Published online Sep 26, 2014. doi: 10.4330/wjc.v6.i9.959
Revised: June 25, 2014
Accepted: July 12, 2014
Published online: September 26, 2014
Processing time: 243 Days and 0.9 Hours
Arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D) is primarily believed to be an inherited cardiomyopathy that subsequently results in significant myocardial fibrosis. The arrhythmogenic consequences that result from the development of fibrosis are similar to other nonischemic cardiomyopathies, but the unique endocardial-epicardial disease process of ARVC/D requires a specialized approach for arrhythmia treatment in the electrophysiology laboratory. Although the association between ARVC/D and development of ventricular arrhythmias has become increasingly clear over the last 2 decades, our understanding of the arrhythmia mechanisms, underlying electrophysiologic substrate, and treatment strategies were significantly limited. Prospective studies performed in the electrophysiology laboratory allowed detailed characterization of the electrophysiologic and electroanatomic substrate underlying ventricular tachycardia in patients with ARVC/D. This has allowed clinician scientists to better characterize the arrhythmia mechanism and develop the necessary strategies to perform successful catheter ablation. Early in this experience, catheter ablation was considered a limited and largely unsuccessful treatment for patients experiencing painful and recurrent defibrillator therapy. Through our increased understanding of the disease process, catheter ablation has evolved to become an effective and preferred therapy for a majority of these patients. Our understanding of the disease and necessary approaches to provide successful treatment continues to evolve as the clinical experience grows. This article will review these important insights from the electrophysiology laboratory and how application of this knowledge has facilitated the development of a methodical approach to successfully perform ventricular tachycardia ablation in patients with ARVC/D.
Core tip: This review article evaluates seminal insights derived from the electrophysiology laboratory and the lessons learned to develop a methodical approach that can utilized to successfully perform ventricular tachycardia ablation in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia.
- Citation: Tschabrunn CM, Marchlinski FE. Ventricular tachycardia mapping and ablation in arrhythmogenic right ventricular cardiomyopathy/dysplasia: Lessons Learned. World J Cardiol 2014; 6(9): 959-967
- URL: https://www.wjgnet.com/1949-8462/full/v6/i9/959.htm
- DOI: https://dx.doi.org/10.4330/wjc.v6.i9.959
Arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D) is a fascinating disease that continues to challenge clinicians and scientists since it was first described in 1728[1]. Though several hypotheses have been proposed for the underlying cause of ARVC/D, it is primarily believed to be an inherited cardiomyopathy resulting from gene mutations that encode desmosomal proteins, the organelle responsible for cell-cell adhesion. Desmosomal dysfunction in patients with ARVC/D leads to inadequate cell adhesion and subsequent myocyte detachment and apoptosis[2,3]. The accumulation of fibrous and adipose tissue predominantly affects the right ventricular free wall and typically extends inward from the epicardium toward the endocardial surface[4,5]. Although this underlying process of ventricular scarring is unique to ARVC/D, the arrhythmogenic consequences that result from the development of fibrosis are similar to other nonischemic cardiomyopathies. The extensive right ventricle (RV) fibrosis results in inhomogeneous conduction with slow and discontinuous electrical propagation in sinus rhythm that serves as the substrate for ventricular arrhythmias. Although the association between ARVC/D and the subsequent development of ventricular arrhythmias had become increasingly clear following early clinical cohort reports, the characterization of arrhythmia mechanisms, the underlying electrophysiologic substrate, and treatment strategies were, until recently, poorly understood and limited.
Much of our understanding of the electrophysiologic and electroanatomic substrate underlying ventricular tachycardia (VT) in patients with ARVC/D was derived from studies performed within the electrophysiology laboratory. Through this experience, much has been learned about the arrhythmia mechanisms and strategies required to facilitate successful catheter ablation. The ability to localize and define the associated abnormalities essential for VT enhanced the effectiveness of catheter ablation procedures. What was once considered a treatment of last resort has now become the preferred therapy for most patients with documented ventricular arrhythmias. In addition, assessment of the anatomic substrate during electrophysiology procedures has shed important light on controversies pertaining to disease pathogenesis.
This review article will evaluate these seminal insights derived from the EP lab and the lessons learned to develop a methodical approach that can be utilized to successfully perform VT ablation in patients with ARVC/D.
Patients are typically diagnosed with ARVC/D after clinical manifestation of signs or symptoms during the second to fifth decade of life. We recommend implantable cardioverter-defibrillator (ICD) implantation to a majority of patients due to the high incidence of ventricular arrhythmias associated with the disease after a definitive diagnosis is made according to the task-force criteria guidelines[6]. Although ICD therapy is routine, management of recurrent VT with frequent device therapy can be difficult. Antiarrhythmic medications are often poorly tolerated and may only provide incomplete VT control. Inadequate arrhythmia control and the use of multiple antiarrhythmic medications is particularly debilitating for these young, and often physically very active patients.
Although techniques used in catheter ablation of VT in patients with ARVC/D have evolved over the last decade, outcomes are still inconsistent, ranging from 50%-90%[7]. This is likely the result of a number of different mapping and ablation strategies with variable endpoints, follow-up assessment, and operator experience[8-13]. In our experience at the University of Pennsylvania, a comprehensive ablation strategy that targets both the endocardial and epicardial substrate with elimination of abnormal electrograms and all inducible VT provides long-term drug-free arrhythmia control in a large majority of patients. For this reason, we offer catheter ablation to all patients with recurrent VT refractory or intolerant to medical therapy.
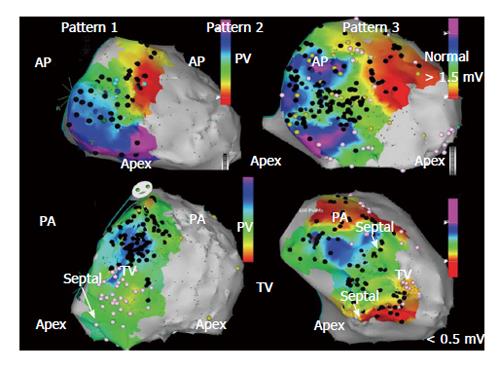
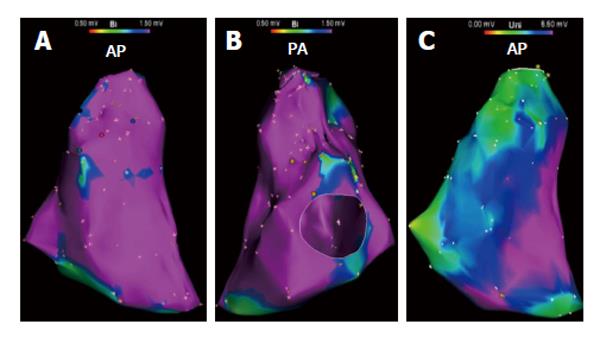
Advances in 3D electroanatomic mapping enabled a more thorough understanding of the complex electrophysiologic substrate in patients with ARVC/D and VT. Abnormal RV endocardial regions can be localized with electroanatomic mapping by identifying regions of low bipolar RV endocardial voltage (< 1.5 mV) and long-duration, low-amplitude, fractionated potentials. These key areas identified have been correlated to relevant histopathologic findings (myocyte loss with fibrofatty replacement) and critical VT circuits confirming the involvement of these areas in the arrhythmogenic mechanism[14]. The endocardial distribution of electroanatomic scar in patients with VT and ARVC/D typically extends from the triscuspid valve and/or the pulmonary valve to the RV free wall. Low-voltage abnormalities can also be found on the septal aspect of the perivalvular region(s), but typically does not include the RV apex (Figure 1)[15].
Although ARVC/D is known to primarily involve the RV, involvement of the left ventricle (LV) is more frequent than previously recognized. LV abnormalities have been documented with electroanatomic mapping and typically involve the basal perivalvular region, which is characteristic of other non-infarct related cardiomyopathies (Figure 2)[15]. Consideration of endocardial LV involvement is of particular importance if right bundle branch block VTs with positive R waves in the precordial leads are seen as this suggests an LV VT exit site of interest.
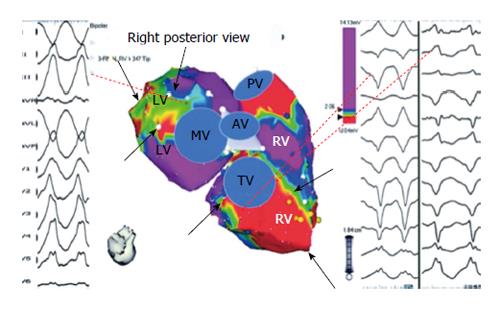
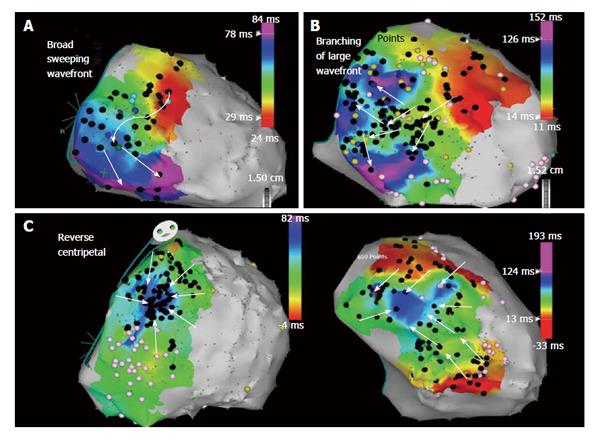
Despite periprocedural advances with irrigated ablation catheter technology and criteria to identify RV endocardial bipolar electroanatomic voltage abnormalities, the endocardial ablation approach provides only modest long-term arrhythmia freedom[15]. The epicardial to endocardial scarring process associated with ARVC/D often results in a more extensive abnormal epicardial substrate that may not be amendable to endocardial ablation alone. Insights from percutaneous epicardial mapping and ablation procedures in patients with ARVC/D and VT have demonstrated the important role of the epicardium. Abnormal epicardial low-voltage areas are typically much larger than the corresponding endocardial region; with extensive networks of late activation and fractioned signals[10,16]. Assessment of the epicardial voltage map should be performed with voltage threshold set to 1.0 mV to identify abnormalities consistent with scar as opposed to epicardial fat (Figure 3)[17]. Due to the widespread extent of confluent scarring in these patients, it is very common to identify multiple VT circuits that may involve both endocardial and epicardial surfaces. In addition, the dense mid-myocardial/sub epicardial fibrosis can create an effective barrier for endocardial to epicardial spread of activation. The resultant layered and delayed activation of the epicardium from the edges of the scar creates the milieu for an isolated VT circuit entirely confined to the epicardium and requiring epicardial access and direct ablation for elimination (Figure 4)[18]. In patients that have failed endocardial ablation, repeat ablation targeting the epicardial circuits was associated with superior long-term success rates[10]. For these reasons, the operator should always anticipate a high likelihood of needing epicardial access for mapping and ablation to achieve a successful outcome.
Although identification of abnormal epicardial substrate is best achieved through a percutaneous pericardial puncture, analysis of unipolar endocardial voltage maps with the associated larger field-of-view, provides information pertaining to the degree of epicardial abnormality present. Areas of unipolar voltage < 5.5 mV are associated with epicardial abnormalities. Unipolar voltage abnormalities identified during RV endocardial mapping that far exceed the bipolar endocardial substrate is highly suggestive of a more extensive epicardial > endocardial substrate that is consistent with the ARVC/D substrate in patients with VT (Figure 5). Additional clues to the requirement for epicardial mapping and ablation include surface ECG morphologies of VT suggesting epicardial exits (QS complex in the inferior leads and/or right precordial leads), the presence of isolated epicardial scar on magnetic resonance or intracardiac echo imaging, and/or prior failed endocardial ablation[16,19,20].
