidence for the presence of prorenin in eye tissue. Invest Ophthalmol Vis Sci. 1988;29:1749-1752. [PubMed] |
| 208. | Ramirez M, Davidson EA, Luttenauer L, Elena PP, Cumin F, Mathis GA, De Gasparo M. The renin-angiotensin system in the rabbit eye. J Ocul Pharmacol Ther. 1996;12:299-312. [PubMed] |
| 209. | Sramek SJ, Wallow IH, Tewksbury DA, Brandt CR, Poulsen GL. An ocular renin-angiotensin system. Immunohistochemistry of angiotensinogen. Invest Ophthalmol Vis Sci. 1992;33:1627-1632. [PubMed] |
| 210. | Vita JB, Anderson JA, Hulem CD, Leopold IH. Angiotensin-converting enzyme activity in ocular fluids. Invest Ophthalmol Vis Sci. 1981;20:255-257. [PubMed] |
| 211. | Weinreb RN, Sandman R, Ryder MI, Friberg TR. Angiotensin-converting enzyme activity in human aqueous humor. Arch Ophthalmol. 1985;103:34-36. [PubMed] |
| 212. | Immonen I, Friberg K, Sorsila R, Fyhrquist F. Concentration of angiotensin-converting enzyme in tears of patients with sarcoidosis. Acta Ophthalmol (Copenh). 1987;65:27-29. [PubMed] |
| 213. | Shiota N, Saegusa Y, Nishimura K, Miyazaki M. Angiotensin II-generating system in dog and monkey ocular tissues. Clin Exp Pharmacol Physiol. 1997;24:243-248. [PubMed] |
| 214. | Geng L, Persson K, Nilsson SF. Angiotensin converting anzyme (ACE) activity in porcine ocular tissue: effects of diet and ACE inhibitors. J Ocul Pharmacol Ther. 2003;19:589-598. [PubMed] |
| 215. | Tikellis C, Johnston CI, Forbes JM, Burns WC, Thomas MC, Lew RA, Yarski M, Smith AI, Cooper ME. Identification of angiotensin converting enzyme 2 in the rodent retina. Curr Eye Res. 2004;29:419-427. [PubMed] |
| 216. | Maruichi M, Oku H, Takai S, Muramatsu M, Sugiyama T, Imamura Y, Minami M, Ueki M, Satoh B, Sakaguchi M. Measurement of activities in two different angiotensin II generating systems, chymase and angiotensin-converting enzyme, in the vitreous fluid of vitreoretinal diseases: a possible involvement of chymase in the pathogenesis of macular hole patients. Curr Eye Res. 2004;29:321-325. [PubMed] |