Published online Aug 26, 2014. doi: 10.4330/wjc.v6.i8.874
Revised: April 30, 2014
Accepted: June 10, 2014
Published online: August 26, 2014
Processing time: 173 Days and 9.4 Hours
Anomalous left coronary artery from the pulmonary artery (ALCAPA) is most commonly diagnosed within the first year of life with congestive heart failure symptomatology reflecting left ventricle (LV) dysfunction. The late diagnosis of ALCAPA is presented in a 5-year-old without significant LV dysfunction, mild LV dilatation and only mild mitral regurgitation that did not change significantly after surgery. The timing of surgical intervention in the late diagnosis of ALCAPA remains unclear despite risks of significant ongoing myocardial injury secondary to coronary artery hypoperfusion and progressive mitral valve dysfunction. Intervention in this case allows for revascularization which may reverse ventricular and valvular dysfunction.
Core tip: This case report presents the rare case of a late diagnosis of anomalous left coronary artery from the pulmonary artery and the associated challenge with determining the need for surgical revascularization in the absence of symptomatology and definitive literature. Late surgical intervention undertaken in this patient may reverse ongoing myocardial dysfunction and prevent permanent left ventricular damage.
- Citation: Lam JC, Giuffre M, Myers KA. Late intervention in an asymptomatic pediatric patient with anomalous left coronary artery. World J Cardiol 2014; 6(8): 874-877
- URL: https://www.wjgnet.com/1949-8462/full/v6/i8/874.htm
- DOI: https://dx.doi.org/10.4330/wjc.v6.i8.874
This case report describes a late diagnosis of anomalous left coronary artery from the pulmonary artery (ALCAPA) in an asymptomatic patient with only mild left ventricular dilatation. Because of a lack of literature to guide management in our patient, the benefits of surgical revascularization were weighed against risks of surgery on a hemodynamically stable patient with relatively preserved ventricular function. Ultimately, the patient underwent surgery in hopes of preventing further suboptimal coronary perfusion.
The patient was initially seen with an asymptomatic 2/6 apical systolic murmur at age two. The electrocardiogram was normal and the initial transthoracic echocardiogram demonstrated good ventricular function, no atrial or ventricular septal defect, and mild mitral regurgitation (MR). There was abnormal flow in the region of the left coronary artery (LCA) suspicious for a LCA fistula.
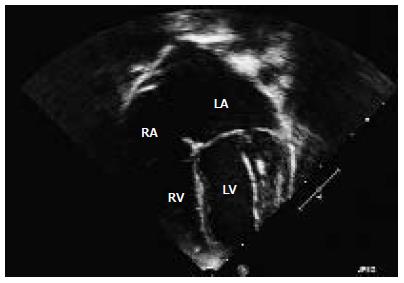
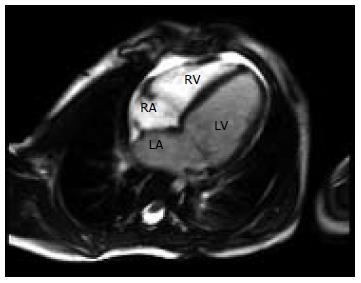
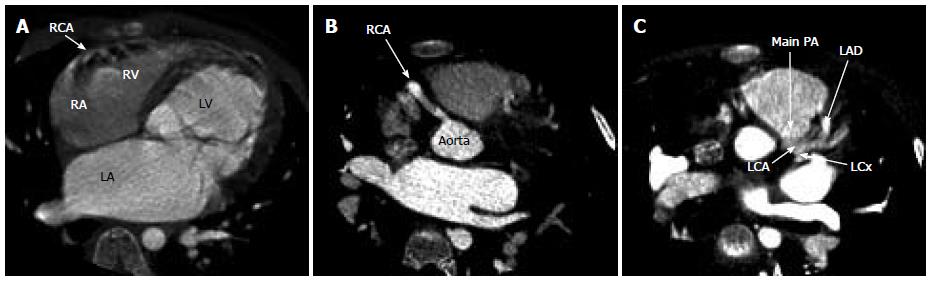
To further visualize the LCA, a transesophageal echocardiogram performed at age two under general anesthetic confirmed normal left and right ventricular function, a small LCA fistula and mild MR with normal ventricular chamber dimensions. Repeated echocardiograms showed evidence of mild dilatation of the left atrium (LA) and left ventricle (LV) with mild MR. Increased echogenicity of the LV myocardium was noted but there was no evidence of endocardial fibroelastosis on these pre-operative echocardiograms (Figure 1). At age 5 further investigation with cardiac magnetic resonance imaging (MRI) saw findings consistent with the previously noted dilated LA and LV, accompanied by normal LV systolic function and mild MR (Figure 2). The LCA system was suboptimally visualized by cardiac MRI but raised the question of an anomalous LCA. Cardiac computed tomography was then performed with angiography demonstrating ALCAPA. Numerous collateral vessels from the right coronary artery to the left coronary system, along with a dilated LA and LV were visualized (Figure 3).
Surgical repair was undertaken with a direct aortic reimplantation performed under cardiopulmonary bypass after arresting the heart with cold blood cardioplegia. The left coronary button was excised and anastomosed with a flap of ascending aorta. The pulmonary root was reconstructed with bovine pericardium before anastomosis to the main pulmonary artery (PA). The patient was weaned from cardiopulmonary bypass uneventfully after a total bypass time of 78 min.
Post-operatively, the patient was hemodynamically stable spending one night in the pediatric intensive care unit. Brief episodes of hypotension were managed routinely with inotropes and intravenous fluid. She was discharged ten days after surgery, weaned off oxygen and prescribed enalapril and aldactazide. Her post-operative course was complicated by mild postpericardiotomy syndrome that responded to acetylsalicylic acid therapy.
The immediate post-operative echocardiograms demonstrated mild MR along with mild dilatation of the LA and LV, and a small pericardial effusion. The patient’s LV function remained preserved with the shortening fraction improved from 35% to 40%. At her two-month post-operative assessment, she was asymptomatic, remaining on low dose enalapril. Her echocardiogram showed mild LA dilatation, mild LV dilatation, and two separate jets of mild MR. Ventricular function was relatively unchanged with a shortening fraction of 38% and ejection fraction of 69%.
Structural assessment of the LV demonstrated a quantitative reduction in LV dilatation following the procedure with the left ventricle internal diameter-diastole reduced from 4.4 cm pre-operatively to 3.9 cm one month after surgery. The degree of MR pre and post-operatively had not changed, with repeat assessment scheduled at six months post-operatively.
ALCAPA is rare congenital anomaly in which the LCA receives deoxygenated blood via the PA. It does not present as a hemodynamic problem in utero as high pulmonary vascular resistance allows for antegrade blood flow from the PA into the LCA. As the pulmonary vascular resistance falls, retrograde flow into the PA develops producing coronary steal or hypoperfusion.
ALCAPA has an incidence of 1/300000 live births[1] and its symptoms of failure to thrive, dyspnea, diaphoresis and findings of left sided heart failure usually present within the first two months of life[2]. Such early diagnoses of ALCAPA usually require prompt surgical revascularization. Without surgery, 90% of infants with ALCAPA will often die in the first year of life due to myocardial ischemia secondary to coronary vessel hypoxia[3].
In order to survive, collateral vessels are necessary to supply the LV myocardium. However, the ALCAPA continues to place stress on the LV, resulting in steadily deteriorating systolic function and LV scarring. Our patient’s MR secondary to subclinical decline of her LV function indicated that the structure of her LV was indeed a concern despite her collateral vasculature. The mildly increased echogenicity of the LV endocardium raised the concern of endocardial fibroelastosis eventually developing with resultant myocardial scarring. This led to the decision for surgery rather than careful cardiac follow-up.
Surgical revascularization is the treatment of choice for ALCAPA. The surgery is most often scheduled immediately after diagnosis of ALCAPA in order to prevent further ischemia and necrosis of cardiac muscle. Most surgeries involve either a Takeuchi operation or direct reimplantation of the LCA[3].
The role of revascularization in an asymptomatic patient with functional collateral coronary vessels and years of subclinical myocardial ischemia remains somewhat contentious. Literature in the adult population suggests that myocardium supplied by longstanding ischemic vasculature may be permanently scarred and unable to regain function despite intervention[4]. This did not appear to be the case with our 5-year-old patient. Surgical intervention places patients at risk of arrhythmias, acute cardiac failure and long-term complications such as intrapulmonary tunnel baffle leaks and suprapulmonary stenosis[3]; however, these did not occur in the case presented, but may develop over more time.
Recovery of LV function is well reported in pediatric patients following revascularization. However, our patient maintained normal ventricular function. Early diagnosis and intervention is correlated with a decreased need of mitral valve repair or replacement in the future[5]. Asymptomatic patients with unrepaired ALCAPA have chronic ischemia despite the production of collateral coronary vessels. The LV continues to undergo adverse remodeling with deterioration in systolic and diastolic function over time. Several studies have demonstrated successful recovery of LV function in patients following surgical intervention for ALCAPA[6,7]. However, patients with poor LV function pre-operatively have higher mortality rates and increased need for mitral valve intervention after ALCAPA surgery.
In the setting of decreased LV function, there is hypothesis regarding the possible existence of hibernating myocardium in ALCAPA. The hypothesis suggests myocytes supplied by the ALCAPA undergo an adaptive change and become hypokinetic rather than necrotic despite being hypoperfused[8]. The function and viability of the cardiomyocytes is preserved because of the successful formation and utilization of a compensatory but inadequate collateral supply. Shivalkar et al[2] demonstrated that although chronically hypoperfused, histology of myocardium from some patients with ALPACA is comprised of viable myocytes with minor morphological changes likely from cellular adaptation to longstanding ischemia.
This case illustrates the considerations in revascularizing an asymptomatic ALCAPA patient with longstanding hypoperfused myocardium but preserved ventricular function in the absence of definitive literature. Surgical intervention was recommended to prevent the potential of further LV dysfunction and mitral valve dysfunction manifesting from further suboptimal myocardial perfusion. The late diagnosis of ALCAPA in an asymptomatic 5-year-old with preserved LV function, mild LV dilatation and mild MR that did not change after surgery further illustrates the clinical dilemma of the timing of surgical intervention.
In conclusion, this case describes the late presentation of a child with subclinical myocardial ischemia secondary to ALCAPA. The patient compensated well hemodynamically for five years with only mild LV dilatation and mild MR. The need for surgical intervention considered the risks of cardiopulmonary bypass on scarred LV myocardium versus the benefits of repair and potential reversal of ongoing myocardial dysfunction and LV myocardial damage, but the timing of such surgery remains somewhat controversial.
A late diagnosis and operative revascularization procedure was performed in a 5-year-old female.
The patient presented with an asymptomatic apical systolic murmur.
Left coronary artery fistula, anomalous left coronary artery arising from the pulmonary artery
Cardiac computed tomography with angiography demonstrated an anomalous left coronary artery from the pulmonary artery.
Surgical revascularization with a direct aortic reimplantation was performed under cardiopulmonary bypass.
The role of revascularization in an older asymptomatic patient with anomalous left coronary artery from the pulmonary artery (ALCAPA) is unclear as there lacks strong clinical literature to guide timing of treatment.
Late surgical intervention ALCAPA may reverse ongoing myocardial dysfunction and prevent permanent left ventricular damage, even in hemodynamically stable patients.
The authors present a rare case report of late diagnosed ALCAPA. Surgical intervention was undertaken and left ventricular function was relatively preserved. The authors emphasized the importance of the timing of surgical intervention from the viewpoint of risks and benefits. The manuscript is clearly written and well organized.
P- Reviewer: Salemi VMC, Tagarakis G, Taguchi I, Ueda H S- Editor: Song XX L- Editor: A E- Editor: Wu HL
| 1. | Keith JD. The anomalous origin of the left coronary artery from the pulmonary artery. Br Heart J. 1959;21:149-161. [PubMed] |
| 2. | Shivalkar B, Borgers M, Daenen W, Gewillig M, Flameng W. ALCAPA syndrome: an example of chronic myocardial hypoperfusion? J Am Coll Cardiol. 1994;23:772-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 53] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Ginde S, Earing MG, Bartz PJ, Cava JR, Tweddell JS. Late complications after Takeuchi repair of anomalous left coronary artery from the pulmonary artery: case series and review of literature. Pediatr Cardiol. 2012;33:1115-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Shapiro BP, Mergo PJ, Austin CO, Kantor B, Gerber TC. Assessing the available techniques for testing myocardial viability: what does the future hold? Future Cardiol. 2012;8:819-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Michielon G, Di Carlo D, Brancaccio G, Guccione P, Mazzera E, Toscano A, Di Donato RM. Anomalous coronary artery origin from the pulmonary artery: correlation between surgical timing and left ventricular function recovery. Ann Thorac Surg. 2003;76:581-588; discussion 588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Schwartz ML, Jonas RA, Colan SD. Anomalous origin of left coronary artery from pulmonary artery: recovery of left ventricular function after dual coronary repair. J Am Coll Cardiol. 1997;30:547-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 118] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 7. | Vouhé PR, Tamisier D, Sidi D, Vernant F, Mauriat P, Pouard P, Leca F. Anomalous left coronary artery from the pulmonary artery: results of isolated aortic reimplantation. Ann Thorac Surg. 1992;54:621-626; discussion 627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 56] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Rein AJ, Colan SD, Parness IA, Sanders SP. Regional and global left ventricular function in infants with anomalous origin of the left coronary artery from the pulmonary trunk: preoperative and postoperative assessment. Circulation. 1987;75:115-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 47] [Article Influence: 1.2] [Reference Citation Analysis (0)] |