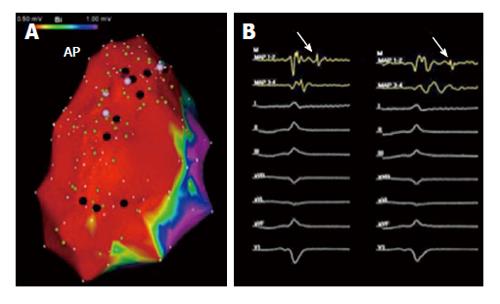
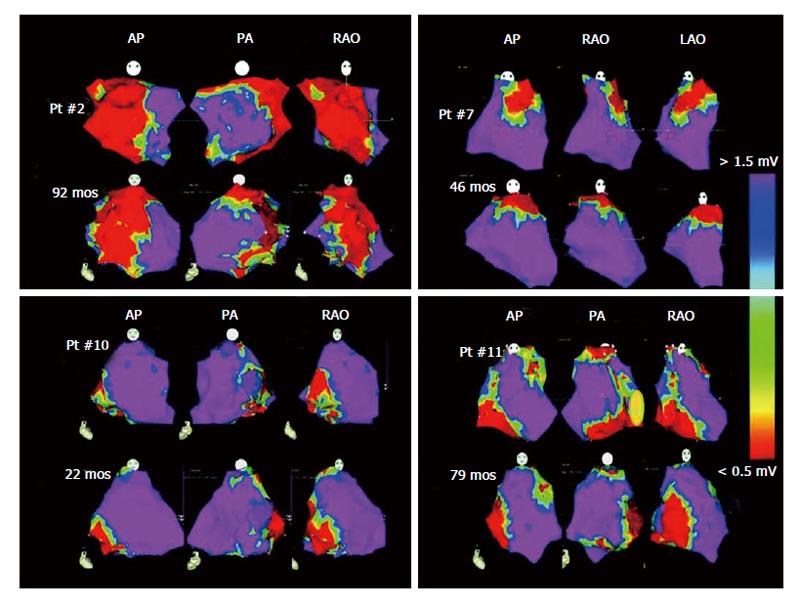
Although much has been learned about the process of fibrosis underlying ARVC/D, there continues to be significant phenotypic variability for reasons that have not been clearly elucidated. Of note, multiple genes that have been implicated in the disease and this may lead to marked variability of phenotypic expression. It is unclear if disease progression is the result of a continuously progressive degenerative process or rather periods of disease stability followed by serial deteriorations associated with a distinct triggering event. The belief that ARVC/D is a degenerative disorder has notably influenced treatment plans, particularly referral for catheter ablation. The degenerative hypothesis has been used as an explanation of unfavorable outcomes, thus labeling catheter ablation as a limited therapeutic option for patients with ARVC/D and VT. We have demonstrated, utilizing detailed electroanatomic mapping, progressive RV dilatation in patients presenting for repeat ablation procedures, but with no or only minimal macroscopic scar progression in a majority of patients (Figure 6)[21]. This data along with the favorable outcome following endocardial/epicardial ablation and the demonstrated complex relationship between various genetic components and possible environmental or acquired factors favors a disease etiology that is not a primary deteriorating process.
Detailed assessment of the endocardial and epicardial electroanatomic maps has provided the much-needed insights into the complex abnormal substrate in patients with ARVC/D and VT. The cornerstone of developing a successful ablation approach in these patients requires a thorough understanding of this underlying substrate, particularly recognizing the importance of the epicardium. Through this evolving process, we have developed a systematic approach to evaluating the substrate and performing catheter ablation in these patients, much of which is centered on important lessons learned from within the electrophysiology laboratory over the last 2 decades.
The general principles common to the ablation of all scar-related VT also apply in ARVC/D. However, in contrast to the post-infarction patient, the operator should anticipate a high likelihood of needing epicardial access for mapping and ablation to achieve a successful outcome for the reasons discussed. Evaluation in the EP laboratory typically begins with patients under conscious sedation to maximize the chances that induced VTs will be hemodynamically tolerated. A detailed RV endocardial voltage map is created in sinus rhythm using the standard 0.5-1.5 mV voltage cutoffs to define the endocardial substrate as previously discussed[13,22]. Special attention is focused on the periannular area and any identified low-voltage areas to ensure adequate sampling has occurred[23-25]. Occasionally, it can be technically challenging to perform catheter manipulation in the periannular tricuspid valve region. It is imperative to ensure adequate catheter contact during mapping to confirm low-voltage areas are from abnormal substrate and not inadequate catheter-tissue contact. This process can be facilitated by (1) using a sheath that extends transvenously to the tricuspid valve and provides stability; and/or (2) looping the mapping catheter in the RV to facilitate acquisition of detailed recording along the free wall adjacent to the annulus. Colored tags are placed on the electroanatomic map when fractionated signals and/or isolated late potentials are identified to keep track of their location[26,27]. Pacemapping is performed at sites of interest with late potentials and other multicomponent electrograms and are carefully analyzed. A match of the pacemap QRS morphology of the VT coupled with a long stimulus to QRS interval will identify additional sites of interest, which are given their own unique color tag. A sudden transition in paced QRS morphology coupled with changes in the stimulus to QRS interval may define anatomic boundaries of the isthmus or if a long stimulus to QRS is still identified a critical isthmus of conduction that will need to be tagged and ultimately targeted for ablation.
After completing the endocardial RV sinus rhythm substrate map and detailed pacemapping, programmed ventricular stimulation is performed. ICD electrograms are also recorded when VT is initiated. Induced VT ECG morphology and ICD electrograms are compared to previously captured clinical arrhythmias in addition to the pacemap morphologies that were obtained during sinus rhythm mapping. Assessment of ICD electrograms may be especially useful if clinical arrhythmia ECG tracings are unavailable[28]. An endocardial ablation strategy is guided primarily by activation and entrainment mapping whenever possible of any hemodynamically tolerated VTs. It is not uncommon for unstable VT to be induced that is characterized by changes in morphology with any catheter manipulation or rates that results in hemodynamic instability. The VTs may not be amenable to localization utilizing conventional activation and entrainment mapping techniques. In these cases, ablation is guided by pacemapping and detailed substrate assessment. Ablation lesions are usually applied with an irrigated tip catheter for a minimum of 90 s. Power delivery begins at 20 Watts and is typically titrated to a maximum of 40 Watts to obtain a 12-15 Ohm impedance drop or approximately 10% decrease from the baseline impedance.
Any VT that can be mapped and successfully ablated from the endocardium is targeted initially. The use of irrigated RF energy delivery may eliminate all induced VTs with endocardial ablation alone[13] though this is less likely in the context of ARVC/D than in ischemic VT. Epicardial mapping is required in cases when inducible VT is still present after endocardial ablation and should be planned for most patients.
When appropriate, the next step in the mapping and ablation procedure is to obtain intrapericardial access and perform epicardial mapping[29]. We prefer to perform detailed endocardial mapping and ablation before proceeding with epicardial access. This has the advantage of eliminating many of the VTs and allowing for some VT control in most patients should the procedure be aborted due to difficulties or complications that may occur from the pericardial puncture.
Patients are usually placed under general anesthesia prior to performing the pericardial puncture, though we have performed the procedure under conscious sedation with remifentanil and midazolam in select cases[30]. A posterior access approach after subxiphoid entry with the Touhy needle is favored as patients with ARVC/D typically have RV dilatation that may increase the risk of RV perforation with an anterior approach (surgical backup should always be readily available throughout the procedure). The posterior epicardial approach should begin just under the rib margin to minimize risk of liver laceration (Supplemental Video). After pericardial access is obtained, a detailed epicardial sinus rhythm voltage map is created in a similar fashion as described for the endocardium, but with voltage threshold set to 1.0 mV to identify abnormalities consistent with scar as previously described[17]. Programmed stimulation is repeated and, if hemodynamically tolerated, the induced VTs are mapped using conventional activation and entrainment techniques. When this is not possible, as is frequently the case and further exacerbated by additional anesthetic vasodilatory effects, a substrate ablation strategy is required. An extensive epicardial lesion set is designed to incorporate sites of pacemap QRS matches to induced or clinical VT morphologies and all markedly abnormal multicomponent or late electrograms within the low voltage area that were identified during the detailed substrate map. On occasion we have been able to map the sequence of late potentials from earliest to latest originating at the scar border (Figures 4 and 7) and targeting the earliest late potential can effectively eliminate a large area of subsequent late potential activation. Occasionally, areas of abnormal electrograms can extend beyond the defined area of low voltage and these signals, late or split electrograms, should be targeted for ablation particularly if associated with a long stimulus to QRS and QRS morphology with pacing that matches the VT. These observations emphasized the crucial importance of paying attention to electrogram characteristics as well as voltage when performing epicardial substrate mapping.
Coronary angiography is performed prior to epicardial ablation to ensure adequate distance between the coronary arteries (particularly the RV marginal branch of the right coronary artery) and ablation catheter. Phrenic nerve proximity to epicardial ablation sites is not an issue in ARVC/D cases although direct diaphragmatic stimulation may occur on the diaphragmatic surface of the RV. Epicardial ablation parameters for energy delivery are generally similar to the endocardium although the irrigation flow-rate is maintained at a lower rate (10-17 mL/min) to avoid unnecessary fluid accumulation with less concern about coagulum formation. Ideally, intracardiac echocardiography (ICE) is used to monitor lesion development and to assess for any complications throughout the procedure. Epicardial fat and thick fibrofatty replacement tissue can make it difficult to create lesions during epicardial ablation. It is important to ensure good contact at the catheter-tissue interface and optimal impedance drops for each lesion.
At the conclusion of the procedure, triamcinolone acetate (2 mg/kg) is injected into the pericardial space and allowed to disperse for 15 min before any suction is applied[31]. This has been shown to minimize the severity of the pericardial inflammatory reaction following epicardial ablation lesion delivery and facilitates future percutaneous pericardial access if required[32]. The pericardial drain is then attached to a passive suction device and removed the following morning after a transthoracic echocardiogram has confirmed no fluid accumulation overnight.
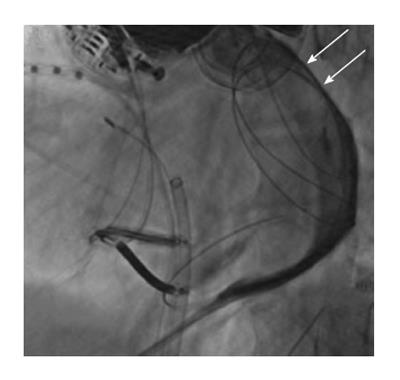
If a repeat procedure is required, the operator should be prepared to encounter pericardial adhesions during repeat epicardial mapping and ablation. These adhesions will be typically limited early after the initial procedure. The adhesions can usually be interrupted with the use of a steerable flexed catheter tip coupled with a steerable sheath. Elimination of the adhesions will allow for detailed mapping[32]. Careful monitoring for bleeding is important when disrupting the adhesions and is facilitated by utilizing ICE throughout this process. It is possible for dense adhesions to compartmentalize the epicardial surface and preclude access to the entire surface without re-accessing the pericardial space with a more anterior puncture (Figure 8)[33]. When adhesions are found to be resistant to dissection, consideration should be made to have the patient undergo an open-chest procedure through a surgical incision, as the adhesions may not be safely lysed if a flexed catheter “u” shape cannot be advanced. A sternotomy may be required to permit dissection of matted pericardium that may not be amenable to catheter dissection.
Our experience has consistently demonstrated that extensive endocardial and epicardial ablation is likely required to abolish all induced VTs. The primary procedure endpoint in these cases is both the elimination of a majority of abnormal electrograms and elimination of all induced VTs. Aggressive programmed stimulation should be performed as part of the evaluation of efficacy, including 2 stimulation sites, the introduction of up to triple extrastimuli, and the use of isoproterenol before the VT is described as non-inducible.
Non-invasive programmed stimulation through the ICD is performed 1-2 d after the ablation off antiarrhythmic drugs (AADs) to ensure continued non-inducibility[34]. Induction of VT suggests the need for further ablation in order to achieve a favorable long-term antiarrhythmic drug-free outcome.
Whenever possible, AADs are discontinued and patients continue with beta-blocker therapy only. Approximately one third of our patients continue to be treated with low dose sotalol therapy, but the effort to discontinue amiodarone has been successful in all but one patient in our experience with 62 ARVC patients.
Ventricular tachycardia in ARVC/D patients can be a difficult clinical problem to manage. Antiarrhythmic medications are often ineffective or not tolerated leaving these young and active patients at high risk for recurrent ICD shocks. Much has been learned about the underlying arrhythmia substrate and the appropriate strategies required to facilitate successful catheter ablation. This comprehensive and extensive ablation strategy that targets both the endocardial and epicardial substrate with elimination of abnormal electrograms offers long-term, drug-free arrhythmia control in a majority of patients.
P- Reviewer: Alzand BSN, Lee TM, Nam GB S- Editor: Wen LL L- Editor: A E- Editor: Liu SQ
| 1. | Lancisi G. De motu cordis et aneurysmatibus posthuman in duas partes divisum. Rome: Giovanni Maria Salvioni 1728; . |
| 2. | Yang Z, Bowles NE, Scherer SE, Taylor MD, Kearney DL, Ge S, Nadvoretskiy VV, DeFreitas G, Carabello B, Brandon LI. Desmosomal dysfunction due to mutations in desmoplakin causes arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circ Res. 2006;99:646-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 224] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 3. | Saffitz JE. Arrhythmogenic cardiomyopathy and abnormalities of cell-to-cell coupling. Heart Rhythm. 2009;6:S62-S65. [PubMed] |
| 4. | Basso C, Thiene G, Corrado D, Angelini A, Nava A, Valente M. Arrhythmogenic right ventricular cardiomyopathy. Dysplasia, dystrophy, or myocarditis? Circulation. 1996;94:983-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 562] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 5. | Corrado D, Basso C, Thiene G, McKenna WJ, Davies MJ, Fontaliran F, Nava A, Silvestri F, Blomstrom-Lundqvist C, Wlodarska EK. Spectrum of clinicopathologic manifestations of arrhythmogenic right ventricular cardiomyopathy/dysplasia: A multicenter study. J Am Coll Cardiol. 1997;30:1512-1520. [RCA] [DOI] [Full Text] [Cited by in Crossref: 696] [Cited by in RCA: 657] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 6. | Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, Calkins H, Corrado D, Cox MG, Daubert JP. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation. 2010;121:1533-1541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1626] [Cited by in RCA: 1435] [Article Influence: 95.7] [Reference Citation Analysis (0)] |
| 7. | Arbelo E, Josephson ME. Ablation of ventricular arrhythmias in arrhythmogenic right ventricular dysplasia. J Cardiovasc Electrophysiol. 2010;21:473-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Dalal D, Jain R, Tandri H, Dong J, Eid SM, Prakasa K, Tichnell C, James C, Abraham T, Russell SD. Long-term efficacy of catheter ablation of ventricular tachycardia in patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy. J Am Coll Cardiol. 2007;50:432-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 164] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 9. | Ellison KE, Friedman PL, Ganz LI, Stevenson WG. Entrainment mapping and radiofrequency catheter ablation of ventricular tachycardia in right ventricular dysplasia. J Am Coll Cardiol. 1998;32:724-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 85] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Garcia FC, Bazan V, Zado ES, Ren JF, Marchlinski FE. Epicardial substrate and outcome with epicardial ablation of ventricular tachycardia in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation. 2009;120:366-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 263] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 11. | Reithmann C, Hahnefeld A, Remp T, Dorwarth U, Dugas M, Steinbeck G, Hoffmann E. Electroanatomic mapping of endocardial right ventricular activation as a guide for catheter ablation in patients with arrhythmogenic right ventricular dysplasia. Pacing Clin Electrophysiol. 2003;26:1308-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Satomi K, Kurita T, Suyama K, Noda T, Okamura H, Otomo K, Shimizu W, Aihara N, Kamakura S. Catheter ablation of stable and unstable ventricular tachycardias in patients with arrhythmogenic right ventricular dysplasia. J Cardiovasc Electrophysiol. 2006;17:469-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Marchlinski FE, Callans DJ, Gottlieb CD, Zado E. Linear ablation lesions for control of unmappable ventricular tachycardia in patients with ischemic and nonischemic cardiomyopathy. Circulation. 2000;101:1288-1296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 769] [Cited by in RCA: 776] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 14. | Kiès P, Bootsma M, Bax J, Schalij MJ, van der Wall EE. Arrhythmogenic right ventricular dysplasia/cardiomyopathy: screening, diagnosis, and treatment. Heart Rhythm. 2006;3:225-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 77] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Marchlinski FE, Zado E, Dixit S, Gerstenfeld E, Callans DJ, Hsia H, Lin D, Nayak H, Russo A, Pulliam W. Electroanatomic substrate and outcome of catheter ablative therapy for ventricular tachycardia in setting of right ventricular cardiomyopathy. Circulation. 2004;110:2293-2298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 182] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 16. | Polin GM, Haqqani H, Tzou W, Hutchinson MD, Garcia FC, Callans DJ, Zado ES, Marchlinski FE. Endocardial unipolar voltage mapping to identify epicardial substrate in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Heart Rhythm. 2011;8:76-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 196] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 17. | Cano O, Hutchinson M, Lin D, Garcia F, Zado E, Bala R, Riley M, Cooper J, Dixit S, Gerstenfeld E. Electroanatomic substrate and ablation outcome for suspected epicardial ventricular tachycardia in left ventricular nonischemic cardiomyopathy. J Am Coll Cardiol. 2009;54:799-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 249] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 18. | Haqqani HM, Tschabrunn CM, Betensky BP, Lavi N, Tzou WS, Zado ES, Marchlinski FE. Layered activation of epicardial scar in arrhythmogenic right ventricular dysplasia: possible substrate for confined epicardial circuits. Circ Arrhythm Electrophysiol. 2012;5:796-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Bazan V, Bala R, Garcia FC, Sussman JS, Gerstenfeld EP, Dixit S, Callans DJ, Zado E, Marchlinski FE. Twelve-lead ECG features to identify ventricular tachycardia arising from the epicardial right ventricle. Heart Rhythm. 2006;3:1132-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 88] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 20. | Bala R, Ren JF, Hutchinson MD, Desjardins B, Tschabrunn C, Gerstenfeld EP, Deo R, Dixit S, Garcia FC, Cooper J. Assessing epicardial substrate using intracardiac echocardiography during VT ablation. Circ Arrhythm Electrophysiol. 2011;4:667-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 21. | Riley MP, Zado E, Bala R, Callans DJ, Cooper J, Dixit S, Garcia F, Gerstenfeld EP, Hutchinson MD, Lin D. Lack of uniform progression of endocardial scar in patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy and ventricular tachycardia. Circ Arrhythm Electrophysiol. 2010;3:332-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Hsia HH, Callans DJ, Marchlinski FE. Characterization of endocardial electrophysiological substrate in patients with nonischemic cardiomyopathy and monomorphic ventricular tachycardia. Circulation. 2003;108:704-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 269] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 23. | Avella A, d’Amati G, Pappalardo A, Re F, Silenzi PF, Laurenzi F, DE Girolamo P, Pelargonio G, Dello Russo A, Baratta P. Diagnostic value of endomyocardial biopsy guided by electroanatomic voltage mapping in arrhythmogenic right ventricular cardiomyopathy/dysplasia. J Cardiovasc Electrophysiol. 2008;19:1127-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | Corrado D, Basso C, Leoni L, Tokajuk B, Bauce B, Frigo G, Tarantini G, Napodano M, Turrini P, Ramondo A. Three-dimensional electroanatomic voltage mapping increases accuracy of diagnosing arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation. 2005;111:3042-3050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 179] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 25. | Marchlinski FE. Perivalvular fibrosis and monomorphic ventricular tachycardia: toward a unifying hypothesis in nonischemic cardiomyopathy. Circulation. 2007;116:1998-2001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | de Bakker JM, van Capelle FJ, Janse MJ, Tasseron S, Vermeulen JT, de Jonge N, Lahpor JR. Fractionated electrograms in dilated cardiomyopathy: origin and relation to abnormal conduction. J Am Coll Cardiol. 1996;27:1071-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 112] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 27. | Nogami A, Sugiyasu A, Tada H, Kurosaki K, Sakamaki M, Kowase S, Oginosawa Y, Kubota S, Usui T, Naito S. Changes in the isolated delayed component as an endpoint of catheter ablation in arrhythmogenic right ventricular cardiomyopathy: predictor for long-term success. J Cardiovasc Electrophysiol. 2008;19:681-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Tschabrunn CM, Anter E, Marchlinski FE. Identifying non-inducible ventricular tachycardia origin utilizing defibrillator electrograms. J Interv Card Electrophysiol. 2013;36:243-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Sosa E, Scanavacca M, d’Avila A, Pilleggi F. A new technique to perform epicardial mapping in the electrophysiology laboratory. J Cardiovasc Electrophysiol. 1996;7:531-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 631] [Cited by in RCA: 649] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 30. | Mandel JE, Hutchinson MD, Marchlinski FE. Remifentanil-midazolam sedation provides hemodynamic stability and comfort during epicardial ablation of ventricular tachycardia. J Cardiovasc Electrophysiol. 2011;22:464-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 31. | d’Avila A, Neuzil P, Thiagalingam A, Gutierrez P, Aleong R, Ruskin JN, Reddy VY. Experimental efficacy of pericardial instillation of anti-inflammatory agents during percutaneous epicardial catheter ablation to prevent postprocedure pericarditis. J Cardiovasc Electrophysiol. 2007;18:1178-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 32. | Tschabrunn CM, Haqqani HM, Cooper JM, Dixit S, Garcia FC, Gerstenfeld EP, Callans DJ, Zado ES, Marchlinski FE. Percutaneous epicardial ventricular tachycardia ablation after noncoronary cardiac surgery or pericarditis. Heart Rhythm. 2013;10:165-169. [PubMed] |
| 33. | Tschabrunn CM, Haqqani HM, Zado ES, Marchlinski FE. Repeat percutaneous epicardial mapping and ablation of ventricular tachycardia: safety and outcome. J Cardiovasc Electrophysiol. 2012;23:744-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Frankel DS, Mountantonakis SE, Zado ES, Anter E, Bala R, Cooper JM, Deo R, Dixit S, Epstein AE, Garcia FC. Noninvasive programmed ventricular stimulation early after ventricular tachycardia ablation to predict risk of late recurrence. J Am Coll Cardiol. 2012;59:1529-1535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |