Published online Sep 26, 2014. doi: 10.4330/wjc.v6.i9.1030
Revised: August 19, 2014
Accepted: September 4, 2014
Published online: September 26, 2014
Processing time: 153 Days and 10.9 Hours
AIM: To evaluate the impact of thrombus aspiration (TA) on procedural outcomes in a real-world ST-segment elevation myocardial infarction (STEMI) registry.
METHODS: From May 2006 to August 2008, 542 consecutive STEMI patients referred for primary or rescue percutaneous coronary intervention were enrolled and the angiographic results and stent implantation characteristics were compared according to the performance of manual TA.
RESULTS: A total of 456 patients were analyzable and categorized in TA group (156 patients; 34.2%) and non-TA (NTA) group (300 patients; 65.8%). Patients treated with TA had less prevalence of multivessel disease (39.7% vs 54.7%, P = 0.003) and higher prevalence of initial thrombolysis in myocardial infarction flow < 3 (P < 0.001) than NTA group. There was a higher rate of direct stenting (58.7% vs 45.5%, P = 0.009), with shorter (24.1 ± 11.8 mm vs 26.9 ± 15.7 mm, P = 0.038) and larger stents (3.17 ± 0.43 mm vs 2.93 ± 0.44 mm, P < 0.001) in the TA group as compared to NTA group. The number of implanted stents (1.3 ± 0.67 vs 1.5 ± 0.84, P = 0.009) was also lower in TA group.
CONCLUSION: In an “all-comers” STEMI population, the use of TA resulted in more efficient procedure leading to the implantation of less number of stents per lesion of shorter lengths and larger sizes.
Core tip: Thrombus embolization is highly detected in ST-segment elevation myocardial infarction (STEMI) leading to unfavorable clinical outcomes. To prevent thrombus embolization, manual thrombus aspiration (TA) receives a high recommendation during primary percutaneous coronary intervention (PCI) by clinical practice guidelines. However, the TASTE trial, recently published, showing no impact of manual TA on short-term mortality, has reopened the debate about the role of this technique in STEMI. This study is one the first showing that manual TA optimizes stent implantation during primary PCI resulted in more efficient procedures, leading to the implantation of fewer, shorter and larger stents.
- Citation: Fernández-Rodríguez D, Alvarez-Contreras L, Martín-Yuste V, Brugaletta S, Ferreira I, De Antonio M, Cardona M, Martí V, García-Picart J, Sabaté M. Does manual thrombus aspiration help optimize stent implantation in ST-segment elevation myocardial infarction? World J Cardiol 2014; 6(9): 1030-1037
- URL: https://www.wjgnet.com/1949-8462/full/v6/i9/1030.htm
- DOI: https://dx.doi.org/10.4330/wjc.v6.i9.1030
ST-segment elevation myocardial infarction (STEMI) occurs as a result of atherosclerotic plaque rupture or erosion and platelet and coagulation activation leading to thrombus formation and complete coronary occlusion[1]. Primary percutaneous coronary intervention (PCI) with stent implantation is the preferred method to restore epicardial flow in STEMI[2,3]. Several thrombectomy devices have been developed with the aim to avoid any suboptimal myocardial reperfusion related to thrombus embolization, which might lead to unfavorable clinical outcome[4].
The randomized clinical trial (RCT) TAPAS, in particular, showed that manual thrombus aspiration (TA) improved myocardial reperfusion and reduced mortality in STEMI patients at 1-year follow-up[5,6]. These results, confirmed by other studies[7-10], including a meta-analysis[11] of 11321 patients from 20 RCT showing lower rates of late mortality, reinfarction and stent thrombosis in patients underwent manual TA compared with conventional primary PCI, led to a recommendation class IIa for manual TA in patients undergoing primary PCI for STEMI[12]. Nevertheless, the use of the thrombectomy devices is still controversial and not routine in STEMI patients, especially because some studies have shown no impact on clinical outcome[13-20], such as the TASTE trial[21]. This RCT, recently published, did not show any impact of manual TA on mortality or any of several other clinical outcomes at 30 d. Furthermore, the potential effect of TA on optimization of stent implantation has not been elucidated yet.
Therefore, we sought to investigate the factors which can lead to the use of the manual TA in STEMI and its impact on acute angiographic success and stent implantation characteristics in a real-world STEMI population.
Between May 2006 and August 2008, all consecutive patients with STEMI referred to our hospital for primary or rescue PCI were enrolled. There were no exclusion criteria. Clinical and angiographic characteristics of all patients were prospectively collected. All patients signed a written informed consent prior to PCI procedure and agreed to be clinically followed. At the time of the study an IRB approval was not formally necessary for observational registries that use a CE-mark approved device.
Patients treated with primary PCI were pretreated with aspirin (300 mg), clopidogrel loading dose (300 mg) and unfractioned heparin adjusted to weight. The use of glycoprotein (GP) IIb/IIIa inhibitors was left at the discretion of the operators in case of significant thrombus, slow or non-reflow of thrombotic complications. PCI was performed according to conventional clinical practice. Manual TA; using the 6-French Pronto V3® aspiration catheter (Vascular Solutions Inc, Minneapolis, MN) and the 6-French Export® aspiration catheter (Medtronic, Minneapolis, MN), was performed according to the operator’s choice; and patients were thereafter classified in TA group and non-thrombus aspiration (NTA) group.
Manual TA technique was performed as follows. The aspiration was started 2-cm before the culprit lesion and the aspiration catheter was advanced very slowly, crossing the lesion with continuous aspiration. The catheter was removed under aspiration even into the guiding catheter, with generous backflow after retrieving the thrombectomy device. At least two or three passages were performed. Manual TA was especially considered, in case of high thrombus burden and initial slow thrombolysis in myocardial infarction (TIMI) flow.
Time to treatment was defined as time from symptom onset to initial intracoronary therapy by TA or balloon inflation of the infarct-related coronary artery[22].
TIMI flow grade was evaluated pre guide-wire and post-PCI[6].
No-reflow was defined as a TIMI flow grade < 2 in absence of coronary dissection, coronary hematoma, occlusive coronary thrombosis or epicardial spasm[10]. Thrombus embolization was defined as circumscribed filling defects and/or abrupt cut off of a vessel distal to the target lesion or in other coronary vessel on the angiogram after PCI[23]. Coronary dissection was defined by the presence of a curvilinear filling defect parallel to the vessel lumen, contrast medium outside of the vessel lumen persisting after passage of contrast medium, or a spiral-shaped filling defect partially or totally obstructing the coronary artery lumen[24].
ST was defined and categorized, according to Academic Research Consortium[25]. Angiographic success was defined as final TIMI flow equals 3 plus absence of any angiographic complication.
The angiographic assessment was performed by consensus of two independent experienced interventional cardiologists. The primary end-point of this study was the rate of angiographic success, as above defined. Secondary end-points included technical and clinical issues related to the procedure as the number of implanted stents, the rates of direct stenting and post-dilatation, the maximal diameter of the implanted stents, the total stented length segment, the final TIMI flow and the resolution of the ST-segment elevation after primary PCI.
A clinical follow-up up to 3 years was performed by a clinical visit or telephone interview. Clinical outcomes were evaluated by measuring the rate of the major adverse cardiac events (MACE) defined as the combination of cardiac death, myocardial infarction (MI) and need for cardiac artery by-pass grafting (CABG) and its individual components, as well as the rate of all-cause death, and the need for target vessel and non-target vessel PCI revascularization. MI was defined according to the World Health Organization extended definition[26].
Continuous variables were explored for normal distribution with the Kolmogorov-Smirnov test. Normally distributed variables were expressed as mean (1 standard deviation) and non-normally distributed variables were expressed as median (inter-quartile range) and were compared using t-student or with Mann-Whitney tests as appropriate. Categorical variables were expressed as count (percentage) and were compared using the χ2 test.
In order to exclude confounding factors in primary end-point (angiographic success), multivariable logistic regression models were fitted to assess independent predictors. The following variables were tested for the predictors of the primary end-point: manual TA, age, gender, smoking history, prior MI, primary PCI, Killip class > I, initial TIMI flow = 0, use of GP IIb/IIIa inhibitors and the use of drug-eluting stents (DES). The result was reported as HR together with the 95%CI.
All P values were 2-tailed, with statistical significance set at a level of < 0.05. Statistical analyses were performed using SPSS Statistics 20.0 (SPSS Inc., Chicago, IL, United States).
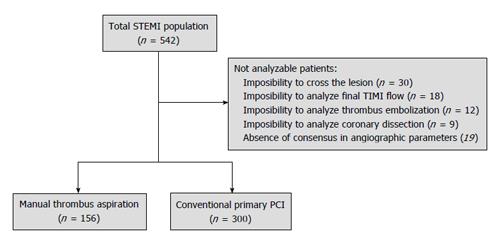
A total of 542 patients were prospectively included during the recruitment period. Of them, 30 patients were not analyzable because impossibility to crossing the culprit lesion by the TA device and 56 patients because inability to analyze the angiographic data. The remaining 456 patients were finally studied and classified in TA (n = 156) and NTA groups (n = 300) (Figure 1).
Baseline characteristics are presented in the Table 1. TA group exhibited lower prevalence of dyslipidemia (19.2% vs 30.7%, P = 0.009) and multivessel disease (39.8% vs 54.7%, P = 0.003) in comparison with NTA group. Conversely, TA was more often used in primary PCI (73.1% vs 68.7%, P = 0.013), in presence of initial TIMI flow < 3 (P < 0.001), and with concomitant use of GP IIb/IIIa inhibitors (65.3% vs 50.6%, P = 0.012) in comparison with NTA group.
| Characteristics | Thrombus aspiration | Conventional PCI | P value |
| n = 156 | n = 300 | ||
| Age, mean ± SD | 63.2 ± 12.8 | 64.3 ± 12.8 | 0.410 |
| Female sex | 38 (24.4) | 62 (20.7) | 0.370 |
| Previous or current smoker | 94 (60.3) | 205 (68.3) | 0.085 |
| Hypertension | 82 (52.6) | 166 (55.3) | 0.570 |
| Dyslipidemia | 30 (19.2) | 92 (30.7) | 0.009 |
| Peripheral vasculopathy | 9 (5.8) | 19 (6.3) | 0.800 |
| Previous MI | 10 (6.7) | 36 (12.3) | 0.065 |
| Previous PCI | 8 (5.1) | 29 (9.7) | 0.092 |
| Previous CABG | 2 (1.3) | 10 (3.3) | 0.190 |
| Indication | 0.013 | ||
| Primary | 114 (73.1) | 206 (68.7) | |
| Rescue | 42 (26.9) | 94 (31.3) | |
| Classification | 0.650 | ||
| Anterolateral | 69 (44.2) | 133 (44.3) | |
| Inferoposterior | 83 (53.2) | 152 (50.7) | |
| Non-Q MI | 3 (1.9) | 12 (4) | |
| LBBB | 1 (0.6) | 3 (1) | |
| Killip | 0.058 | ||
| I | 182 (86.3) | 228 (76.5) | |
| II | 13 (8.5) | 35 (11.7) | |
| III | 1 (0.7) | 7 (2.3) | |
| IV | 7 (4.6) | 28 (9.4) | |
| Number of diseased vessels | 0.003 | ||
| 1 | 94 (60.3) | 136 (45.3) | |
| 2 | 43 (27.6) | 95 (31.7) | |
| 3 | 19 (12.2) | 69 (23) | |
| Infarct related artery | 0.650 | ||
| LAD | 68 (43.6) | 137 (45.7) | |
| LCx | 15 (9.6) | 35 (11.7) | |
| RCA | 69 (44.2) | 116 (38.7) | |
| LM | 4 (2.6) | 8 (2.75) | |
| Bypass | 0 | 1 (0.3) | |
| GP IIb/IIIa inhibitors | 100 (65.3) | 150 (50.6) | 0.012 |
| IABP | 7 (4.5) | 25 (8.4) | 0.120 |
Main procedural results are presented in the Table 2. Patients included in TA group showed higher prevalence of angiographic success (78.8% vs 68%, P = 0.015) and better final TIMI flow (TIMI flow 3: 85.9% vs 78.3%, P = 0.04) in comparison with NTA group. Patients treated with TA received higher rate of direct stenting (58.7% vs 45.5%, P = 0.009), less number of stents implanted (1.3 ± 0.67 vs 1.5 ± 0.84, P = 0.009), with larger (3.17 ± 0.43 mm vs 2.93 ± 0.44 mm, P < 0.001) and shorter sizes (24.1 ± 11.8 mm vs 26.9 ± 15.7 mm, P = 0.038). The use of DES was lower in the TA group (DES; 11.3% vs 16.3%, P = 0.008). In multivariate analysis, TA was associated with angiographic success (HR = 2.3; 95%CI: 1.2-4.3) (Table 3).
| Characteristics | Thrombus aspiration | Conventional PCI | P value |
| n = 156 | n =300 | ||
| Time to treatment, median (IQR) | 273 (170-477) | 300 (180-480) | 0.610 |
| Initial TIMI flow | < 0.001 | ||
| 0 | 111 (71.2) | 143 (48.8) | |
| 1 | 9 (5.8) | 16 (5.5) | |
| 2 | 16 (10.3) | 38 (13) | |
| 3 | 20 (12.8) | 96 (32.8) | |
| Initial TIMI flow < 3 | 136 (87.2) | 204 (67.2) | < 0.001 |
| Final TIMI flow | 0.140 | ||
| 0 | 2 (1.3) | 9 (3.1) | |
| 1 | 1 (0.6) | 6 (2.1) | |
| 2 | 19 (12.3) | 50 (17.1) | |
| 3 | 133 (85.8) | 227 (77.7) | |
| Final TIMI flow < 3 | 22 (14.1) | 65 (21.7) | 0.040 |
| Angiographic complication | 0.450 | ||
| Non-reflow | 6 (3.8) | 16 (5.4) | |
| Thrombus embolization | 7 (4.5) | 22 (7.4) | |
| Coronary dissection | 2 (1.3) | 7 (2.4) | |
| Angiographic success | 123 (78.8) | 200 (68) | 0.015 |
| Direct stenting | 88 (58.7) | 131 (45.5) | 0.009 |
| Type of stent | 0.008 | ||
| BMS | 133 (88.7) | 238 (79.3) | |
| DES | 17 (11.3) | 62 (20.7) | |
| Length of stented segment (mm), mean ± SD | 24.1 ± 11.8 | 26.9 ± 15.7 | 0.038 |
| Diameter of stented segment (mm), mean ± SD | 3.17 ± 0.43 | 2.93 ± 0.44 | < 0.001 |
| Number of stents, mean ± SD | 1.3 ± 0.67 | 1.5 ± 0.84 | 0.009 |
| LVEF, mean ± SD | 49.6 ± 9.8 | 49 ± 10.4 | 0.610 |
| HR (95%CI) | P | |
| Thrombus aspiration | 2.3 (1.2-4.3) | 0.007 |
| Primary PCI | 4.4 (2.1-9) | < 0.001 |
| Active smoking | 1.76 (0.9-3.4) | 0.093 |
| Age | 1.031 (1.001-1.063) | 0.044 |
| Initial TIMI flow = 0 | 0.46 (0.25-0.84) | 0.012 |
In-hospital and long-term data are presented in the Table 4. No difference in major cardiac events was observed between groups during hospitalization. The only difference was a significantly higher CK peak [2563 (1284-4542) UI/L vs 1517 (744-3816) UI/L, P = 0.02] observed by the use of TA.
| Thrombus aspiration | Conventional PCI | P value | |
| n = 156 | n = 300 | ||
| In-hospital | |||
| CK peak UI/L, median (IQR) | 2563 (1284-4542) | 1517 (744-3816) | 0.020 |
| ST resolution at 30 min | 75 (71.4) | 174 (74) | 0.610 |
| Intra-procedural death | 2 (1.3) | 5 (1.7) | 1.000 |
| In-hospital cardiac death | 15 (9.6) | 22 (7.3) | 0.400 |
| Non-target vessel PCI revascularization | 12 (7.7) | 41 (13.8) | 0.059 |
| CABG | 0 (0) | 1 (0.3) | 1.000 |
| Follow-up | |||
| MACE | 25 (17.0) | 61 (21.6) | 0.250 |
| All-cause death | 26 (17.0) | 57 (19.6) | 0.500 |
| Cardiac death | 13 (8.3) | 23 (7.9) | 0.830 |
| MI | 10 (6.8) | 28 (10) | 0.270 |
| CABG | 2 (1.4) | 10 (3.5) | 0.390 |
| Target vessel PCI revascularization | 8 (5.4) | 25 (8.9) | 0.200 |
| Non-target vessel PCI revascularization | 7 (4.8) | 16 (5.7) | 0.680 |
| Definitive stent thrombosis | 2 (1.4) | 12 (4.4) | 0.150 |
| Probable stent thrombosis | 1 (0.7) | 2 (0.7) | 1.000 |
| Possible stent thrombosis | 2 (1.4) | 6 (2.2) | 0.720 |
At three years clinical follow-up (36 ± 7 mo), no differences between manual TA and conventional PCI were observed in the rates of MACE (17.0% vs 21.6%, P = 0.25), all-cause death (17.0% vs 19.6%, P = 0.5), cardiac death (8.3% vs 7.9%, P = 0.83), MI (6.8% vs 10%, P = 0.27), need for CABG revascularization (1.4% vs 3.5%, P = 0.39), target vessel PCI revascularization (5.4% vs 8.9%, P = 0.2), and non-target vessel PCI revascularization (4.8% vs 5.7%, P = 0.68) and definite ST (1.4% vs 4.4%, P = 0.15).
The major findings of this study were: (1) manual TA was used more often in primary PCI and in patients with worse TIMI flow; (2) its use was subsequently related to optimization of procedural technique; and (3) TA was independently associated with acute angiographic success.
According to clinical trials and real-world registries, our work confirms that manual TA is more often used in the presence of high thrombus burden, such as in patients with initial low TIMI flow (0-1) or primary PCI indication. This registry confirms as well that use of TA achieves better angiographic results than conventional PCI, with greater reduction in thrombus burden and higher rate of final TIMI flow 3. Of note is the recent article by Ahn et al[27] which showed that the addition of IIb/IIIa inhibitors (Abciximab) to manual TA improves the index of microcirculatory resistance and the microvascular obstruction assessed by cardiac magnetic resonance. This leads us to hypothesize that the optimal strategy to optimize myocardial perfusion would be the synergistic use of these two therapeutic options.
Moreover, it appeared that the use of TA allowed immediate good angiographic results before stent implantation, so that fewer, larger and shorter stents could be more often implanted. Previous clinical trials and real-world registries failed to show any differences in the length, diameter and number of implanted stents between patients treated with or without TA[6,7,10,20,28] except for one brief work[29] that demonstrated a higher stent diameter after manual TA, in STEMI patients treated with bare metal stents. Recently, the TASTE trial[21] also showed the need for fewer stents per procedure in manual TA group in comparison with conventional PCI. It is well known that intra-stent restenosis and ST are directly related to the characteristics of the stents[30,31]. Thus; optimizing on stent implantation using fewer stents and stents of larger diameter and smaller length, during STEMI could have long-term prognostic implications by reducing the intra-stent restenosis and ST.
Besides, in light of these results we might hypothesize that TA may be cost-saving. Therefore, further studies on cost-effectiveness implications by the use of manual TA in primary PCI are warranted.
This registry reflected real-world clinical practice in STEMI population as no exclusion criteria was applied. Additionally, both primary and rescue PCI patients were included.
Unlike other studies with strict inclusion criteria[6,10], this registry demonstrated no impact of TA on both short and long-term outcomes. In the TAPAS trial[5,6], only patients with primary PCI were included; in another real-world registry[10], only patients with primary PCI indication and TIMI flow 0-1 were included. Conversely, our clinical results are consistent with studies with broad inclusion criteria, such as the TASTE trial[21], that evaluated the primary end-point at short-term and with the largest published real-world registry in manual TA[20] that had a very extended follow-up. Both studies included patients with initial TIMI flow from 0 to 3 and rescue or primary PCI indication. Thus, differences in inclusion criteria and in follow-up periods between the various trials and inherent selection bias induced in clinical registries may explain the different impact of the TA on long-term outcome.
Furthermore, it is noteworthy that in our study MACE rate was numerically higher in NTA group, although it did not reach statistical significance, probably due to the small number of patients included in our registry.
Of note is that no difference in target-vessel revascularization or stent thrombosis was found between the two groups, despite implantation of larger and shorter stent in TA than NTA group: this finding may be explained by the higher rate of DES implanted in NTA group than TA group.
This interesting controversy will continue until the publication of the results of the TOTAL trial[32]. The TOTAL trial is a multicenter, prospective, open, international, randomized trial with blinded assessment of outcomes which will recruit 10700 STEMI patients to compare routine manual TA with the Export aspiration catheter vs conventional primary PCI alone. The primary outcome will be the composite of cardiovascular death, recurrent myocardial infarction, cardiogenic shock, or new or worsening New York Heart Association class IV heart failure up to 180 d.
First, this study is a non-randomized, prospective registry and there were differences in baseline clinical and angiographic characteristics that could lead to a worse baseline risk profile in NTA group. Second, the use of GP IIb/IIIa inhibitors was higher in the TA group and this difference could also affect angiographic results in this group. Third, in our study manual TA was only used in one third of cases, whereas current use of manual TA in recent all-comer RCT[33-35] is around two thirds of patients. This was related to the relatively lack of evidence of manual thrombectomy at the time of the recruitment of the registry. Fourth, the relative small number of patients included in our study could preclude any conclusions regarding clinical efficacy of TA.
In this all-comer registry, TA was able to optimize stent implantation technique, leading to the implantation of less number of stents per lesion of shorter lengths and larger sizes, and was associated with angiographic success following PCI for STEMI.
In ST-segment elevation myocardial infarction (STEMI) patients, manual thrombus aspiration (TA) is effective to reduce thrombus burden. Nevertheless, the effect on optimization of stent implantation has not been elucidated yet. Therefore, the objective of this study is to evaluate the impact of manual TA on acute angiographic success and stent implantation characteristics in a real-world STEMI.
Manual TA reduces thrombotic burden, receiving a recommendation class IIa during the performance of primary percutaneous coronary intervention. However, the TASTE trial, recently published, showing no impact of manual TA on 30-d mortality, has reopened the debate about the role of this technique in STEMI setting.
Thrombus embolization is detected up to 15% of STEMI population and is responsible for suboptimal myocardial reperfusion, leading to unfavorable clinical outcomes. Manual TA reduces thrombotic burden and receives a high recommendation during the performance of primary percutaneous coronary intervention. The TASTE trial, demonstrating absence of impact of manual TA on short-term mortality, has reopened the debate about the use of this technique in STEMI patients. In the present study the authors want to investigate, in a real-world STEMI population, the factors which can lead to the use of manual thrombectomy in STEMI and its impact on angiographic and stent implantation characteristics.
The study results suggest that manual TA during primary percutaneous coronary intervention is associated with a higher rate of angiographic success and optimization on stent implantation compared with conventional primary percutaneous coronary intervention, in a real-world population. However, it seems to have no impact on long-term clinical outcomes.
STEMI: It is a type of acute coronary syndromes, which occurs when a coronary artery becomes totally blocked by a blood clot, causing the heart muscle supplied by the artery to die; Primary percutaneous coronary intervention: It is a non-surgical procedure used to open the occluded coronary arteries during STEMIs; Manual TA device: It is a type of thrombectomy device, which comprises a monorail catheter with a central lumen connected proximally to a syringe for manual aspiration, designed to extract thrombotic material during percutaneous coronary intervention.
In this study, Diego et al reported that the thrombus aspiration therapy in patients with AMI were associated with high procedure success and contributed to optimize the implantation of stents. As a non-randomized, prospective registry study, it provide their some new insights about the use of thrombus aspiration in the real world.
P- Reviewer: Gong KZ, Lazzeri C, Patanè S S- Editor: Song XX L- Editor: A E- Editor: Liu SQ
| 1. | Van de Werf F, Ardissino D, Betriu A, Cokkinos DV, Falk E, Fox KA, Julian D, Lengyel M, Neumann FJ, Ruzyllo W. Management of acute myocardial infarction in patients presenting with ST-segment elevation. The Task Force on the Management of Acute Myocardial Infarction of the European Society of Cardiology. Eur Heart J. 2003;24:28-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 902] [Cited by in RCA: 842] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 2. | Wijns W, Kolh P, Danchin N, Di Mario C, Falk V, Folliguet T, Garg S, Huber K, James S, Knuuti J. Guidelines on myocardial revascularization. Eur Heart J. 2010;31:2501-2555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1660] [Cited by in RCA: 1715] [Article Influence: 114.3] [Reference Citation Analysis (0)] |
| 3. | Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361:13-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2931] [Cited by in RCA: 2774] [Article Influence: 126.1] [Reference Citation Analysis (1)] |
| 4. | Valgimigli M, Campo G, Malagutti P, Anselmi M, Bolognese L, Ribichini F, Boccuzzi G, de Cesare N, Rodriguez AE, Russo F. Persistent coronary no flow after wire insertion is an early and readily available mortality risk factor despite successful mechanical intervention in acute myocardial infarction: a pooled analysis from the STRATEGY (Single High-Dose Bolus Tirofiban and Sirolimus-Eluting Stent Versus Abciximab and Bare-Metal Stent in Acute Myocardial Infarction) and MULTISTRATEGY (Multicenter Evaluation of Single High-Dose Bolus Tirofiban Versus Abciximab With Sirolimus-Eluting Stent or Bare-Metal Stent in Acute Myocardial Infarction Study) trials. JACC Cardiovasc Interv. 2011;4:51-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Svilaas T, Vlaar PJ, van der Horst IC, Diercks GF, de Smet BJ, van den Heuvel AF, Anthonio RL, Jessurun GA, Tan ES, Suurmeijer AJ. Thrombus aspiration during primary percutaneous coronary intervention. N Engl J Med. 2008;358:557-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 799] [Cited by in RCA: 741] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 6. | Vlaar PJ, Svilaas T, van der Horst IC, Diercks GF, Fokkema ML, de Smet BJ, van den Heuvel AF, Anthonio RL, Jessurun GA, Tan ES. Cardiac death and reinfarction after 1 year in the Thrombus Aspiration during Percutaneous coronary intervention in Acute myocardial infarction Study (TAPAS): a 1-year follow-up study. Lancet. 2008;371:1915-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 630] [Cited by in RCA: 595] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 7. | Sardella G, Mancone M, Bucciarelli-Ducci C, Agati L, Scardala R, Carbone I, Francone M, Di Roma A, Benedetti G, Conti G. Thrombus aspiration during primary percutaneous coronary intervention improves myocardial reperfusion and reduces infarct size: the EXPIRA (thrombectomy with export catheter in infarct-related artery during primary percutaneous coronary intervention) prospective, randomized trial. J Am Coll Cardiol. 2009;53:309-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 269] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 8. | De Luca G, Dudek D, Sardella G, Marino P, Chevalier B, Zijlstra F. Adjunctive manual thrombectomy improves myocardial perfusion and mortality in patients undergoing primary percutaneous coronary intervention for ST-elevation myocardial infarction: a meta-analysis of randomized trials. Eur Heart J. 2008;29:3002-3010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 179] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 9. | Burzotta F, De Vita M, Gu YL, Isshiki T, Lefèvre T, Kaltoft A, Dudek D, Sardella G, Orrego PS, Antoniucci D. Clinical impact of thrombectomy in acute ST-elevation myocardial infarction: an individual patient-data pooled analysis of 11 trials. Eur Heart J. 2009;30:2193-2203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 181] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 10. | Mangiacapra F, Wijns W, De Luca G, Muller O, Trana C, Ntalianis A, Heyndrickx G, Vanderheyden M, Bartunek J, De Bruyne B. Thrombus aspiration in primary percutaneous coronary intervention in high-risk patients with ST-elevation myocardial infarction: a real-world registry. Catheter Cardiovasc Interv. 2010;76:70-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Kumbhani DJ, Bavry AA, Desai MY, Bangalore S, Byrne RA, Jneid H, Bhatt DL. Aspiration thrombectomy in patients undergoing primary angioplasty: Totality of data to 2013. Catheter Cardiovasc Interv. 2014;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC), Steg PG, James SK, Atar D, Badano LP, Blömstrom-Lundqvist C, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fernandez-Aviles F, Gershlick AH, Giannuzzi P, Halvorsen S, Huber K, Juni P, Kastrati A, Knuuti J, Lenzen MJ, Mahaffey KW, Valgimigli M, van 't Hof A, Widimsky P, Zahger D. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569-2619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3540] [Cited by in RCA: 3702] [Article Influence: 284.8] [Reference Citation Analysis (0)] |
| 13. | Chao CL, Hung CS, Lin YH, Lin MS, Lin LC, Ho YL, Liu CP, Chiang CH, Kao HL. Time-dependent benefit of initial thrombosuction on myocardial reperfusion in primary percutaneous coronary intervention. Int J Clin Pract. 2008;62:555-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Chevalier B, Gilard M, Lang I, Commeau P, Roosen J, Hanssen M, Lefevre T, Carrié D, Bartorelli A, Montalescot G. Systematic primary aspiration in acute myocardial percutaneous intervention: a multicentre randomised controlled trial of the export aspiration catheter. EuroIntervention. 2008;4:222-228. [PubMed] |
| 15. | Liistro F, Grotti S, Angioli P, Falsini G, Ducci K, Baldassarre S, Sabini A, Brandini R, Capati E, Bolognese L. Impact of thrombus aspiration on myocardial tissue reperfusion and left ventricular functional recovery and remodeling after primary angioplasty. Circ Cardiovasc Interv. 2009;2:376-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Silva-Orrego P, Colombo P, Bigi R, Gregori D, Delgado A, Salvade P, Oreglia J, Orrico P, de Biase A, Piccalò G. Thrombus aspiration before primary angioplasty improves myocardial reperfusion in acute myocardial infarction: the DEAR-MI (Dethrombosis to Enhance Acute Reperfusion in Myocardial Infarction) study. J Am Coll Cardiol. 2006;48:1552-1559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 177] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 17. | Mongeon FP, Bélisle P, Joseph L, Eisenberg MJ, Rinfret S. Adjunctive thrombectomy for acute myocardial infarction: A bayesian meta-analysis. Circ Cardiovasc Interv. 2010;3:6-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 18. | Burzotta F, Testa L, Giannico F, Biondi-Zoccai GG, Trani C, Romagnoli E, Mazzari M, Mongiardo R, Siviglia M, Niccoli G. Adjunctive devices in primary or rescue PCI: a meta-analysis of randomized trials. Int J Cardiol. 2008;123:313-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | De Luca L, Sardella G, Davidson CJ, De Persio G, Beraldi M, Tommasone T, Mancone M, Nguyen BL, Agati L, Gheorghiade M. Impact of intracoronary aspiration thrombectomy during primary angioplasty on left ventricular remodelling in patients with anterior ST elevation myocardial infarction. Heart. 2006;92:951-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 114] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 20. | Hachinohe D, Jeong MH, Saito S, Kim MC, Cho KH, Ahmed K, Hwang SH, Lee MG, Sim DS, Park KH. Clinical impact of thrombus aspiration during primary percutaneous coronary intervention: results from Korea Acute Myocardial Infarction Registry. J Cardiol. 2012;59:249-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Fröbert O, Lagerqvist B, Olivecrona GK, Omerovic E, Gudnason T, Maeng M, Aasa M, Angerås O, Calais F, Danielewicz M. Thrombus aspiration during ST-segment elevation myocardial infarction. N Engl J Med. 2013;369:1587-1597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 778] [Cited by in RCA: 827] [Article Influence: 68.9] [Reference Citation Analysis (0)] |
| 22. | Tarantini G, Cacciavillani L, Corbetti F, Ramondo A, Marra MP, Bacchiega E, Napodano M, Bilato C, Razzolini R, Iliceto S. Duration of ischemia is a major determinant of transmurality and severe microvascular obstruction after primary angioplasty: a study performed with contrast-enhanced magnetic resonance. J Am Coll Cardiol. 2005;46:1229-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 165] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 23. | Henriques JP, Zijlstra F, Ottervanger JP, de Boer MJ, van ‘t Hof AW, Hoorntje JC, Suryapranata H. Incidence and clinical significance of distal embolization during primary angioplasty for acute myocardial infarction. Eur Heart J. 2002;23:1112-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 461] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 24. | Lincoff AM, Popma JJ, Ellis SG, Hacker JA, Topol EJ. Abrupt vessel closure complicating coronary angioplasty: clinical, angiographic and therapeutic profile. J Am Coll Cardiol. 1992;19:926-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 264] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 25. | Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, Steg PG, Morel MA, Mauri L, Vranckx P. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344-2351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4265] [Cited by in RCA: 4676] [Article Influence: 259.8] [Reference Citation Analysis (0)] |
| 26. | Vranckx P, Cutlip DE, Mehran R, Kint PP, Silber S, Windecker S, Serruys PW. Myocardial infarction adjudication in contemporary all-comer stent trials: balancing sensitivity and specificity. Addendum to the historical MI definitions used in stent studies. EuroIntervention. 2010;5:871-874. [PubMed] |
| 27. | Ahn SG, Lee SH, Lee JH, Lee JW, Youn YJ, Ahn MS, Kim JY, Yoo BS, Yoon J, Choe KH. Efficacy of combination treatment with intracoronary abciximab and aspiration thrombectomy on myocardial perfusion in patients with ST-segment elevation myocardial infarction undergoing primary coronary stenting. Yonsei Med J. 2014;55:606-616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Burzotta F, Trani C, Romagnoli E, Mazzari MA, Rebuzzi AG, De Vita M, Garramone B, Giannico F, Niccoli G, Biondi-Zoccai GG. Manual thrombus-aspiration improves myocardial reperfusion: the randomized evaluation of the effect of mechanical reduction of distal embolization by thrombus-aspiration in primary and rescue angioplasty (REMEDIA) trial. J Am Coll Cardiol. 2005;46:371-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 258] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 29. | Bulum J, Ernst A, Strozzi M. The impact of successful manual thrombus aspiration on in-stent restenosis after primary PCI: angiographic and clinical follow-up. Coron Artery Dis. 2012;23:487-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Brodie B, Pokharel Y, Garg A, Kissling G, Hansen C, Milks S, Cooper M, McAlhany C, Stuckey T. Predictors of early, late, and very late stent thrombosis after primary percutaneous coronary intervention with bare-metal and drug-eluting stents for ST-segment elevation myocardial infarction. JACC Cardiovasc Interv. 2012;5:1043-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 31. | Cristea E, Stone GW, Mehran R, Kirtane AJ, Brener SJ. Changes in reference vessel diameter in ST-segment elevation myocardial infarction after primary percutaneous coronary intervention: implications for appropriate stent sizing. Am Heart J. 2011;162:173-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Jolly SS, Cairns J, Yusuf S, Meeks B, Shestakovska O, Thabane L, Niemelä K, Steg PG, Bertrand OF, Rao SV, Avezum A, Cantor WJ, Pancholy SB, Moreno R, Gershlick A, Bhindi R, Welsh RC, Cheema AN, Lavi S, Rokoss M, Džavík V. Design and rationale of the TOTAL trial: a randomized trial of routine aspiration ThrOmbecTomy with percutaneous coronary intervention (PCI) versus PCI ALone in patients with ST-elevation myocardial infarction undergoing primary PCI. Am Heart J. 2014;167:315-321.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 33. | Sabate M, Cequier A, Iñiguez A, Serra A, Hernandez-Antolin R, Mainar V, Valgimigli M, Tespili M, den Heijer P, Bethencourt A. Everolimus-eluting stent versus bare-metal stent in ST-segment elevation myocardial infarction (EXAMINATION): 1 year results of a randomised controlled trial. Lancet. 2012;380:1482-1490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 349] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 34. | Räber L, Kelbæk H, Ostojic M, Baumbach A, Heg D, Tüller D, von Birgelen C, Roffi M, Moschovitis A, Khattab AA. Effect of biolimus-eluting stents with biodegradable polymer vs bare-metal stents on cardiovascular events among patients with acute myocardial infarction: the COMFORTABLE AMI randomized trial. JAMA. 2012;308:777-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 238] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 35. | Sabaté M, Brugaletta S, Cequier A, Iñiguez A, Serra A, Hernádez-Antolín R, Mainar V, Valgimigli M, Tespili M, den Heijer P. The EXAMINATION trial (Everolimus-Eluting Stents Versus Bare-Metal Stents in ST-Segment Elevation Myocardial Infarction): 2-year results from a multicenter randomized controlled trial. JACC Cardiovasc Interv. 2014;7:64-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |