Published online Aug 26, 2014. doi: 10.4330/wjc.v6.i8.771
Revised: May 15, 2014
Accepted: May 28, 2014
Published online: August 26, 2014
Processing time: 263 Days and 14.4 Hours
Alcohol is the most frequently consumed toxic substance in the world. Low to moderate daily intake of alcohol has been shown to have beneficial effects on the cardiovascular system. In contrast, exposure to high levels of alcohol for a long period could lead to progressive cardiac dysfunction and heart failure. Cardiac dysfunction associated with chronic and excessive alcohol intake is a specific cardiac disease known as alcoholic cardiomyopathy (ACM). In spite of its clinical importance, data on ACM and how alcohol damages the heart are limited. In this review, we evaluate available evidence linking excessive alcohol consumption with heart failure and dilated cardiomyopathy. Additionally, we discuss the clinical presentation, prognosis and treatment of ACM.
Core tip: Cardiac dysfunction associated with excessive alcohol intake is a specific cardiac disease known as alcoholic cardiomyopathy. In spite of its clinical importance, data on alcoholic cardiomyopathy and how alcohol damages the heart are limited. In this review, we evaluate available evidence linking excessive alcohol consumption with heart failure and dilated cardiomyopathy. Additionally, we discuss the clinical presentation, prognosis and treatment of alcoholic cardiomyopathy.
- Citation: Guzzo-Merello G, Cobo-Marcos M, Gallego-Delgado M, Garcia-Pavia P. Alcoholic cardiomyopathy. World J Cardiol 2014; 6(8): 771-781
- URL: https://www.wjgnet.com/1949-8462/full/v6/i8/771.htm
- DOI: https://dx.doi.org/10.4330/wjc.v6.i8.771
Daily consumption of low to moderate amounts of alcohol has beneficial effects on cardiovascular health among both ischemic and non-ischemic patients[1-3]. In contrast, chronic and excessive alcohol consumption could lead to progressive cardiac dysfunction and heart failure (HF)[3].
HF is most frequently related to the presence of arterial hypertension and ischemic cardiomyopathy[4,5]. In younger individuals, however, where HF is less prevalent, a heterogeneous group of cardiac diseases, collectively known as cardiomyopathies, represent the leading cause of HF and heart transplant in the world[6]. Among cardiomyopathies, the variety that most often leads to HF and the first cause of heart transplant among young patients is dilated cardiomyopathy (DCM)[6]. DCM is defined as left ventricular systolic dysfunction and dilatation, which may or may not be associated with a similar right ventricular dysfunction. Excessive alcohol consumption is prominent among the multiple aetiologies causing DCM and has been considered the major cause of non-ischemic DCM in Western countries[7-12].
Despite the key clinical importance of alcohol as a cause of DCM, relatively few studies have investigated the effects of alcohol on the heart and the clinical characteristics of DCM caused by excessive alcohol consumption (known as alcoholic cardiomyopathy). Moreover, conflicting results are available regarding several factors related to alcoholic cardiomyopathy (ACM), such as the precise amount of alcohol necessary to cause the disease, whether the long-term prognosis of ACM is similar to that of other forms of DCM, or whether complete alcohol abstinence is necessary to improve clinical outcomes.
In this review, we evaluate the available evidence linking alcohol consumption with HF and DCM. We also discuss the clinical presentation, prognosis and treatment of ACM.
The depressing effect of alcohol on the heart has been known for some time. Indeed, the first account of the possible harmful effects of alcohol specifically on heart muscle was reported in the latter half of the 19th century. Expressions referring to “the heart of a wine drinker in Tubingen” and particularly a “Munich beer heart” were used and known in Germany during this time[13].
Bollinger, a pathologist in Munich in the late 19th century, was perhaps the first to suspect a possible link between excessive alcohol consumption and sudden death in young individuals, an occurrence that alarmed public opinion at the time. The diagnosis of the source of those deaths was found after performing autopsies and discovering the characteristic left ventricular dilatation and hypertrophy. The findings that led Bollinger to establish a causal relationship between alcohol consumption and these structural abnormalities were both of a clinical and an epidemiological nature. Thus, he identified that the incidence of alcohol-related DCM was much higher in Munich, where alcohol intake was greater, than in other German cities. Indeed, he found 42 cases of ACM from among 1500 autopsies performed in Munich, contrasting with a single case in Berlin from 809 hearts analysed. Also, he observed that these individuals often presented co-morbidities closely associated with alcohol consumption, including delirium tremens and cirrhosis of the liver, and that 22 of the 42 deceased individuals were regular patrons of beer houses in Munich, where they could drink from 6 to 12 L of beer per day[13].
Later, in 1902, William McKenzie, in his treatise on arterial and venous pulse and heart movements, described the existence of individuals who, in association with alcohol consumption, developed an accelerated heart pulse or swelling and engorgement of the veins, and according to his experience they had a poor prognosis with progressive heart failure. In their autopsies, he described finding dilated cavities of the heart and fatty degeneration of the ventricular walls[14].
Since those initial descriptions, reports on several isolated cases or in small series of patients with HF due to DCM and high alcohol intake have been published[15-17]. Some of these papers have also described the recovery of LVEF in many subjects after a period of alcohol withdrawal[15-17].
At present ACM is considered a specific disease both by the European Society of Cardiology (ESC) and by the American Heart Association (AHA)[18,19]. In the ESC consensus document on the classification of cardiomyopathies, ACM is classified among the acquired forms of DCM[19].
The diagnosis of ACM is usually one of exclusion in a patient with DCM with no identified cause and a long history of heavy alcohol abuse. According to most studies, the alcohol consumption required to establish a diagnosis of ACM is over 80 g per day during at least 5 years[9-12].
Data on the amount of alcohol consumption required to cause ACM are limited and controversial.
The first study, which specifically focused on the amount of alcohol necessary to cause ACM, was conducted by Koide et al[20] in 1975. The authors examined the prevalence of cardiomegaly by means of chest x-rays and related it to alcohol consumption among a consecutive series of Japanese males of working age. They found that 2 of the 6 individuals (33%) whose alcohol consumption exceeded 125 mL/d had cardiomegaly. In contrast, an enlarged heart was found in only 1 of 25 subjects with moderate consumption (4%), in 6 of 105 very mild consumers (5.7%), and in 4.5% of non-drinking individuals.
A second set of studies that are quoted when addressing this topic are those conducted in individuals who started an alcohol withdrawal program[21-24]. In these studies, the authors estimated the amount and chronicity of alcohol intake and subsequently related the figures to a number of echocardiographic measurements and parameters. Although all of the studies reported an increase in left ventricular mass and volume, it cannot generally be stated that they provided the alcohol consumption dosage required to cause ACM.
Askanas et al[21] found a significant increase in the myocardial mass and of the pre-ejection periods in drinkers of over 12 oz of whisky (approximately 120 g of alcohol) compared to a control group of non-drinkers. However, no differences were found in these parameters between the sub-group of individuals who had been drinking for 5 to 14 years and the sub-group of individuals who had a drinking history of over 15 years. Kino et al[22] found increased ventricular thickness when consumption exceeded 75 mL/d (60 g) of ethanol, and the increase was higher among those subjects who consumed over 125 mL/d (100 g), without specifying the duration of consumption. In another study on this topic, Lazarević et al[23] divided a cohort of 89 asymptomatic individuals whose consumption exceeded 80 g/d (8 standard units) into 3 groups according to the duration of their alcohol abuse. Subjects with a shorter period of alcohol abuse, from 5 to 10 years, had a significant increase in left ventricular diameter and volume compared to the control group. However, a systolic impairment was not found as the years of alcoholic abuse continued.
Unfortunately Lazarević et al[23], as in most of these studies, systematically excluded patients with a history of heart disease or with HF symptoms. It is therefore possible that most of these studies may have also consistently omitted most alcohol abusers in whom alcohol had already caused significant ventricular dysfunction.
One of the exceptions in these accounts is the study conducted by Urbano-Márquez et al[24], in which 46 asymptomatic alcohol abusers who were beginning an alcohol withdrawal program were studied together with 6 alcoholics identified at the emergency department due to HF symptoms. This is the only study describing the existence of a direct linear relationship between accumulated alcohol consumption throughout life and left ventricular mass (r = 0.42), fractional shortening (r = 0.35), and ejection fraction (r = 0.46) (all P < 0.001). A large number of studies, however, never reproduced this relationship, and it has been suggested that this relationship could correspond to the existence of a threshold dose above which the risk of suffering this disease increases[25]. Kupari et al[25], after reviewing the research by Urbano-Márquez, suggested a lifetime cumulative cut-off dose of alcohol of 20 kg/kg of weight. Actually, in the research by Urbano-Márquez et al[24], slight dysfunction of the left ventricle had already appeared due to cumulative doses of 10 kg of alcohol per kg of weight.
Finally, it should be noted that a large majority of studies on the long-term prognosis of ACM used the cut-off point of 80 g/d for a minimum of 5 years to consider alcohol as the cause of DCM. Although this figure may be sufficient to cause the structural alterations described above, we must stress that this value is arbitrary and is not based on robust experimental or epidemiological data; also, the average consumption of the individuals included in the research was always much greater[9-12] .
Additionally, the accepted ACM definition does not take into account a patient’s sex or body mass index (BMI). As women typically have a lower BMI than men, a similar amount of alcohol would reach a woman’s heart after consuming smaller quantities of alcohol.
For many decades, ACM has been considered one of the main causes of left ventricular dysfunction in developed countries. Specifically in the United States, ACM was declared the leading cause of non-ischemic DCM[7]; a fact related to the high consumption of alcoholic beverages worldwide, which is particularly elevated in Western countries[26] .
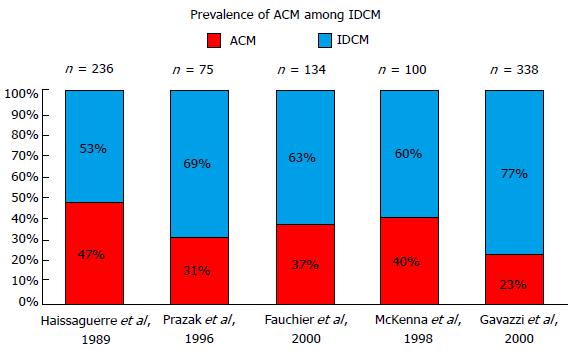
Studies that have assessed the prevalence of ACM among IDCM patients have found high alcohol consumption in 3.8% to 47% of DCM patients. The lowest prevalence of ACM among DCM (3.8%) was obtained from a series of 673 patients admitted to hospital consecutively due to HF in the state of Maryland[27]. This study included not only DCM, but also all causes of left ventricular dysfunction, including hypertensive heart disease, ischemic cardiomyopathy and heart valve disease. Furthermore, the inclusion criteria for ACM were very strict and required a minimum consumption of 8 oz of alcohol (200 g or 20 standard units) each day for over 6 mo. In contrast, European studies focusing on the prevalence of ACM included only subjects diagnosed with DCM and applied the consumption threshold of 80 g/d for ≥ 5 years, finding an ACM prevalence of 23%-47% among idiopathic DCM patients[9-12] (Figure 1).
Finally, it should be noted that McKenna and co-workers, in one of the most frequently cited papers in the ACM field, reported an incidence of 40% in 100 individuals suffering from idiopathic DCM, but in this case the consumption threshold used was only 30-40 g/d[8].
EVIDENCE LINKING EXCESSIVE ALCOHOL CONSUMPTION AND DCM
The existence of a direct causal link between excessive alcohol consumption and the development of DCM is a controversial issue. While some consider that this toxin alone is able to cause such a disease[18,19], others contend that it is just a trigger or an agent favouring DCM[3,21,22].
At present, however, ACM is considered to be a disease in its own right[18,19].
The evidence that allows this link to be established arises from 6 categories of research: (1) epidemiological studies; (2) experimental studies with controlled alcohol administration; (3) haemodynamic/echocardiographic studies analysing the effects generated by alcohol consumption on myocardial structure and function; (4) histological studies; (5) basic research studies identifying the mechanisms of alcohol-induced damage to the cardiomyocyte; and (6) studies analysing the positive clinical response to alcohol withdrawal.
Epidemiological studies analysing the relationship between excessive alcohol consumption and the development of DCM have found the existence of a reciprocal link between both disorders.
In this respect, a higher prevalence of excessive alcohol consumption has been reported among individuals diagnosed with DCM than in the general population[8].
In 1986, Komajda et al[28] reported that DCM patients admitted due to HF had higher alcohol consumption levels than patients admitted to undergo surgical procedures (101 mL/d vs 64 mL/d; RR = 7.6, P < 0.001).
Furthermore, Gillet published a similar study in which a cohort of 23 patients with DCM reported higher average daily alcohol consumption (82 g/d vs 30 g/d; P < 0.001) and a greater duration of that consumption (34 vs 22 years, P < 0.001) than a second group of 46 individuals suffering from other forms of heart disease[29]. Also, in 1998 McKenna described an incidence of excessive alcohol consumption of 40% in a group of 100 DCM patients compared to 23% found in a control group of 211 healthy subjects[8].
Furthermore, Fernández-Solá et al[30], when analysing a population of alcoholics, found a higher prevalence of DCM in alcoholics than among the general population. Specifically, among alcoholics they found a prevalence of DCM of 0.43% in women and 0.25% in men, whereas the described prevalence of DCM in the general population is 0.03% to 0.05%[18,19].
Experimental studies analysing the depressive properties of alcohol on the cardiac muscle invariably use similar approaches[31-39]. Accordingly, a given amount of alcohol is administered to volunteers or alcoholics, followed by the measurement of a number of haemodynamic parameters and, in some cases, echocardiographic parameters. Generally, following alcohol intake, healthy, non-drinking individuals showed an increase in cardiac output due to a decline in peripheral arterial resistance and an increase in cardiac frequency[31]. However, during the time that these haemodynamic changes appeared, some researchers identified a possible decrease in the ejection fraction and other parameters related to systolic function[32-39]. This was questioned by other authors, who pointed out that these conclusions could not be drawn, as alcohol itself also induces changes in the pre-load and after-load conditions, which influence cardiac contractility[35]. However, in this context, experimental in vitro studies using cardiomyocytes have shown that alcohol depresses the contractile capacity of the myocardium, regardless of the sympathetic tone and the haemodynamic conditions[36].
The capacity of alcohol to depress cardiac contractility became evident in studies carried out with chronic alcoholics and in patients with left ventricular dysfunction. In these patients, alcohol, in spite of causing vasodilatation and an increase in the heart rate, did not produce an increase in heart output or, if it did, it was lower than in healthy non-drinking individuals[32,34]. Together, this suggests a depressed contractile capacity. This was specifically addressed by Regan, who found that, after an intake of 81 g of alcohol, the heartbeat volume of a group of chronic alcoholics was reduced and the end diastolic pressure increased, indicating that in these individuals there was a reduction in the left ventricular contractile reserve[32]. This impairment of contractile capacity among chronic alcoholics was demonstrated in the same study using an after-load test with angiotensin. Results showed that the end diastolic pressure increased to a greater extent in alcoholics and was associated with a lower beat volume than in non-drinkers[32].
Myocardial impairment following chronic excessive alcohol intake has been evaluated using echocardiographic and haemodynamic measurements in a significant number of reports. In these studies, haemodynamic and echocardiographic parameters were measured in individuals starting an alcohol withdrawal program. The findings were analysed taking into account the amount and chronicity of intake and they were compared with the same parameters measured in a control group of non-drinkers.
The majority of the echocardiographic studies performed on asymptomatic alcoholics found only mild changes in their hearts with no clear impairment of the systolic function. For example, a slight increase in the pre-ejection period/left ventricular ejection time ratio (PEP/LVET) was found by some authors, suggesting a sub-clinical impairment of systolic function[21,33]. Mathews and Kino found a small, but significant increase in left ventricular mass in individuals consuming at least 12 oz of whisky during 6 years and 60 g of ethanol per day, respectively[22,40]. More recently, Lazarevic found a modest increase in end-systolic and diastolic left ventricular volumes and a subsequent thickening of the posterior wall in a cohort of alcoholics consuming at least 80 g during 5 years[23]; however, no differences in systolic function were observed. Finally, only Urbano-Márquez et al[24] found a clear decrease in the ejection fraction, in a cohort of 52 alcoholics, which was directly proportional to the accumulated alcohol intake throughout the patients’ lives.
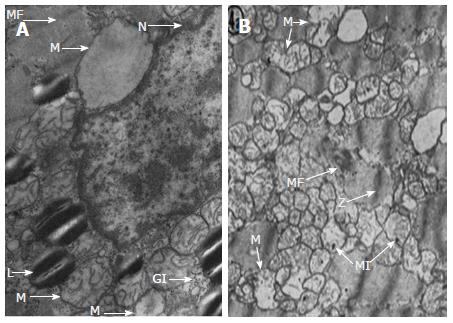
Alterations caused by heavy alcohol intake have also been studied from the perspective of histopathology. Emmanuel Rubin analysed muscle biopsies from individuals who were previously non-drinkers and were submitted to a balanced diet with heavy alcohol intake during one month[41]. Although no significant changes were found using conventional microscopy, when electron microscopy was employed he discovered intracellular swelling, glycogen and lipid accumulation, and alterations in the structure of the sarcoplasmic reticulum and of the mitochondria (Figure 2). These changes, though subtle, were similar to those found by Ferrans and Hibbs in eight deceased individuals diagnosed with ACM[42,43]. On histological examination, various degrees of fibrosis, patchy areas of endocardial fibroelastosis, intramural blood clots and focal collections of swollen cells in both the epicardium and endocardium were found. Also, there were significant size variations in the myofibrils and they showed a relative decrease in the number of striations, in addition to swelling, vacuolisation and hyalinisation. Cell nuclei were larger than normal, morphologically difficult to define and they occasionally showed hyperpigmentation. The authors highlighted the presence of an extensive intracellular accumulation of neutral lipids, principally in the form of small cytoplasmic droplets. In a subsequent study using electron microscopy, the authors found histological features that could be superimposed onto those found in hearts that had suffered hypoxia, anoxia or ischemia[43]. Analogous to the sarcoplasmic reticulum, the mitochondria were swollen or oedema was present, with crest alterations and intra-mitochondrial inclusions suggesting degenerative processes (Figure 2). Moreover, myofibrils showed a progressively distorted structure, resulting in a homogeneous mass.
Despite these features, the structural changes do not seem to be specific, furthermore, they are not qualitatively different from those found in idiopathic DCM and they do not allow us to differentiate between the two conditions[44]. It also appears that the changes emerging in ACM patients only differ from idiopathic DCM in quantitative terms, with histological changes being more striking in idiopathic DCM than in ACM[44].
Basic research studies have described an abundance of mechanisms that could underscore the functional and structural alterations found in ACM. Because of this, their origin could be multifactorial and linked both to the alcohol molecule and to its main metabolite, acetaldehyde.
Coinciding with the histological studies mentioned above, the majority of research on molecular mechanisms describes dysfunctions of intracellular organelles prompting alterations in the lipid-energetic metabolism and in calcium homeostasis, which are especially relevant for the contractile activity of myofibrils.
In spite of numerous studies, the sequence of events that occur in alcohol-induced myocardial damage is still highly controversial. Although some authors contend that the initial event is the appearance of hypertrophy, the majority accept that the core event is the loss of cardiomyocytes.
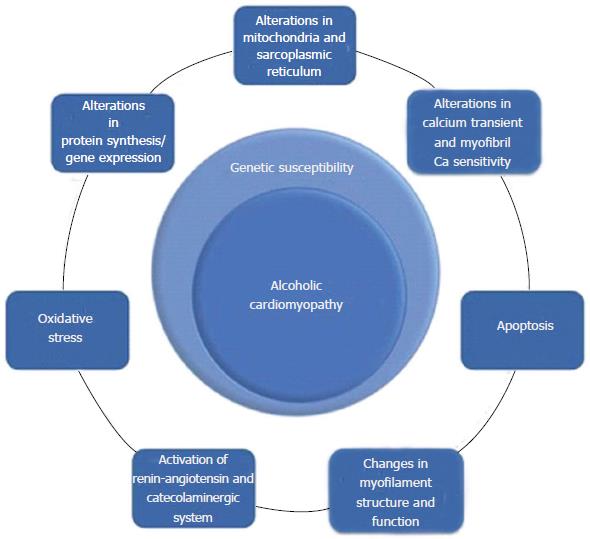
The mechanisms described to date are shown in Figure 3 and they include: apoptosis[45,46], alterations of the excitation-contraction coupling in cardiac myocytes[47], structural and functional alterations of the mitochondria and sarcoplasmic reticulum[41-43], changes in cytosolic calcium flows[48], changes in calcium sensitivity of myofilaments[49,50], alterations of mitochondrial oxidation[37,38,46], deregulation of protein synthesis[51-53], decrease of contractile proteins and disproportion between the different types of myofibrils[54-56], changes in the regulation of myosin ATPase[51], up-regulation of the L-type calcium channels[57], increase of oxidative stress[58,59], induction of ANP and p21 mRNA expression in ventricular myocardium[45], and activation of the renin-angiotensin system and of the sympathetic nervous system[60-62]. Additionally, it has been proposed that mechanisms of a genetic nature play a determining role in the pathophysiology of this disease.
The suspicion that there may be an individual susceptibility to this disease is underscored by the finding that only a small group of alcoholics develop ACM, and that a proportional relationship between myocardial damage and alcohol intake has not been proven.
Although some studies have detailed structural and functional damage in proportion to the amount of alcohol consumed during a patient’s lifetime[24], a large majority of authors have discarded this theory[21-23,25]. Both the absence of a direct correlation and the theory of the existence of a threshold dose (above which some alcoholics develop ACM) require the presence of individual susceptibility to alcohol induced cardiac damage[63]. It is unknown whether individual susceptibility would be related to increased vulnerability at the myocardial level and/or to impaired alcohol metabolism.
One of the few papers analysing genetic susceptibility in ACM was published by Fernández-Solà et al[64] in 2002. He compared the prevalence of different polymorphisms of the angiotensin-converting enzyme gene in 30 ACM patients and in 27 alcoholics with normal ventricular function. The DD genotype was more frequent among ACM patients (56% vs 8%). Furthermore, 89% of the alcoholics with a DD genotype developed ACM, whereas only 13% of those with an II or ID genotype developed this condition. However, this individual susceptibility mediated by polymorphisms of the angiotensin-converting enzyme gene does not appear to be specific to ACM insofar as several diseases, including some that are not of a cardiologic origin, have been related to this genetic finding[65].
Regarding individual susceptibility based on alcohol metabolism, data are scarce, but provocative findings arose from a study published in 2002 which showed that the cardio-depressive power of alcohol in mice varied according to the activity of the enzymes involved in the metabolism of alcohol[66]. In this study, alcohol caused greater cardiomyocyte impairment in mice genetically modified with higher alcohol dehydrogenase activity. The mechanism by which cardiac damage occurred was not fully elucidated, but it was proposed that it was due to the accumulation of acetaldehyde. Furthermore, mice that received an aldehyde dehydrogenase inhibitor experienced an additional impairment in contractility[66]. Regrettably, the role of gene mutations in alcohol or aldehyde dehydrogenase and genetic polymorphisms including ADH1B (*) 2, ALDH2 (*) 2 in humans have not yet been studied.
Finally, it is worth stressing that a large majority of studies on the physiopathology and prognosis of ACM were conducted some years ago, prior to the development of our current understanding regarding the role of genetics in DCM[67]. According to recent data, a genetic form of DCM could be present in up to 50% of idiopathic DCM cases, and other specific forms of DCM such as peripartum cardiomyopathy have been shown to have a genetic basis in a significant number of cases[68]. It is therefore possible that patients with ACM could also harbour a genetic substrate that predisposes them to this form of cardiomyopathy.
Further research is required to determine the definitive role of genetics on ACM pathophysiology.
In spite of the high prevalence of excessive alcohol consumption and of its consideration as one of the main causes of DCM, only a small number of studies have analysed the long-term natural history of ACM. Unfortunately, all the available reports were completed at a time when a majority of the current heart failure therapies were not available (Table 1).
| Ref. | Definition alcohol intake/cardiopathy criteria | Number of patients | NYHA III-IV | % Abstinent | Follow-up | Mortality or heart transplant |
| McDonald et al[69] | > 6 beers/d or 2 quarts of wine/wk or 1 fifth of whisky/wk (44 patients consumed > 6 yr) Cardiomegaly with HF signs or symptoms | 48 | N/A | N/A | N/A | 40% |
| Demakis et al[70] | > 8 oz or 1 L wine or 2 L of beer (ca. 90 g) ≥ 5 yr < 50 years old, HF and cardiothoracic ratio > 0.5 | 57 | 100% | 31% | 40.5 mo | 57% in persistent drinkers 24% in non-drinkers |
| Haissaguerre et al[9] | > 80 g/d; ≥ 5 yr LVEF< 55% or LVEF 55%-59% and LVEDV 115 mL/m2 | 110 | N/A | 44% | 38.8 mo | 50% in persistent drinkers 6% in non-drinkers |
| Prazak et al[12] | > 80 g/d; ≥ 5 yr Heart failure and LVEF < 50% | 23 | 52% | N/A | N/A | 19% (10-yr survival) |
| Fauchier et al[11] | > 80 g/d; ≥ 5 yr DCM: WHO definition and hospitalization or arrhythmia | 50 | 44% | 45% | 47 ± 40 mo | 50% non-drinkers 70% in persistent drinkers |
| Gavazzi et al[10] | > 80 g/d; ≥ 5 yr or 100 mg/d 2 yr LVEF < 50 and HF or arrhythmia | 79 | 35% | 74% | 59 ± 35 mo | Overall: 59% 55% in non-drinkers, 73% in persistent drinkers |
Furthermore, there are conflicting data among studies regarding the prognosis of the condition, with some showing overall mortality near 60% and others showing a mortality rate of only 19% (Table 1).
The first paper to assess the natural history and long-term prognosis of ACM was published by McDonald et al[69] in 1971. He recruited 48 patients admitted to hospital with cardiomegaly without a clear aetiology and severe alcoholism. Patients were treated with diuretics, digitalis and vitamin B. During the follow-up, which varied significantly, 19 patients died (40%). The only factor to predict a poor outcome was the duration of symptoms before admission.
Demakis in 1974 recruited 57 patients with ACM[70]. The patients were drinkers of an amount of alcohol equivalent to > 90-100 g of alcohol per day for at least 5 years. During an average follow-up period of 40.5 mo, 24 deaths occurred among the 57 patients (42%). The adverse prognostic factors found in this study were lasting severe alcohol intake and the duration of HF symptoms.
In 1996, Prazak compared the evolution of a cohort of 42 individuals with idiopathic DCM and that of another group of 23 patients diagnosed with ACM who were seen between the years 1981 and 1992[12]. The populations were homogeneous and showed no clinical or haemodynamic differences at the beginning of the study. After 10 years of follow-up, the authors concluded that patients with ACM had better prognosis than patients with idiopathic DCM. Survival rates after 1, 5 and 10 years were 100%, 81% and 81%, respectively, in the ACM group, and 89%, 48% and 30% among those with idiopathic DCM. The predictive factors of poor prognosis that were identified were of a clinical nature: New York Heart Association (NYHA) functional class III-IV, presence of hepatojugular reflux and congestion. The left ventricular volumes, ejection fraction and filling pressures were only predictors of prognosis among patients with idiopathic DCM.
The latest two papers to be published, unlike previous papers, reported worse outcomes for ACM patients compared to DCM patients. In the first of these studies, Fauchier et al[11] studied 50 patients with ACM and 84 patients with DCM between 1986 and 1997. Although up to 81% of ACM patients received an ACEI, none received beta-blockers and the use of spironolactone was not specified, although it was probably quite low. Also, current common cardiac therapies such as ICD and CRT devices were not used because of the period when the study was conducted. After a follow-up period of 47 mo, a significantly higher survival rate was observed among patients with DCM compared to patients with ACM. In this study, the only independent predictor of cardiac death was alcohol abstinence.
In the second study, Gavazzi led a multicentre study in which, from 1986 to 1995, 79 patients with ACM and 259 patients with DCM were recruited[10]. The average duration of follow-up was 59 ± 35 mo. Transplant-free survival after 7 years was worse among patients with ACM than among those with DCM (41% vs 53%). Among patients who continued drinking heavily, transplant-free survival was significantly worse than in non-drinkers (27% vs 45%). No other predictors were described.
Considering all the works conducted to date, it is clear that new studies on the natural history of ACM are needed, including patients treated with contemporary heart failure therapies. In light of the available data, new studies will help to clarify the current prognosis of ACM compared to DCM and to determine prognostic factors in ACM that might differ from known prognostic factors in DCM.
To date, none of the ACM studies have proposed a treatment for ACM other than that recommended for DCM in current HF guidelines.
From the data provided in the available ACM studies, it appears that patients who received an ACEI globally showed improved prognosis. In contrast, beta-blockers, similar to aldosterone inhibitors, however beneficial they may be, have thus far not yielded sufficient data on their efficacy in relation to this disease.
Regarding ICD and CRT implantation, the same criteria as in DCM are used in ACM, although it is known that excessive alcohol intake is specifically linked to ventricular arrhythmia and sudden cardiac death[71]. As it is not uncommon in ACM for patients to experience a significant recovery of systolic function, it is particularly challenging in this disease to decide the most appropriate time to implant an ICD and whether it is necessary to replace a previously implanted device. Future studies in ACM should also address this topic, which has important economic consequences.
Complete alcohol withdrawal is usually recommended to all patients with ACM. For tens of years, the literature has documented many clinical cases or small series of patients who have undergone a full recovery of ejection fraction and a good clinical evolution after a period of complete alcoholic abstinence. The need for complete withdrawal, however, is still disputed.
Demakis et al[70] in 1974 divided a cohort of 57 ACM patients according to the evolution of their symptoms during follow-up. The sub-group of patients in whom symptoms improved was made up of a larger proportion of non-drinkers (73%), compared to 25% in the group who did not improve, or 17% in the group whose condition worsened. However, a possible confusion factor was identified because the group with clinical improvement also exhibited a shorter evolution of the symptoms and the disease.
Guillo et al[17] in 1997 described the evolution of 9 ACM patients who had been admitted. He divided this cohort into two groups according to the evolution of the ejection fraction during 36 mo in which no deaths were recorded. The 6 subjects who experienced a clear improvement in their ejection fraction had fully refrained from drinking. Conversely, the 3 subjects recording a less satisfactory evolution had persisted in their consumption of alcohol. It should be noted that a moderate drinker included in this latter group showed an improvement of his ejection fraction.
The natural history and long-term prognosis studies of Gavazzi et al[10] and Fauchier et al[11] compared the evolution of ACM patients according to their degree of withdrawal. These authors found a relationship between the reduction or cessation of alcohol consumption and higher survival rates without a heart transplant.
Fauchier et al[11] found that after 47 mo of follow-up, the transplant-free survival of DCM patients was better than that of patients with ACM, but these differences were no longer significant when comparing the DCM group with the alcoholics who refrained from drinking or significantly reduced their alcohol consumption[11].
In the study by Gavazzi et al[10], ACM patients who continued drinking exhibited worse transplant-free survival rates after 7 years than those who stopped drinking alcohol (27% vs 45%)[10].
Ballester specifically analysed the effects of alcohol withdrawal on the myocardium using antimyosin antibodies labelled with Indium-111[72]. This radiotracer has been acknowledged as an indicator of irreversible myocardial damage. Of the 56 patients included in the study, 28 were former drinkers and 28 continued consuming alcohol during the study. Absorption levels of Indium-111 were high in 75% of patients who continued drinking and in only 32% of those who had withdrawn from consuming alcohol.
Of the 19 patients who were studied 9 ± 4 mo after withdrawal, the average absorption level decreased from an average HLR of 1.76 ± 0.17 to 1.55 ± 0.19 and this was associated with a significant improvement in the ejection fraction, from 30% ± 12% to 43% ± 16% (P < 0.01).
Data supporting the beneficial effect of continuing with alcohol intake at moderate levels in ACM patients arose from the observation that published studies evaluating the effect of alcohol abstinence included ACM patients who reduced their alcohol intake to low/moderate levels alongside ACM patients who stopped their alcohol intake altogether[9-12]. Also, low to moderate daily alcohol intake was proved to be a predictor of better prognosis for both ischemic cardiomyopathy and heart failure regardless of the presence of coronary disease[1,2].
Additionally, echocardiographic data suggest that subjects who do not fully withdraw from alcohol consumption, but who reduce it to moderate amounts recover LVEF in a similar manner to strict non-drinkers. Thus, Nicolás et al[73] studied the evolution of the ejection fraction in 55 patients with ACM according to their degree of withdrawal. The population was divided into 3 groups according to their intake volume during the follow-up period. At the end of the first year, no differences were found among the non-drinkers, who improved by 13.1%, and among those who reduced consumption to 20-60 g/d (with an average improvement of 12.2%). Conversely, those whose consumption remained in excess of 80 g/d showed an average decline of 3.8% in their ejection fraction.
Thus, although there is a certain degree of consensus regarding the recommendation of full alcohol withdrawal in ACM, it is yet to be resolved whether moderate alcohol consumption is sufficient to achieve an improvement in the prognosis of these patients.
Future studies with a strict classification of non-drinkers and drinkers will help clarify whether complete abstinence is mandatory for ACM patients. In the interim it seems appropriate to continue discouraging any alcohol consumption in these patients, as it would be difficult for them to maintain a limited alcohol intake considering their history of alcohol dependence and abuse.
In all ACM studies, inclusion of patients is based on patients’ self-reported alcohol drinking habits, which may lead to an underestimation of the prevalence of ACM together with problematic identification of patients who abstain and those who continue drinking. Although analytical markers of alcohol consumption, such as average erythrocyte volume and serum gamma-glutamyltranspeptidase levels, could be an aid to establish abstinence or persistence of alcohol intake in patients, the quantity of alcohol intake is dependent on the patients’ report. Furthermore, in many of these reports, comorbid conditions, especially myocarditis and other addictions such as cocaine and nicotine, were not reported.
As pointed out before, the current accepted definition of ACM probably underestimates the number of women affected by the disease. Alcohol affects heart function and is dependent on the quantity of alcohol that the heart is exposed to. Women typically have a lower BMI than men, and therefore the same alcohol exposure can be achieved with lower alcohol intake.
ACM is an important clinical entity known since the 19th century. Epidemiological and experimental studies link excessive alcohol intake to the development of DCM. Although not based on solid experimental or epidemiological data, the currently accepted definition of ACM requires chronic exposure to > 80 g/d of alcohol for > 5 years. There is a surprising paucity of clinical data on ACM prognosis and particularly on ACM evolution under modern HF therapies. In the absence of robust data, current therapy of ACM should include complete alcohol abstinence along with all the therapies recommended to treat DCM. Further studies in the field of ACM are required.
P- Reviewer: Alzand BSN, Ghanem A, Li XP, Ueda H, Xu Y S- Editor: Wen LL L- Editor: Webster JR E- Editor: Wu HL
| 1. | Bryson CL, Mukamal KJ, Mittleman MA, Fried LP, Hirsch CH, Kitzman DW, Siscovick DS. The association of alcohol consumption and incident heart failure: the Cardiovascular Health Study. J Am Coll Cardiol. 2006;48:305-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 2. | Abramson JL, Williams SA, Krumholz HM, Vaccarino V. Moderate alcohol consumption and risk of heart failure among older persons. JAMA. 2001;285:1971-1977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 183] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 3. | Movva R, Figueredo VM. Alcohol and the heart: to abstain or not to abstain? Int J Cardiol. 2013;164:267-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Kannel WB. Incidence and epidemiology of heart failure. Heart Fail Rev. 2000;5:167-173. [PubMed] |
| 5. | Gottdiener JS, Arnold AM, Aurigemma GP, Polak JF, Tracy RP, Kitzman DW, Gardin JM, Rutledge JE, Boineau RC. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35:1628-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 658] [Cited by in RCA: 646] [Article Influence: 25.8] [Reference Citation Analysis (1)] |
| 6. | Stehlik J, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, Dipchand AI, Dobbels F, Kirk R, Rahmel AO, Hertz MI. The Registry of the International Society for Heart and Lung Transplantation: 29th official adult heart transplant report--2012. J Heart Lung Transplant. 2012;31:1052-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 441] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 7. | Graves EJ. Detailed diagnoses and procedures, National Hospital Discharge Survey, 1993. Vital Health Stat 13. 1995;1-288. [PubMed] |
| 8. | McKenna CJ, Codd MB, McCann HA, Sugrue DD. Alcohol consumption and idiopathic dilated cardiomyopathy: a case control study. Am Heart J. 1998;135:833-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 66] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 9. | Haissaguerre M, Fleury B, Gueguen A, Bonnet J, Lorente P, Nakache JP, Broustet JP, Dallochio M, Besse P. [Mortality of dilated myocardiopathies as a function of continuation of alcohol drinking. Multivariate analysis concerning 236 patients]. Presse Med. 1989;18:711-714. [PubMed] |
| 10. | Gavazzi A, De Maria R, Parolini M, Porcu M. Alcohol abuse and dilated cardiomyopathy in men. Am J Cardiol. 2000;85:1114-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 86] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Fauchier L, Babuty D, Poret P, Casset-Senon D, Autret ML, Cosnay P, Fauchier JP. Comparison of long-term outcome of alcoholic and idiopathic dilated cardiomyopathy. Eur Heart J. 2000;21:306-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 92] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 12. | Prazak P, Pfisterer M, Osswald S, Buser P, Burkart F. Differences of disease progression in congestive heart failure due to alcoholic as compared to idiopathic dilated cardiomyopathy. Eur Heart J. 1996;17:251-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 42] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Bollinger O. Ueber die haufigkeit und ursachen der idiopathischen herzhypertrophie in munchen. Deutsch Med Wchnschr. 1884;180e1. |
| 14. | Mackenzie W. The study of the pulse, arterial, venous, and hepatic, and of the movements of the heart. Am J Med Sci. 1902;124. |
| 15. | Mansourati J, Forneiro I, Genet L, Le Pichon J, Blanc JJ. Regression of dilated cardiomyopathy in a chronic alcoholic patient after abstinence from alcohol. Arch Mal Coeur Vaiss. 1990;83:1849-1852; discussion 1853. [PubMed] |
| 16. | Mølgaard H, Kristensen BO, Baandrup U. Importance of abstention from alcohol in alcoholic heart disease. Int J Cardiol. 1990;26:373-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Guillo P, Mansourati J, Maheu B, Etienne Y, Provost K, Simon O, Blanc JJ. Long-term prognosis in patients with alcoholic cardiomyopathy and severe heart failure after total abstinence. Am J Cardiol. 1997;79:1276-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, Moss AJ, Seidman CE, Young JB. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113:1807-1816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2284] [Cited by in RCA: 2241] [Article Influence: 117.9] [Reference Citation Analysis (0)] |
| 19. | Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F, Charron P, Dubourg O, Kühl U, Maisch B, McKenna WJ. Classification of the cardiomyopathies: a position statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2008;29:270-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1766] [Cited by in RCA: 1891] [Article Influence: 105.1] [Reference Citation Analysis (0)] |
| 20. | Koide T, Ozeki K. The incidence of myocardial abnormalities in man related to the level of ethanol consumption. A proposal of a diagnostic criterion of alcoholic cardiomyopathy. Jpn Heart J. 1974;15:337-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Askanas A, Udoshi M, Sadjadi SA. The heart in chronic alcoholism: a noninvasive study. Am Heart J. 1980;99:9-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 65] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Kino M, Imamitchi H, Morigutchi M, Kawamura K, Takatsu T. Cardiovascular status in asymptomatic alcoholics, with reference to the level of ethanol consumption. Br Heart J. 1981;46:545-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 23. | Lazarević AM, Nakatani S, Nesković AN, Marinković J, Yasumura Y, Stojicić D, Miyatake K, Bojić M, Popović AD. Early changes in left ventricular function in chronic asymptomatic alcoholics: relation to the duration of heavy drinking. J Am Coll Cardiol. 2000;35:1599-1606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 95] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Urbano-Marquez A, Estruch R, Navarro-Lopez F, Grau JM, Mont L, Rubin E. The effects of alcoholism on skeletal and cardiac muscle. N Engl J Med. 1989;320:409-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 415] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 25. | Kupari M, Koskinen P. Relation of left ventricular function to habitual alcohol consumption. Am J Cardiol. 1993;72:1418-1424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | World Health Organization. Management of Substance Abuse Team. Global status report on alcohol and health. Geneva, Switzerland: World Health Organization 2011; . |
| 27. | Kasper EK, Agema WR, Hutchins GM, Deckers JW, Hare JM, Baughman KL. The causes of dilated cardiomyopathy: a clinicopathologic review of 673 consecutive patients. J Am Coll Cardiol. 1994;23:586-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 172] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 28. | Komajda M, Richard JL, Bouhour JB, Sacrez A, Bourdonnec C, Gerbaux A, Rozensztajn L, Lablanche JM, Matinat D, Morand P. Dilated cardiomyopathy and the level of alcohol consumption: a planned multicentre case-control study. Eur Heart J. 1986;7:512-519. [PubMed] |
| 29. | Gillet C, Juilliere Y, Pirollet P, Aubin HJ, Thouvenin A, Danchin N, Cherrier F, Paille F. Alcohol consumption and biological markers for alcoholism in idiopathic dilated cardiomyopathy: a case-controlled study. Alcohol Alcohol. 1992;27:353-358. [PubMed] |
| 30. | Fernández-Solà J, Estruch R, Nicolás JM, Paré JC, Sacanella E, Antúnez E, Urbano-Márquez A. Comparison of alcoholic cardiomyopathy in women versus men. Am J Cardiol. 1997;80:481-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 88] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 31. | Juchems R, Klobe R. Hemodynamic effects of ethyl alcohol in man. Am Heart J. 1969;78:133-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Regan TJ, Levinson GE, Oldewurtel HA, Frank MJ, Weisse AB, Moschos CB. Ventricular function in noncardiacs with alcoholic fatty liver: role of ethanol in the production of cardiomyopathy. J Clin Invest. 1969;48:397-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 154] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 33. | Zambrano SS, Mazzotta JF, Sherman D, Spodick DH. Cardiac dysfunction in unselected chronic alcoholic patients: noninvasive screening by systolic time intervals. Am Heart J. 1974;87:318-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 34. | Greenberg BH, Schutz R, Grunkemeier GL, Griswold H. Acute effects of alcohol in patients with congestive heart failure. Ann Intern Med. 1982;97:171-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Kupari M. Acute cardiovascular effects of ethanol A controlled non-invasive study. Br Heart J. 1983;49:174-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 36. | Kupari M, Heikkilä J, Tolppanen EM, Nieminen MS, Ylikahri R. Acute effects of alcohol, beta blockade, and their combination on left ventricular function and hemodynamics in normal man. Eur Heart J. 1983;4:463-471. [PubMed] |
| 37. | Kupari M, Koskinen P, Suokas A, Ventilä M. Left ventricular filling impairment in asymptomatic chronic alcoholics. Am J Cardiol. 1990;66:1473-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 49] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 38. | Delgado CE, Gortuin NJ, Ross RS. Acute effects of low doses of alcohol on left ventricular function by echocardiography. Circulation. 1975;51:535-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 78] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 39. | Cameli M, Ballo P, Garzia A, Lisi M, Bocelli A, Mondillo S. Acute effects of low doses of ethanol on left and right ventricular function in young healthy subjects. Alcohol Clin Exp Res. 2011;35:1860-1865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 40. | Mathews EC, Gardin JM, Henry WL, Del Negro AA, Fletcher RD, Snow JA, Epstein SE. Echocardiographic abnormalities in chronic alcoholics with and without overt congestive heart failure. Am J Cardiol. 1981;47:570-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 86] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 41. | Rubin E. Alcoholic myopathy in heart and skeletal muscle. N Engl J Med. 1979;301:28-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 135] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 42. | Ferrans VJ, Hibbs RG, Weilbaecher DG, Black WC, Walsh JJ, Burch GE. Alcoholic cardiomyopathy; a histochemical study. Am Heart J. 1965;69:748-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 81] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 43. | Hibbs RG, Ferrans VJ, Black WC, Weilbaecher DG, Burch GE. Alcoholic cardiomyopathy; an electron microscopic study. Am Heart J. 1965;69:766-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 119] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 44. | Teragaki M, Takeuchi K, Takeda T. Clinical and histologic features of alcohol drinkers with congestive heart failure. Am Heart J. 1993;125:808-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 45. | Jänkälä H, Eklund KK, Kokkonen JO, Kovanen PT, Linstedt KA, Härkönen M, Mäki T. Ethanol infusion increases ANP and p21 gene expression in isolated perfused rat heart. Biochem Biophys Res Commun. 2001;281:328-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 46. | Chen DB, Wang L, Wang PH. Insulin-like growth factor I retards apoptotic signaling induced by ethanol in cardiomyocytes. Life Sci. 2000;67:1683-1693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 47. | Danziger RS, Sakai M, Capogrossi MC, Spurgeon HA, Hansford RG, Lakatta EG. Ethanol acutely and reversibly suppresses excitation-contraction coupling in cardiac myocytes. Circ Res. 1991;68:1660-1668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 64] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 48. | Thomas AP, Sass EJ, Tun-Kirchmann TT, Rubin E. Ethanol inhibits electrically-induced calcium transients in isolated rat cardiac myocytes. J Mol Cell Cardiol. 1989;21:555-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 49. | Figueredo VM, Chang KC, Baker AJ, Camacho SA. Chronic alcohol-induced changes in cardiac contractility are not due to changes in the cytosolic Ca2+ transient. Am J Physiol. 1998;275:H122-H130. [PubMed] |
| 50. | Piano MR, Rosenblum C, Solaro RJ, Schwertz D. Calcium sensitivity and the effect of the calcium sensitizing drug pimobendan in the alcoholic isolated rat atrium. J Cardiovasc Pharmacol. 1999;33:237-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 51. | Siddiq T, Salisbury JR, Richardson PJ, Preedy VR. Synthesis of ventricular mitochondrial proteins in vivo: effect of acute ethanol toxicity. Alcohol Clin Exp Res. 1993;17:894-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 52. | Tiernan JM, Ward LC. Acute effects of ethanol on protein synthesis in the rat. Alcohol Alcohol. 1986;21:171-179. [PubMed] |
| 53. | Schreiber SS, Oratz M, Rothschild MA. Alcoholic cardiomyopathy: the effect of ethanol and acetaldehyde on cardiac protein synthesis. Recent Adv Stud Cardiac Struct Metab. 1975;7:431-442. [PubMed] |
| 54. | Preedy VR, Patel VB, Why HJ, Corbett JM, Dunn MJ, Richardon PJ. Alcohol and the heart: biochemical alterations. Cardiovasc Res. 1996;31:139-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 55. | Meehan J, Piano MR, Solaro RJ, Kennedy JM. Heavy long-term ethanol consumption induces an alpha- to beta-myosin heavy chain isoform transition in rat. Basic Res Cardiol. 1999;94:481-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 56. | Capasso JM, Li P, Guideri G, Malhotra A, Cortese R, Anversa P. Myocardial mechanical, biochemical, and structural alterations induced by chronic ethanol ingestion in rats. Circ Res. 1992;71:346-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 54] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 57. | Guppy LJ, Littleton JM. Effect of calcium, Bay K 8644, and reduced perfusion on basic indices of myocardial function in isolated hearts from rats after prolonged exposure to ethanol. J Cardiovasc Pharmacol. 1999;34:480-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 58. | Amici A, Levine RL, Tsai L, Stadtman ER. Conversion of amino acid residues in proteins and amino acid homopolymers to carbonyl derivatives by metal-catalyzed oxidation reactions. J Biol Chem. 1989;264:3341-3346. [PubMed] |
| 59. | Paradis V, Kollinger M, Fabre M, Holstege A, Poynard T, Bedossa P. In situ detection of lipid peroxidation by-products in chronic liver diseases. Hepatology. 1997;26:135-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 159] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 60. | Adams MA, Hirst M. Metoprolol suppresses the development of ethanol-induced cardiac hypertrophy in the rat. Can J Physiol Pharmacol. 1990;68:562-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 61. | Cheng CP, Cheng HJ, Cunningham C, Shihabi ZK, Sane DC, Wannenburg T, Little WC. Angiotensin II type 1 receptor blockade prevents alcoholic cardiomyopathy. Circulation. 2006;114:226-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 62. | Kim SD, Beck J, Bieniarz T, Schumacher A, Piano MR. A rodent model of alcoholic heart muscle disease and its evaluation by echocardiography. Alcohol Clin Exp Res. 2001;25:457-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 63. | Kupari M, Koskinen P, Suokas A. Left ventricular size, mass and function in relation to the duration and quantity of heavy drinking in alcoholics. Am J Cardiol. 1991;67:274-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 64. | Fernández-Solà J, Nicolás JM, Oriola J, Sacanella E, Estruch R, Rubin E, Urbano-Márquez A. Angiotensin-converting enzyme gene polymorphism is associated with vulnerability to alcoholic cardiomyopathy. Ann Intern Med. 2002;137:321-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 65. | Gard PR. Implications of the angiotensin converting enzyme gene insertion/deletion polymorphism in health and disease: a snapshot review. Int J Mol Epidemiol Genet. 2010;1:145-157. [PubMed] |
| 66. | Duan J, McFadden GE, Borgerding AJ, Norby FL, Ren BH, Ye G, Epstein PN, Ren J. Overexpression of alcohol dehydrogenase exacerbates ethanol-induced contractile defect in cardiac myocytes. Am J Physiol Heart Circ Physiol. 2002;282:H1216-H1222. [PubMed] |
| 67. | Garcia-Pavia P, Cobo-Marcos M, Guzzo-Merello G, Gomez-Bueno M, Bornstein B, Lara-Pezzi E, Segovia J, Alonso-Pulpon L. Genetics in dilated cardiomyopathy. Biomark Med. 2013;7:517-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 68. | van Spaendonck-Zwarts KY, van Tintelen JP, van Veldhuisen DJ, van der Werf R, Jongbloed JD, Paulus WJ, Dooijes D, van den Berg MP. Peripartum cardiomyopathy as a part of familial dilated cardiomyopathy. Circulation. 2010;121:2169-2175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 157] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 69. | McDonald CD, Burch GE, Walsh JJ. Alcoholic cardiomyopathy managed with prolonged bed rest. Ann Intern Med. 1971;74:681-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 70. | Demakis JG, Proskey A, Rahimtoola SH, Jamil M, Sutton GC, Rosen KM, Gunnar RM, Tobin JR. The natural course of alcoholic cardiomyopathy. Ann Intern Med. 1974;80:293-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 145] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 71. | George A, Figueredo VM. Alcohol and arrhythmias: a comprehensive review. J Cardiovasc Med (Hagerstown). 2010;11:221-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 72. | Ballester M, Martí V, Carrió I, Obrador D, Moya C, Pons-Lladó G, Bernà L, Lamich R, Aymat MR, Barbanoj M. Spectrum of alcohol-induced myocardial damage detected by indium-111-labeled monoclonal antimyosin antibodies. J Am Coll Cardiol. 1997;29:160-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 73. | Nicolás JM, Antúnez E, Thomas AP, Fernández-Solà J, Tobías E, Estruch R, Urbano-Márquez A. Ethanol acutely decreases calcium transients in cultured human myotubes. Alcohol Clin Exp Res. 1998;22:1086-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |